Abstract
Chicory is a perennial plant grown in different parts of the world, used as forage for livestock, as folklore remedies, or as a vegetable addition in human diets. There are several varieties of the chicory plant, known differently globally due to its numerous medicinal, culinary, and nutritional qualities. Most parts of the plant contain a potpourri of nutrients ranging within carbohydrates, proteins, vitamins, minerals, soluble fiber, trace elements, and bioactive phenolic compounds, which are responsible for the various nutritive, prophylactic, and therapeutic qualities of chicory. Inulin, coumarins, tannins, monomeric flavonoids, and sesquiterpene lactones are some of the major phytocompounds mostly found in chicory plants. The health-promoting activities attributed to chicory comprise, among others, anti-inflammatory, anticarcinogenic, antiviral, antibacterial, antimutagenic, antifungal, anthelmintic, immune-stimulating, and antihepatotoxic and its antioxidative qualities. As a versatile plant, chicory's chemical composition and use as a suitable livestock feed supplement or as an alternative feed ingredient (AFI) are thus reviewed.
1. Introduction
The use of cereals in animal feeds competes with human nutrition, and some of the cereals are too expensive. Therefore, the introduction of alternative feedstuffs that could overcome the challenges of production cost in the livestock industry as well as impact positively animal health, production yield, and product quality has become necessary. Some of these AFI such as chicory parts (seeds, leaves, and roots) (Figure 1) have successfully been used as supplements in small ruminant diets, without compromising animal performance. Cichorium intybus, a perennial herbaceous plant of the Asteraceae family, has been used for ages as livestock forage in various parts of the world. Popularity of chicory is steadily growing owing to its numerous medicinal, culinary, and nutritional qualities.
Figure 1.
Useable parts of the chicory plant. (a) Seeds, (b) flower, (c) roots, (d) roasted roots, and (e) chicory field [28].
The leaves and flowers are usually used as vegetables in salads and the roots are used as a coffee substitute, livestock feedstuff, or pet food [1]. Chicory extracts are sometimes added to alcoholic and nonalcoholic beverages to improve taste [2], while the inulin rich tuberous roots can be converted to alcohol [3]. In some countries, parts of the chicory plant are used as ethnoveterinary remedy for bodily ailments and disorders and for prophylactic purposes in both humans and livestock [4, 5]. Since fresh chicory roots have a rather bitter taste, the roots are normally debittered by boiling, drying, baking or roasting, and soaking in water or citric acid solution and then chopped or milled before being used as coffee blends, feed, or a functional food ingredient [1, 6–8].
Chicory root contains some phytochemicals such as inulin (starch-like polysaccharide), coumarins, flavonoids, sesquiterpene lactones (lactucin and lactucopicrin), tannins, alkaloids, vitamins, minerals, and volatile oils [9]. The secondary metabolites (flavonoids, tannins, and coumarins) found in chicory have been reported to demonstrate some biological activities such as antioxidant, anticancer, anti-inflammatory, antiparasitic, antihepatotoxic, which impact positive health effect on humans and livestock [10, 11]. Inulin is a polymer of fructose with β-(2-1)-glycosidic-linkage [12], which accounts for up to 68% of the total compounds present in fresh chicory roots [13, 14]. As a prebiotic, inulin is low in calorie and dietary fiber, making it a good replacement for sugar and an ideal component for diabetic nutrition [15, 16].
Furthermore, animal performance on forage chicory is similar to that of legumes and superior to grass‐based pastures [11]. According to [17], chicory increases milk production when offered as a supplement to pasture. As reported elsewhere [18–21], grazing chicory is known to decrease some internal parasites in livestock and therefore has potential to reduce the use of anthelmintics. This paper reviews and summarizes research opinions on chicory's chemical constituents and associated nutritional properties, which makes it an ideal complementary and/or alternative livestock feed supplement.
2. Phytochemical Constituents of Chicory
A recent reduction in the incidences of chronic diseases is largely associated with high dietary ingestions of varied vegetables and fruits [22]. The health-promoting factors in these plant parts, commonly called phytochemicals, have extensive biological activities such as antioxidant, anticancer, anti-inflammatory, and α-glucosidase inhibition. The very common phytocompounds are phenolic acids, which include chlorogenic acids, and flavonoids (anthocyanins, flavonols, flavanone, and flavan-3-ols). The plant polyphenols usually occur as glycosides, which makes them less reactive and easier to store in the cell vacuole [23]. Cleavage of the glycosidic-linkage and associated rearrangement reactions releases the following sugar residues: hexose, glucose or galactose, deoxyhexose, rhamnose, pentose, xylose or arabinose, and glucuronic acid [23]. The anthocyanins have been reported to reduce the risk of coronary heart disease in animals by exhibiting arterial protection, inhibition of platelet aggregation, and protection of endothelial tissues [23–26].
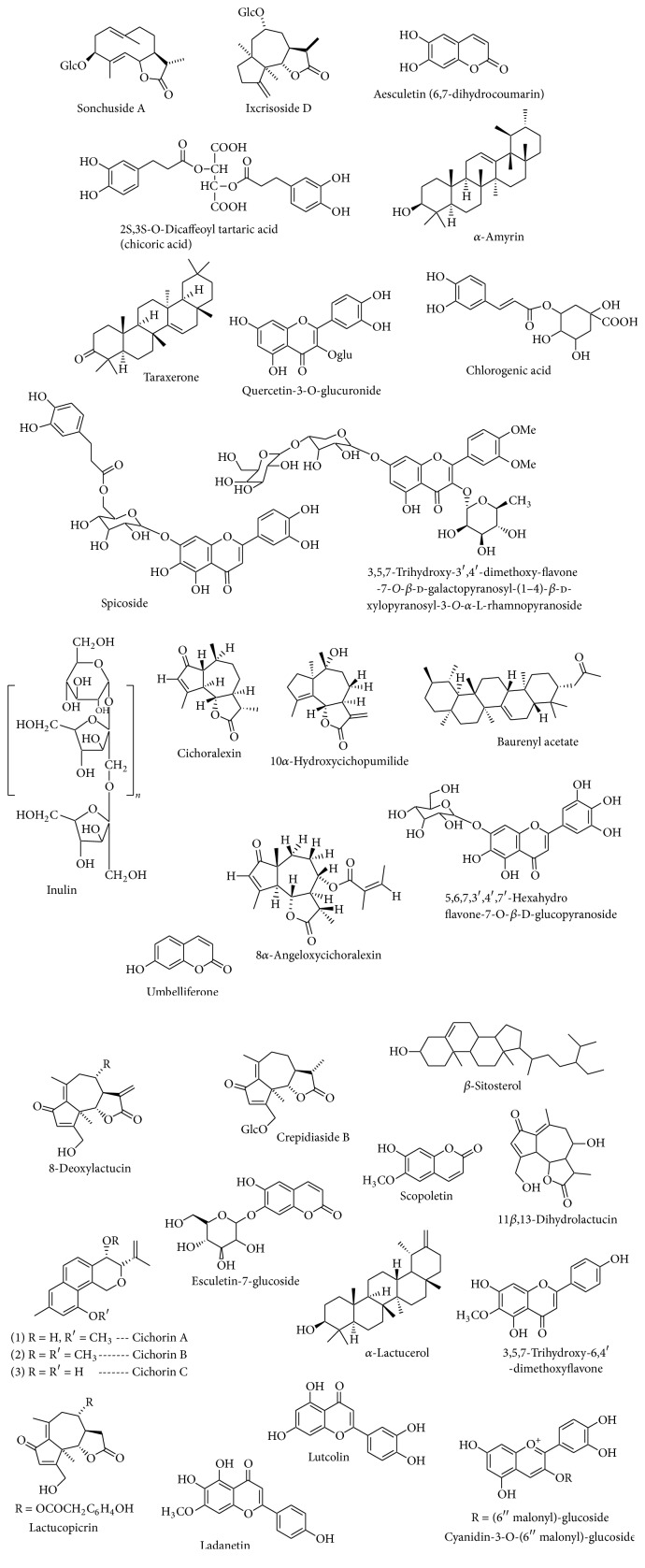
Many studies have shown that chicory is an embodiment of animal health-promoting phytocompounds, which includes condensed tannins. However, when the tannins are present in high concentrations in the diet, it can negatively affect animal productivity [27]. Another study by Carazzone et al. (2013) [23] recorded the extraction of phenolic acids and flavonoids from several types of Cichorium intybus var. silvestre and the characterization of the compounds using high-performance liquid chromatography-electrospray ionisation/mass spectrometry. Sixty-four compounds were detected, which include several hydroxycinnamic acid derivatives comprising eight mono- and dicaffeoylquinic acids, three tartaric acid derivatives, thirty-one flavonol and two flavone glycosides, and ten anthocyanins as well as several isomers of caffeic acid derivatives. The recent review report of [11] focused on ethnomedicinal, botanical, and phytopharmacological aspects of Cichorium intybus and identification of some phytoconstituents of chicory as presented in Figure 2.
Figure 2.
Identified phytoconstituents of chicory [11].
Massoud et al. (2009) [1] investigated the chemical composition of the leaves and roots of Cichorium Intybus and the representative phytoconstituents presented as macronutrients, micronutrients (essential minerals), and phenolic compounds in Tables 1, 2, and 3, respectively.
Table 1.
Macronutrient composition of chicory plant∗ [1].
| Chemical composition% | Roots | Leaves |
|---|---|---|
| Moisture content | 75.63 ± 0.39 | 83.06 ± 1.55 |
| Crude protein | 4.65 ± 0.25 | 14.70 ± 1.03 |
| Crude ether extract | 1.69 ± 0.71 | 3.68 ± 0.19 |
| Ash | 4.25 ± 0.11 | 10.91 ± 1.86 |
| Total carbohydrates | 89.41 ± 1.07 | 70.71 ± 3.08 |
| Total soluble sugars | 11.06 ± 1.00 | 7.80 ± 1.45 |
| Inulin | 44.69 ± 0.88 | 10.95 ± 2.56 |
| Crude fiber | 5.12 ± 1.55 | 16.78 ± 2.20 |
| Dietary fiber (DF) | ||
| Insoluble DF | 30.73 ± 0.33 | ND∗∗ |
| Soluble DF | 0.42 ± 0.07 | ND∗∗ |
| Total DF | 31.15 | ND∗∗ |
∗On dry weight basis; mean ± 5.0 (each value represents the average of three determinations ± standard deviation); ∗∗ND: not determined.
Table 2.
Mineral content (mg/100 g) of chicory plant (leaves and roots) in comparison with RDA∗ [1].
| Chicory plant | Macroelements | Microelements | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | K | Mg | Na | Fe | Cu | Mn | Zn | Pb | |
| Roots | 181.26 ± 4.40 | 103.7 ± 4.62 | 20.14 ± 1.69 | 67.42 ± 2.45 | 1.77 ± 0.21 | 0.36 ± 0.02 | 0.31 ± 0.10 | 0.39 ± 0.03 | 0.04 ± 0.003 |
| Leaves | 292.61 ± 13.35 | 166.57 ± 3.43 | 6.944 ± 5.86 | 88.84 ± 2.58 | 9.178 ± 0.85 | 0.60 ± 0.06 | 0.90 ± 0.01 | 0.91 ± 0.03 | 0.03 ± 0.01 |
| RDA mg/100 g | 1000–1300 | - | 240–420 | 1600 | 8–11 | 0.8–1.2 | 1.6–2.3 | 12–15 | - |
∗RDA recommended daily requirement for men and women.
Table 3.
Chicory extracts (%) as identified by HPLC [1].
| Chicory | Methanolic extracts (%) | Total phenolic content∗ | Phenolic compound (%) | |
|---|---|---|---|---|
| Leaves | 23.16 | 26.4 ± 1.05 | Gallic acid | 1.96 |
|
| ||||
| Roots | 10.75 | 20.0 ± 0.9 | Protocatechuic acid | 2.50 |
| Chlorogenic acid | 17.84 | |||
| p-Hydroxybenzoic acid | 11.04 | |||
| Caffeic acid | 35.22 | |||
| Isovanillic acid | 1.97 | |||
| p-Coumaric acid | 9.65 | |||
| Unknown compound | 19.46 | |||
| Protocatechuic acid | 1.77 | |||
| Chlorogenic acid | 10.85 | |||
| Caffeic acid | 24.36 | |||
| m-Coumaric acid | 27.90 | |||
| p-Coumaric acid | 25.03 | |||
| Unknown compound | 10.09 | |||
∗Values expressed as mg GAE g−1 dry extract (mean of three replicates ± standard deviation).
Many researchers noted that fresh chicory roots contain, by dry weight, 68% of inulin (a polysaccharide similar to starch), 14% sucrose, 5% cellulose, 6% protein, 4% ash, and 3% other compounds [13, 30–32]. These researchers also noted that dry chicory root extract contains, by weight, approximately 98% inulin and 2% of other compounds. A study by Soobo (2005) [33], also agreed that chicory root is high in inulin-type fructan and oligofructose. Chemically, inulin is a polydisperse-(2,1)-fructan and can be converted to fructose and glucose by hydrolysis. Chicory contains affluent quantities of fructose (up to 94%), which is a long chain carbohydrate, consisting of 22–60 fructose molecules with a terminal glucose molecule [34]. High quantities of sesquiterpene lactones, such as lactucin, 8-deoxylactucin, lactucopicrin, and 11ß-dihydro-derivatives, are hugely responsible for their bitterness [35]. This was confirmed by some researchers [36], who isolated sesquiterpene lactones, a (+)-germacrene from chicory roots. According to a study by [36], roasted chicory roots contain various compounds like 2-acetylpyrrole, furfural, phenyl acetaldehyde, phenyl acetic acid, vanillin, pyrazines, benzothiazoles, aldehydes, aromatic hydrocarbons, furans, phenols, organic acids, and small quantities of insole alkaloid (ß-carbolines), harmane and norharmane. Chicory root extract, produced by removing the insoluble fraction of milled dry root in water by filtration and centrifugation, contains volatile oils, fatty acids, alkaloids, triterpenes, flavonoids, latex, tannins, and saponins [37]. Three new benzo-isochromenes (Cichorins A, B, and C) were isolated from chicory roots [38, 39]. According to these researchers, the roots also contain tannins, fatty acids (mostly palmitic and linoleic), pectin, α-lactucerol (taraxasterol), cichoriin (esculetin-7-glucoside), sugars (especially fructose and mannose), fixed oils, choline, and others.
Chicory seeds contain a rich potpourri of nutrients ideal for both ruminant and monogastric nutrition. Ying and Gui (2012) [40] stated that most varieties of chicory seeds have high amounts of crude protein which is more than 19% of the dry weight and 1.6–2.4 times higher than the value in most conventional grains, like wheat, rice, corn, and barley. These authors also noted that good sources of most essential amino acids like methionine, lysine, leucine, isoleucine, phenylalanine, and so on, which are recommended for an ideal dietary protein, can be obtained from chicory seeds. Moreover, the seeds contain abundant demulcent oils, a good source of both saturated and unsaturated fatty acids [41], having the essential linoleic acid (18:2n-6) content of more than 76% of the total fatty acids profile which includes the monounsaturated oleic acid (18:1n-9), stearic acid (18:0), and palmitic acid (16:0) [40]. Relatively high levels of essential minerals such as potassium (K), calcium (Ca), magnesium (Mg), selenium (S), and zinc (Zn) are found in chicory seeds, when compared with those of alfalfa seeds [42]. Elsewhere, [40] identified phosphorus, potassium, calcium, magnesium, sodium (mg g−1), iron (mg g−1), copper (μg g−1), zinc (μg g−1), and manganese (μg g−1) as the minerals present in chicory seeds. Some researchers [43] isolated cichotyboside, a sesquiterpene glycoside, from Cichorium intybus seeds, which was verified to exhibit a significant hepatoprotective activity in rats against carbon tetrachloride induced hepatic damage. Chicory seed can therefore be said to be a healthy alternative and/or complementary nutritional component in animal and human diets.
In 2002, Nørbæk and coworkers [44] identified anthocyanins as the contributory factor for the blue colour of the perianth in chicory flower and [45, 46] later reported that chicory flower contains saccharides, flavonoids, cichorine, methoxycoumarin, and essential oils. Earlier, these scientists [2] established that chicory flowers, leaves, and shoots contain inulin, fructose, choline, resin, chicoric acid (dicaffeoyl tartaric acid), esculetin, esculin (esculetin-6-glucoside), cichoriin, umbelliferone, scopoletin, 6,7-dihydrocoumarin, and sesquiterpene lactones and their glycosides. Also, some investigators [47, 48] isolated vitamins A, B6, and K from red chicory which contains carotenoids and some minerals as well. In a food chemistry study by [23, 49], chicoric acid was found to be the major compound derived from the methanolic extracts of chicory. Similarly, earlier investigations [45] noted that octane, n-nonadecane, pentadecanone, and hexadecane have been found to be the primary volatile components of chicory. Aliphatic compounds and their derivatives make up the main fraction of the chicory plant, while the minor constituents are mostly terpenoids [50].
3. Ethnomedicinal Benefit of Chicory in Livestock Production
Inulin found in chicory is a source of soluble dietary fiber, a prototype prebiotic especially beneficial in monogastric nutrition and also used as a functional food additive [14, 51, 52]. Various studies have reported that prebiotics stimulates the growth of host-beneficial gut bacteria, such as lactobacilli and bifidobacteria for overall beneficial health [53, 54]. In addition, a prebiotic may stimulate the immune system, decrease the levels of pathogenic bacteria in the intestine [55], relieve constipation, decrease the risk of osteoporosis by increasing essential mineral absorption [56] especially calcium, and reduce the risk of atherosclerosis by lowering the synthesis of triglycerides and fatty acids in the liver as well as lowering their serum levels [54]. Interestingly, probiotic inulin has been indicated to reduce boar taint and odorous compounds in colon and rectal contents when included in male pig diets [20, 57, 58].
Some of the plant secondary metabolites (PSM) of the chicory plant have been reported to possess some ethnomedicinal properties. These PSM serve as herbal remedies for ailments and body conditioning in both humans and animals. Saeed et al. (2017) [59] summarized previous opinions on the health benefits of chicory herb, emphasizing its role as a hepatoprotective agent. The nutritional benefits of Cichorium intybus in livestock mitigating peroxidation of lipids in serum and organs, as well as improving growth efficiency, reproduction, and health, were explained. Other bioactive properties such as antioxidant, anti-inflammatory, anticancer, antiprotozoal, antidiabetic, antimicrobial, immunological, cardiovascular, hypolipidemic, gastroprotective, analgesic, anthelmintic, productive and reproductive enhancer, and wound healing properties were also described [59]. Volatile oils are found in all parts of the plant but are more concentrated in the roots which have been found to be effective at eliminating intestinal worms [20, 21, 32]. The studies of Hassan et al. (2014) [60] on the effects of different concentrations of chicory (Cichorium intybus) and wormwood (Artemisia absinthium) extracts against ovine gastrointestinal nematodes showed high effectiveness against ovine gastrointestinal nematode. The concentration of chicory extract (50 mg/ml) and wormwood extract (25 mg/ml) against adult Haemonchus contortus in vitro caused death of all the worms after 4 hours [60]. Another study on anthelmintic effect of forage chicory against gastrointestinal nematode parasites established that feeding forage (⩾70% of chicory DM) significantly reduces worm burdens and fecal egg counts of Ostertagia ostertagi in experimentally infected calves. Sesquiterpene lactones identified in both the fresh and silage chicory forage were suspected to contribute to the observed anthelmintic effects of dietary chicory [61]. Scientific works by Milala et al. (2009) [62] and other investigators posited that the polyphenolic acid of chicory roots expresses a wide range of health-promoting activities such as anticarcinogenic, anti-inflammatory, antiviral, antibacterial, antimutagenic, antifungal, anthelmintic, immune-stimulating, and antihepatotoxic activity [43, 63, 64] and its antioxidant properties [65, 66]. Likewise, different researchers [63, 67–69] have submitted that chicory PSM can act against the Human Immunodeficiency Virus (HIV), protect the alimentary tract, and influence the reduction of serum cholesterol. Therefore, the supplementary or complementary inclusions of preparations rich in prebiotic saccharides and polyphenols produced from chicory can be used to promote the healthy properties of a diet and, at the same time, act as food and herbal medicament. A study [70] asserted that chicory (C. intybus) is an herb that can be used as a source of fibre in pig diets. According to diverse groups of studies [11, 18–21], chicory root is well known for its toxicity to internal parasites. It has since been reported that feeding inulin suppresses parasites such as Ascaris [71] and Trichuris [72] in pigs. The lowering of the intestinal pH, which is not favorable for the development of the parasite embryo, has been suggested as a possible mechanism. Two different studies [18, 21] established that ingestion of chicory by farm animals resulted in the reduction of worm burdens, which have prompted its widespread use as a feed supplement with a low fiber concentration. The total number of helminths in the abomasum of lambs grazed on chicory was reported to be remarkably reduced in the study conducted by Marley and coworkers, (2003) [73]. Chicory also contains a low quantity of condensed tannins [32, 74–76] and sesquiterpene lactones [77] which may affect protein utilization efficiency in ruminants and can as well reduce intestinal parasites in animals. It is also noted that sesquiterpene lactones in chicory extract inhibited the hatching of sheep's Haemonchus contortus eggs [78]. Earlier, some workers [79] reported that a dose-dependent anthelmintic action of extracts rich in condensed tannins and sesquiterpenes from C. intybus were indicated to reduce larval motility of lungworm and gastrointestinal nematodes, using a larval migration and inhibition assay. A study on pigs in Denmark [80] has shown chicory to have a positive effect on parasites and Lawsonia bacteria and it is relatively inexpensive but when used too much, it can cause a feeling of congestion in the digestive tract.
4. Chicory: Specialist Livestock Forage
A study [81] revealed that the nutritional value of a chicory crop vary at different parts of the plant, with different growth stages, crop's condition, and the plant's environment as depicted by Figure 3.
Figure 3.
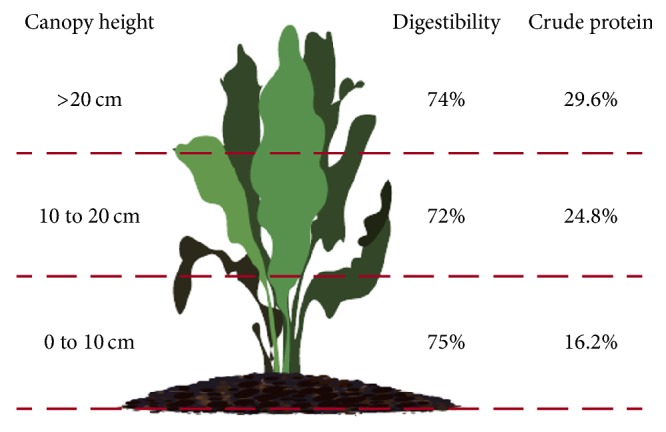
Nutritive values of different parts of a chicory plant [81].
Literature has demonstrated that chicory is a suitable forage for lamb finishing. The herb offers exceptional qualities such as thriving well at a minimum annual rainfall of 600 mm and low soil pH levels. The nutritive value of the forage is appreciably high: 14–24% CP, 70–80% digestibility in the leaves, and ME of 13.7 MJ/kg DM [82]. Chicory provides condensed tannins and other secondary metabolites, which positively affect the internal parasites in lambs, lowers the amount of methane production, and increase the reproductive rate in sheep. It is noted in a comparative study that faster growth rate is observed in lambs grazing on chicory (190–370 g/day) than those grazing on other pastures (e.g., ryegrass at 160–230 g/day or lucerne at 170–300 g/day) [83]. Hopkins et al. (1995) [84] also recorded that lambs that grazed chicory on their finishing phase showed no change in meat quality and were not fatter than the lambs fed lucerne forage.
In 2004, Brown and Moot's study [85] established that combined herbage quality and greater herbage consumptions of lucerne forage afforded 30% greater annual crude protein (CP) and ME intake for sward than chicory or red clover. This indicates that lucerne has greater potential to improve livestock production. The report also revealed that chicory, lucerne, and red clover gave similar high ME (10.9–11.3 MJ/kg DM) contents. Lucerne and red clover gave high CP contents of 0.25–0.29 g/g DM, while chicory had a lower CP content of 0.18 g/g DM. However, another study [86] pointed out that the chicory's greater efficiency of use in the rumen can compensate for its lower CP content influence.
In New Zealand, chicory stays leafy in the first summer but in a second summer it goes to seed, which reduces the feed quality [29]. A comparative study between chicory and ryegrass established that herbage quality of chicory of 0–20% stem has a lower DM content than ryegrass pasture (Table 4). Also, chemical constituents of chicory comprises less fibre, more protein, more soluble sugars, and greater mineral content (P, K, S, Ca, Mg, Na, Zn, Cu, and B) than the ryegrass pasture. Though chicory's high digestibility and low fibre content are not suitable as a sole diet for cows, its high digestibility permits it to be more quickly removed from the cow than perennial ryegrass, providing an opportunity to increase voluntary feed intake [29]. The use of chicory in summer in New Zealand varies with pasture availability and quality. A drop in pasture quality in summer warrants the inclusion of chicory, which relatively increases milk solids production per cow compared to a pasture diet [29]. Though chicory can generate high nitrate levels, which rarely pose toxicity issues (probably because chicory is a small portion of the diet), it is still used seasonally to boost pasture quality and quantity in some countries.
Table 4.
Herbage quality of chicory and pasture [29].
| DM (%) | Protein (% DM) | Soluble sugars + starch (% DM) | Fibre (% DM) | Digestibility (% DM) | ME (MJ/kg DM) | |
|---|---|---|---|---|---|---|
| Chicory | 7–15 | 16–27 | 10–22 | 20–30 | 72–83 | 11.5–13.0 |
| Ryegrass∗ | 10–30 | 12–28 | 8–21 | 40–55 | 65–85 | 9.5–12.5 |
DM = dry matter; ME = metabolisable energy. Quality may be outside these ranges depending on pasture/crop management. ∗Spring to autumn.
A recent review of the problems of feeding dairy cows in Australia and New Zealand indicated that dairy farms that rely on pastures are usually challenged with feed shortages in summer-autumn seasons because of soil moisture deficits [87]. As a result, farmers would require supplementary feed to maintain milk production. Literature has shown that, in summer, chicory and plantain afford more DM and better nutritive qualities than perennial grasses [74]. Another report [17] indicated that chicory and plantain mixture also increases milk production in livestock.
5. Antinutritional Properties of Cichorium intybus
Some researchers have reported the possibilities of some PSM having detrimental or toxic effects on the growth and performance of livestock once they have exceeded an optimal intake level [88]. Alkaloids, terpenes, saponins, lactones, glycosides, and phenolic compounds are classes of active PSM whose excessive consumption can harmfully affect the health of both ruminants and nonruminants and, in extreme cases, can threaten their survival [21]. For example, the consumption of tannins and other PSM at high intake rates have been associated with reduced feed intake and dry matter digestibility and impaired rumen metabolism when included in the diets of ruminants at more than 4-5% of dry matter [89]. Weight loss, toxicity, and death of animals have also been reported [90, 91]. Earlier studies [21, 88, 90, 92–94] have proved that saponins and condensed tannins are also responsible for mucosal toxicity, reduction in nutrient absorption, and, subsequently, growth impairment. They also recounted that these PSM have been associated with haemolytic action and bloat in ruminants as well. Prior to this, it was purported that excessive consumption of alkaloids, glycosides, and terpenoids by animals can result in lesions in the nervous system [95].
Similarly, monogastrics are not immune to the detrimental fallouts of these phytochemicals. In poultry, tannin levels from 0.5–2.0% can suppress growth and egg production. Between 3 and 7% inclusion levels of tannins in their diet may be lethal [93, 96, 97]. Similar harmful effects of condensed tannins and other plant chemicals have also been reported in swine [93]. However, despite their antinutritional properties, low levels of PSM have been noted to increase growth and milk yield in sheep and cattle [61, 92–94].
6. Conclusion
Cichorium intybus is grown and used in many parts of the world for various purposes. It is often used for its therapeutic and prophylactic quality, or for maintaining general wellbeing. As a very versatile plant, it is beneficial to both animals and humans due to its high amounts of proteins, carbohydrates, minerals, and phytobioactive elements. In livestock production, it has been noted that some of its phytoconstituents possess properties that improve the welfare of animals either in a parasitized state or otherwise. This makes chicory an ideal, cheap, natural, and sustainable livestock supplement or alternative feed material. However, caution should be exercised when chicory is included in diets or grazed by ruminants to prevent toxicity in high concentrations of PSM. Further research on the multipurpose properties of the phytobioactive elements found in chicory, their antinutritional effects, effective dose of inclusion in animal diets, mechanism of action involved, and the biochemical description of the active PSM is strongly recommended.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Massoud M. I., Amin W. A., Elgindy A. A. Chemical and technological studies on Chicory (Cichorium Intybus L) and its applications in some functional food. Journal of Advanced Agricultural Research. 2009;14(3):735–756. [Google Scholar]
- 2.Bais H. P., Ravishankar G. A. Cichorium intybus L.—cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. Journal of the Science of Food and Agriculture. 2001;81(5):467–484. doi: 10.1002/jsfa.817. [DOI] [Google Scholar]
- 3.Chandra S., Kumar M., Dwivedi P., Arti K. Studies on industrial importance and medicinal value of chicory plant (Cichorium intybus L.) International Journal of Advance Research. 2016;4(1):1060–1071. [Google Scholar]
- 4.Schillhorn van Veen T. W. Ethnoveterinary Research and Development. London, UK: Intermediate Technology Publications; 1996. Sense or nonsense? Traditional methods of animal disease prevention and control in the African savannah; pp. 25–36. [Google Scholar]
- 5.Van der Merwe D., Swan G. E., Botha C. J. Use of ethnoveterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province of South Africa. Journal of the South African Veterinary Association. 2001;72(4):189–196. doi: 10.4102/jsava.v72i4.651. [DOI] [PubMed] [Google Scholar]
- 6.Baek H. H., Cadwallader K. R. Roasted chicory aroma evaluation by gas chromatography/mass spectrometry/olfactometry. Journal of Food Science. 1998;63(2):234–237. [Google Scholar]
- 7.Desprez B. F., Delesalle L., Dhellemmes C., Desprez M. F., Rambaud C., Vasseur J. Genetics and breeding of industrial chicory, a historical review. Proceedings of the Eighth Seminar on Inulin; 1999; Lille, France. [Google Scholar]
- 8.Leite Toneli J. T. C., Mürr F. E. X., Martinelli P., Dal Fabbro I. M., Park K. J. Optimization of a physical concentration process for inulin. Journal of Food Engineering. 2007;80(3):832–838. doi: 10.1016/j.jfoodeng.2006.07.012. [DOI] [Google Scholar]
- 9.Varotto S., Lucchin M., Parrini P. Immature embryos culture in Italian red chicory (Cichorium intybus C) Plant Cell, Tissue and Organ Culture. 2000;62(1):75–77. doi: 10.1023/A:1006468229414. [DOI] [Google Scholar]
- 10.Hoste H., Jackson F., Athanasiadou S., Thamsborg S. M., Hoskin S. O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends in Parasitology. 2006;22(6):253–261. doi: 10.1016/j.pt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Das S., Vasudeva N., Sharma S. Cichorium intybus: a concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Development and Therapeutics. 2016;7(1):1–12. [Google Scholar]
- 12.Hui Ru Y., Shaoh H., Yingli Y. The extraction and purification of inulin. Natural Product Research and Development. 2002;14:p. 65. [Google Scholar]
- 13.Kim M., Shin H. K. The water-soluble extract of chicory reduces glucose uptake from the perfused jejunum in rats. Journal of Nutrition. 1996;126:2236–2242. doi: 10.1093/jn/126.9.2236. [DOI] [PubMed] [Google Scholar]
- 14.Madrigal L., Sangronis E. Inulin and derivates as key ingredients in functional foods: a review. Archivos Latinoamericanos de Nutrición. 2007;57:387–396. [PubMed] [Google Scholar]
- 15.Park K. J., de Oliveira R. A., Brod F. P. R. Drying operational parameters influence on chicory roots drying and inulin extraction. Food and Bioproducts Processing. 2007;85(3 C):184–192. doi: 10.1205/fbp07016. [DOI] [Google Scholar]
- 16.Li D., Kim J. M., Jin Z., Zhou J. Prebiotic effectiveness of inulin extracted from edible burdock. Anaerobe. 2008;14(1):29–34. doi: 10.1016/j.anaerobe.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Waugh C. D., Clark D., Harris S. L., Thom E. R., Copemanand. P. J. A., Napper A. R. Chicory for milk production. Proceedings of the New Zealand Grassland Association. 1998;60:33–37. [Google Scholar]
- 18.Tzamaloukas O., Athanasiadou S., Kyriazakis I., Huntley J. F., Jackson F. The effect of chicory (Cichorium intybus) and sulla (Hedysarum coronarium) on larval development and mucosal cell responses of growing lambs challenged with Teladorsagia circumcincta. Parasitology. 2006;132(3):419–426. doi: 10.1017/S0031182005009194. [DOI] [PubMed] [Google Scholar]
- 19.Heckendorn F., Häring D. A., Maurer V., Zinsstag J., Langhans W., Hertzberg H. Effect of sainfoin (Onobrychis viciifolia) silage and hay on established populations of Haemonchus contortus and Cooperia curticei in lambs. Veterinary Parasitology. 2006;142(3-4):293–300. doi: 10.1016/j.vetpar.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Van Loo J. How chicory fructans contribute to zootechnical performance and well-being in livestock and companion animals. Journal of Nutrition. 2007;137(11):2594S–2597S. doi: 10.1093/jn/137.11.2594S. [DOI] [PubMed] [Google Scholar]
- 21.Athanasiadou S., Githiori J., Kyriazakis I. Medicinal plants for helminth parasite control: Facts and fiction. Animal. 2007;1(9):1392–1400. doi: 10.1017/S1751731107000730. [DOI] [PubMed] [Google Scholar]
- 22.Margett B., Buttriss J. Plants: Diet and health. Oxford, UK: Blackwell Publishing; 2003. Epidemiology linking consumption of plant foods and their constituents with health; pp. 49–60. [Google Scholar]
- 23.Carazzone C., Mascherpa D., Gazzani G., Papetti A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chemistry. 2013;138(2-3):1062–1071. doi: 10.1016/j.foodchem.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda T., Horio F., Uchida K., Aoki H., Osawa T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. Journal of Nutrition. 2003;133(7):2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Mazza G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor α in LPS/IFN-γ-activated RAW 264.7 macrophages. Journal of Agricultural and Food Chemistry. 2002;50(15):4183–4189. doi: 10.1021/jf011613d. [DOI] [PubMed] [Google Scholar]
- 26.Youdim K. A., McDonald J., Kalt W., Joseph J. A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. The Journal of Nutritional Biochemistry. 2002;13(5):282–288. doi: 10.1016/S0955-2863(01)00221-2. [DOI] [PubMed] [Google Scholar]
- 27.Makkar H. P. S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Ruminant Research. 2003;49(3):241–256. doi: 10.1016/S0921-4488(03)00142-1. [DOI] [Google Scholar]
- 28.Images for chicory parts and uses. https://en.wikipedia.org/wiki/Chicory.
- 29.Farmfacts, DairyNZ. 2013. Chicory management. [Google Scholar]
- 30.Nadkarni A. K. Indian Materia. Bombay, India: Medica; 1976. [Google Scholar]
- 31.Varier P. S. Indian Medicinal Plants a Compendium of 500 Species. Chennai, India: Orient Longman; 1994. Cichorium intybus Linn; p. p. 74. [Google Scholar]
- 32.Wilson R. G., Smith J. A., Yonts C. D. Chicory root yield and carbohydrate composition is influenced by cultivar selection, planting, and harvest date. Crop Science. 2004;44(3):748–752. doi: 10.2135/cropsci2004.0748. [DOI] [Google Scholar]
- 33.Soobo S. Effects of prebiotics, probiotics andsynbiotics in the diet of young pigs [Ph.D. thesis] Wageningen, Netherlands: Wageningen University; 2005. [Google Scholar]
- 34.Phelps C. F. The physical properties of inulin solutions. Biochemical Journal. 1965;95:41–47. doi: 10.1042/bj0950041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters A. M., Van Amerongen A. Relationship between levels of sesquiterpene lactones in chicory and sensory evaluation. Journal of the American Society for Horticultural Science. 1998;123(2):326–329. [Google Scholar]
- 36.De Kraker J.-W., Franssen M. C. R., De Groot A., König W. A., Bouwmeester H. J. (+)-Germacrene A biosynthesis - The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiology. 1998;117(4):1381–1392. doi: 10.1104/pp.117.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nandagopal S., Kumari B. D. Phytochemical and antibacterial studies of chicory (CichoriumintybusL.) A multipurpose medicinal plant. Advances in Biological Research. 2007;1(1-2):17–21. [Google Scholar]
- 38.Hussain H., Hussain J., Saleem M., et al. Cichorin A: A new benzo-isochromene from Cichorium intybus. Journal of Asian Natural Products Research. 2011;13(6):566–569. doi: 10.1080/10286020.2011.573789. [DOI] [PubMed] [Google Scholar]
- 39.Hussain H., Hussain J., Ali S., et al. Cichorins B and C: Two new benzo-isochromenes from Cichorium intybus. Journal of Asian Natural Products Research. 2012;14(4):297–300. doi: 10.1080/10286020.2011.652953. [DOI] [PubMed] [Google Scholar]
- 40.Ying G. W., Gui L. J. Chicory seeds: a potential source of nutrition for food and feed. Journal of Animal and Feed Sciences. 2012;13(2):1736–1746. [Google Scholar]
- 41.Marcone M. F., Jahaniaval F., Aliee H., Kakuda Y. Chemical characterization of Achyranthes bidentata seed. Food Chemistry. 2003;81(1):7–12. doi: 10.1016/S0308-8146(02)00250-9. [DOI] [Google Scholar]
- 42.Plaza L., De Ancos B., Cano M. P. Nutritional and health-related compounds in sprouts and seeds of soybean (Glycine max), wheat (Triticum aestivum.L) and alfalfa (Medicago sativa) treated by a new drying method. European Food Research and Technology. 2003;216(2):138–144. doi: 10.1007/s00217-002-0640-9. [DOI] [Google Scholar]
- 43.Ahmed B., Khan S., Masood M. H., Siddique A. H. Anti-hepatotoxic activity of cichotyboside, a sesquiterpene glycoside from the seeds of Cichorium intybus. Journal of Asian Natural Products Research. 2008;10(3-4):223–231. doi: 10.1080/10286020701590764. [DOI] [PubMed] [Google Scholar]
- 44.Nørbæk R., Nielsen K., Kondo T. Anthocyanins from flowers of Cichorium intybus. Phytochemistry. 2002;60(4):357–359. doi: 10.1016/s0031-9422(02)00055-9. [DOI] [PubMed] [Google Scholar]
- 45.Judžentiene A., Budiene J. Volatile constituents from aerial parts and roots of Cichorium intybus L. (chicory) grown in Lithuania. Chemija. 2008;19(2):25–28. [Google Scholar]
- 46.Abbas Z. K., Saggu S., Sakeran M. I., Zidan N., Rehman H., Ansari A. A. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi Journal of Biological Sciences. 2014;22(3):322–326. doi: 10.1016/j.sjbs.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ćustić M., Poljak M., Toth N. Effects of nitrogen nutrition upon the quality and yield of head chicory (Cichorium intybus L. var.foliosum) Acta Horticulturae. 2000;533:401–410. doi: 10.17660/ActaHortic.2000.533.50. [DOI] [Google Scholar]
- 48.Mulabagal V., Wang H., Ngouajio M., Nair M. G. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. European Food Research and Technology. 2009;230(1):47–53. doi: 10.1007/s00217-009-1144-7. [DOI] [Google Scholar]
- 49.Drazen J. M. Inappropriate advertising of dietary supplements. The New England Journal of Medicine. 2003;348(9):777–778. doi: 10.1056/NEJMp030021. [DOI] [PubMed] [Google Scholar]
- 50.Street R. A., Sidana J., Prinsloo G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/579319.579319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mejer H. Transmission, infection dynamics and alternative control of helminths in organic swine [Ph.D. thesis] Danish Centre for Experimental Parasitology, Royal Veterinary and Agricultural University; 2006. [Google Scholar]
- 52.Roberfroid M. B. Inulin-type fractans: functional food ingredients. The Journal of Nutrition. 2007;137:2493S–2502S. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- 53.Roberfroid M. B. Prebiotics: preferential substrates for specific germs? American Journal of Clinical Nutrition. 2001;73(2):406S–409S. doi: 10.1093/ajcn/73.2.406s. [DOI] [PubMed] [Google Scholar]
- 54.Kaur N., Gupta A. K. Applications of inulin and oligofructose in health and nutrition. Journal of Biosciences. 2002;27(7):703–714. doi: 10.1007/BF02708379. [DOI] [PubMed] [Google Scholar]
- 55.Liu H., Ivarsson E., Dicksved J., Lundh T., Lindberg J. E. Inclusion of Chicory (Cichorium intybus L.) in pigs' diets affects the intestinal microenvironment and the gut microbiota. Applied and Environmental Microbiology. 2012;78(12):4102–4109. doi: 10.1128/AEM.07702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberfroid M. B., Cumps J., Devogelaer J. P. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. Journal of Nutrition. 2002;132(12):3599–3602. doi: 10.1093/jn/132.12.3599. [DOI] [PubMed] [Google Scholar]
- 57.Jensen M. T., Hansen L. L. Feeding with chicory roots reduces the amount of odorous compounds in colon and rectal contents of pigs. Journal of Animal Science. 2006;82(3):369–376. doi: 10.1079/ASC200649. [DOI] [Google Scholar]
- 58.Rasmussen M. Regulation of porcine hepatic cytochrome P450 by chicory root implication for boar taint [Ph.D. thesis] Aarhus, Denmark: Department of Food Science, Faculty of Science and Technology, Aarhus University; 2012. [Google Scholar]
- 59.Saeed M., Abd El-Hac M. E., Alagawany M., et al. Chicory (Cichorium intybus) Herb: chemical composition, pharmacology, nutritional and healthical applications. International Journal of Pharmacology. 2017;13(4):351–360. doi: 10.3923/ijp.2017.351.360. [DOI] [Google Scholar]
- 60.Hassan A. I., Osman S. A., Al-Gaabary M. H., Abo El-Soud K. M. Effects of chicory (Cichoriumintybus) and Artemisiaabsenthium extracts against ovine gastrointestinal nematodes. International Journal of Food, Agriculture and Veterinary Sciences. 2014;4(2):43–53. [Google Scholar]
- 61.Peña-Espinoza M., Thamsborg S. M., Desrues O., Hansen T. V. A., Enemark H. L. Anthelmintic effects of forage chicory (Cichorium intybus) against gastrointestinal nematode parasites in experimentally infected cattle. Parasitology. 2016;143(10):1279–1293. doi: 10.1017/S0031182016000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milala J., Grzelak K., Król B., Juśkiewicz J., Zduńczyk Z. Composition and properties of chicory extracts rich in fructans and polyphenols. Polish Journal of Food and Nutrition Sciences. 2009;59(1):35–43. [Google Scholar]
- 63.Kim M. The water-soluble extract of chicory reduces cholesterol uptake in gut- perfused rats. Nutrition Research. 2000;20(7):1017–1026. doi: 10.1016/S0271-5317(00)00192-5. [DOI] [Google Scholar]
- 64.Ahmed B., Al-Howiriny T. A., Siddiqui A. B. Antihepatotoxic activity of seeds of Cichorium intybus. Journal of Ethnopharmacology. 2003;87(2-3):237–240. doi: 10.1016/S0378-8741(03)00145-4. [DOI] [PubMed] [Google Scholar]
- 65.Papetti A., Daglia M., Grisoli P., Dacarro C., Gregotti C., Gazzani G. Anti- and pro-oxidant activity of Cichorium genus vegetables and effect of thermal treatment in biological systems. Food Chemistry. 2006;97(1):157–165. doi: 10.1016/j.foodchem.2005.03.036. [DOI] [Google Scholar]
- 66.Peschel W., Sánchez-Rabaneda F., Diekmann W., et al. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chemistry. 2006;97(1):137–150. doi: 10.1016/j.foodchem.2005.03.033. [DOI] [Google Scholar]
- 67.Wang M., Simon J. E., Aviles I. F., He K., Zheng Q.-Y., Tadmor Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.) Journal of Agricultural and Food Chemistry. 2003;51(3):601–608. doi: 10.1021/jf020792b. [DOI] [PubMed] [Google Scholar]
- 68.Innocenti M., Gallori S., Giaccherini C., Ieri F., Vincieri F. F., Mulinacci N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. Journal of Agricultural and Food Chemistry. 2005;53(16):6497–6502. doi: 10.1021/jf050541d. [DOI] [PubMed] [Google Scholar]
- 69.Mares D., Romagnoli C., Tosi B., Andreotti E., Chillemi G., Poli F. Chicory extracts from Cichorium intybus L. as potential antifungals. Mycopathologia. 2005;160(1):85–92. doi: 10.1007/s11046-004-6635-2. [DOI] [PubMed] [Google Scholar]
- 70.Ivarsson E., Frankow-Lindberg B. E., Andersson H. K., Lindberg J. E. Growth performance, digestibility and faecal coliform bacteria in weaned piglets fed a cereal-based diet including either chicory (Cichorium intybus L) or ribwort (Plantago lanceolata L) forage. Animal. 2011;5(4):558–564. doi: 10.1017/S1751731110002193. [DOI] [PubMed] [Google Scholar]
- 71.Petkevicius S., Knudsen K., Nansen P., Roepstorff A., Skjoth F., Jensen K. The impact of diets varying in carbohydrates resistant to endogenous enzymes and lignin on populations of Ascarissuum and Oesophagostomumdentatum in pigs. Parasitology. 1997;114(6):555–568. [PubMed] [Google Scholar]
- 72.Thomsen L. E., Petkevičius S., Bach Knudsen K. E., Roepstorff A. The influence of dietary carbohydrates on experimental infection with Trichuris suis in pigs. Parasitology. 2005;131(6):857–865. doi: 10.1017/S0031182005008620. [DOI] [PubMed] [Google Scholar]
- 73.Marley C. L., Cook R., Keatinge R., Barrett J., Lampkin N. H. The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Veterinary Parasitology. 2003;112(1-2):147–155. doi: 10.1016/S0304-4017(02)00412-0. [DOI] [PubMed] [Google Scholar]
- 74.Li G., Kemp P. D. Forage Chicory (Cichorium intybus L.): a review of its agronomy and animal production. Advances in Agronomy. 2005;88:187–222. doi: 10.1016/S0065-2113(05)88005-8. [DOI] [Google Scholar]
- 75.Kidane A., Houdijk J. G. M., Athanasiadou S., Tolkamp B. J., Kyriazakis I. Effects of maternal protein nutrition and subsequent grazing on chicory (Cichorium intybus) on parasitism and performance of lambs. Journal of Animal Science. 2010;88(4):1513–1521. doi: 10.2527/jas.2009-2530. [DOI] [PubMed] [Google Scholar]
- 76.Lombardi D., Vasseur E., Berthiaume R., DeVries T. J., Bergeron R. Feeding preferences and voluntary feed intake of dairy cows: effect of conservation and harvest time of birdsfoot trefoil and chicory. Journal of Dairy Science. 2015;98(10):7238–7247. doi: 10.3168/jds.2015-9427.73852 [DOI] [PubMed] [Google Scholar]
- 77.Miller M. C., Duckett S. K., Andrae J. G. The effect of forage species on performance and gastrointestinal nematode infection in lambs. Small Ruminant Research. 2011;95(2-3):188–192. doi: 10.1016/j.smallrumres.2010.09.006. [DOI] [Google Scholar]
- 78.Foster J. G., Cassida K. A., Turner K. E. In vitro analysis of the anthelmintic activity of forage chicory (Cichorium intybus L.) sesquiterpene lactones against a predominantly Haemonchus contortus egg population. Veterinary Parasitology. 2011;180(3-4):298–306. doi: 10.1016/j.vetpar.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Molan A. L., Duncan A. J., Barry T. N., McNabb W. C. Effects of condensed tannins and crude sesquiterpene lactones extracted from chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. Parasitology International. 2003;52(3):209–218. doi: 10.1016/S1383-5769(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 80.Roepstorff A., Mejer H., Thomson L. E., Thamsborg S. M., Byrene S. M. Chicory root improves the taste and odour of organic pork. Newsletter from Danish Research Centre for Organic Farming. 2005;1(3):1–4. [Google Scholar]
- 81.Laws D., Genever L. Using Chicory and Plantain in Beef and Sheep Systems. Kenilworth, UK: AHDB Beef & Lamb Stoneleigh Park; 2016. [Google Scholar]
- 82.Burnett V., Ponnampalam E. Specialist Forages Lamb Finishing Guidelines. Victoria, UK: The Future Farming Systems Research; 1996. [Google Scholar]
- 83.Holst P. J., Kemp M., Hall D. G. Summer lamb production from puna chicory (Cichoriumintybus) and lucerne (Medicago sativa). Proceedings of the Australian Society of Animal Production; 1998; pp. 145–148. [Google Scholar]
- 84.Hopkins D. L., Holst P. J., Hall D. G., Atkinson W. R. Carcass and meat quality of second-cross cryptorchid lambs grazed on chicory (Cichorium intybus) or lucerne (medicago sativa) Australian Journal of Experimental Agriculture. 1995;35(6):693–697. doi: 10.1071/EA9950693. [DOI] [Google Scholar]
- 85.Brown H. E., Moot D. J. Quality and quantity of chicory, lucerne and red clover production under irrigation. Proceedings of the New Zealand Grassland Association. 2004;66:257–264. [Google Scholar]
- 86.Komolong M., Nicol A. M., Poppi D. P., Fraser T. J., Kirsopp S. Nutrient supply for lamb growth from Grasslands Puna chicory (Cichoriumintybus) and Wana cocksfoot (Dactylisglomerata) Proceedings of the New Zealand Society of Animal Production. 1992;52:85–87. [Google Scholar]
- 87.Wales W. J., Kolver E. S. Challenges of feeding dairy cows in Australia and New Zealand. Animal Production Science. 2017;57(7):1366–1383. doi: 10.1071/AN16828. [DOI] [Google Scholar]
- 88.Githiori J. B., Athanasiadou S., Thamsborg S. M. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Veterinary Parasitology. 2006;139(4):308–320. doi: 10.1016/j.vetpar.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 89.Barry T. N., McNabb W. C. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. British Journal of Nutrition. 1999;81(4):263–272. [PubMed] [Google Scholar]
- 90.Milgate J., Roberts D. C. K. The nutritional & biological significance of saponins. Nutrition Research. 1995;15(8):1223–1249. doi: 10.1016/0271-5317(95)00081-S. [DOI] [Google Scholar]
- 91.Waghorn G. C., McNabb W. C. Consequences of plant phenolic compounds for productivity and health of ruminants. Proceedings of the Nutrition Society. 2003;62(2):383–392. doi: 10.1079/PNS2003245. [DOI] [PubMed] [Google Scholar]
- 92.Applebaum S., Birk Y. Herbivores: Their Interactions with Secondary Plant Metabolites. London, UK: Academic Press; 1979. Saponins; pp. 539–566. [Google Scholar]
- 93.Reed J. D. Nutritional toxicology of tannins and related polyphenols in forage legumes. Journal of Animal Science. 1995;73(5):1516–1528. doi: 10.2527/1995.7351516x. [DOI] [PubMed] [Google Scholar]
- 94.Dawson J. M., Buttery P. J., Jenkins D., Wood C. D., Gill M. Effects of dietary quebracho tannin on nutrient utilisation and tissue metabolism in sheep and rats. Journal of the Science of Food and Agriculture. 1999;79(11):1423–1430. doi: 10.1002/(SICI)1097-0010(199908)79:11<1423::AID-JSFA383>3.0.CO;2-8. doi: 10.1002/(SICI)1097-0010(199908)79:11<1423::AID-JSFA383>3.0.CO;2-8. [DOI] [Google Scholar]
- 95.Conn E. Herbivores: Their Interactions with Secondary Plant Metabolites. London, UK: Academic Press; 1979. Cyanide and cyanogenic glycosides; p. p. 452. [Google Scholar]
- 96.Haslam E. Plant Polyphenols: Chemistry and Significance of Condensed Tannins. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 97.Giner-Chavez B. Condensed Tannins in Tropical Forages. Ithaca, NY, USA: Cornell University; 1996. [Google Scholar]