Abstract
Oxidative stress has both detrimental and beneficial effects. Kallistatin, a key component of circulation, protects against vascular and organ injury. Serum kallistatin levels are reduced in patients and animal models with hypertension, diabetes, obesity, and cancer. Reduction of kallistatin levels is inversely associated with elevated thiobarbituric acid-reactive substance. Kallistatin therapy attenuates oxidative stress and increases endothelial nitric oxide synthase (eNOS) and NO levels in animal models. However, kallistatin administration increases reactive oxygen species formation in immune cells and bacterial killing activity in septic mice. High oxygen inhibits kallistatin expression via activating the JNK-FOXO1 pathway in endothelial cells. Conversely, mild oxygen/hyperoxia stimulates kallistatin, eNOS, and hypoxia-inducible factor-1 (HIF-1) expression in endothelial cells and in the kidney of normal mice. Likewise, kallistatin stimulates eNOS and HIF-1, and kallistatin antisense RNA abolishes oxygen-induced eNOS and HIF-1 expression, indicating a role of kallistatin in mediating mild oxygen's stimulation on antioxidant genes. Protein kinase C (PKC) activation mediates HIF-1-induced eNOS synthesis in response to hyperoxia/exercise; thus, mild oxygen through PKC activation stimulates kallistatin-mediated HIF-1 and eNOS synthesis. In summary, oxidative stress induces down- or upregulation of kallistatin expression, depending on oxygen concentration, and kallistatin plays a novel role in mediating oxygen/exercise-induced HIF-1-eNOS-NO pathway.
1. Introduction
Kallistatin was first identified in human plasma as a tissue kallikrein-binding protein (KBP) and characterized as a serine proteinase inhibitor (serpin) [1–3]. Tissue kallikrein (TK) is a serine proteinase that cleaves low molecular weight kininogen substrate to release vasodilating kinin peptides [4]. Kallistatin consists of two structural elements, an active site and a heparin-binding domain, which exert pleiotropic activities by regulating differential signaling pathways [5, 6]. Kallistatin through its active site forms a covalent complex with TK and inhibits TK activity [5, 7]. Kallistatin via its heparin-binding site interacts with cell surface heparan sulfate proteoglycans and thereby antagonizes the signaling pathways mediated by vascular endothelial growth factor, tumor necrosis factor-α, transforming growth factor-β, and Wnt [8–11]. Moreover, kallistatin exerts a wide spectrum of biological effects independent of TK. For example, kallistatin is a potent vasodilator unrelated to the tissue kallikrein-kinin system [12]. Transgenic mice overexpressing kallistatin have lower blood pressure compared to control mice and are resistant to lipopolysaccharide-induced mortality [13, 14]. Kallistatin is mainly expressed in the liver and is widely distributed in the kidney, heart, and blood vessel [15–18]. Circulating kallistatin levels are markedly reduced under pathological conditions, such as in hypertension, liver disease, sepsis, cardiac and renal injury, severe pneumonia, obesity, and cancer in patients and in animal models [19]. Kallistatin administration by gene or protein delivery alleviates hypertension, multiorgan damage, and cancer development by reducing oxidative stress, inflammation, angiogenesis, apoptosis, fibrosis, tumor growth, and metastasis in rodents [8, 20–25]. These findings indicate that kallistatin therapy has beneficial effects in various disease states.
Kallistatin belongs to the serpin family, which includes α1-antitrypsin and α1-antichymotrypsin [2]. In contrast to α1-antitrypsin, kallistatin is a negative acute-phase protein [26]. Kallistatin levels are markedly reduced in animals after endotoxin shock or experimental inflammation [26]. Oxidative stress downregulates kallistatin expression by activating c-Jun NH2-terminal kinase- (JNK-) dependent FOXO1 signaling in cultured endothelial cells [27]. However, hyperoxia treatment markedly stimulates kallistatin expression in breast cancer cells [28]. Moreover, kallistatin exhibits antioxidative actions. Kallistatin via its heparin-binding site antagonizes cytokine-induced reactive oxygen species (ROS) formation, and its active site is responsible for the upregulation of antioxidant gene expression in endothelial cells [9, 10, 29]. On the other hand, kallistatin stimulates ROS formation in immune cells, leading to marked bacterial killing activity in septic mice [28, 30]. Moreover, kallistatin's vasodilating activity is partly mediated by H2O2 formation [28]. Therefore, oxidative stress plays opposite roles in the regulation of kallistatin synthesis, and kallistatin possesses a dual role in modulating oxidative stress.
2. Reduced Circulating Kallistatin Levels Are Inversely Associated with Oxidative Stress
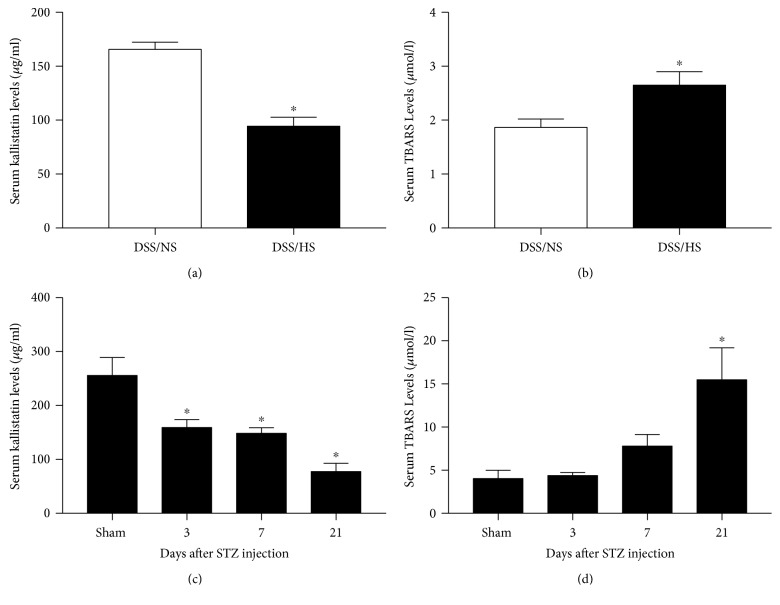
Circulatory kallistatin levels are markedly reduced in spontaneous hypertensive and arterial hypertensive rats [15, 31, 32]. Reduced kallistatin levels are associated with increased oxidative organ damage in animal models of hypertension and cardiovascular and renal dysfunction. Likewise, plasma kallistatin levels are reduced in patients with liver disease, sepsis, pulmonary pneumonia, obesity, and cancer [19]. Moreover, serum kallistatin levels are decreased in Dahl salt-sensitive (DSS) hypertensive rats receiving a high-salt diet (HS), compared with DSS rats with a normal-salt diet (NS), and are reduced time dependently in rats receiving streptozotocin (STZ) injection, a model of diabetes (Figures 1(a) and 1(c)). Reduced kallistatin levels are inversely associated with elevated serum thiobarbituric acid reactive substances (TBARs, an indicator of lipid peroxidation) in hypertensive DSS rats and in STZ-induced diabetic rats (Figures 1(a), 1(b), 1(c), and 1(d)). These findings indicate that kallistatin levels are reduced in diseased states and, consequently, negatively associated with oxidative stress.
Figure 1.
Circulating kallistatin levels are reduced, and TBARS levels are increased in DSS hypertensive rats and STZ-induced diabetic rats. ∗P < 0.05 versus control group.
3. Kallistatin Treatment Reduces Oxidative Stress and Organ Damage
Oxidative stress is a key contributor to the pathogenesis of hypertension, inflammation, fibrosis, and multiorgan injury [33–35]. Kallistatin administration attenuates cardiovascular and renal damage associated with reduced superoxide formation, inflammation, and increased endothelial nitric oxide synthase (eNOS) and NO levels in animal models of acute and chronic myocardial damage and salt-induced hypertension [21, 36, 37]. Moreover, kallistatin treatment inhibits liver fibrosis via antioxidative stress [38]. Conversely, depletion of endogenous kallistatin by neutralizing antibody injection augments cardiovascular and renal injury, in conjunction with increased oxidative stress, inflammation, endothelial cell loss, and fibrosis in hypertensive rats [39]. Kallistatin acts as a potent antioxidant as it prevents oxidative NO inactivation induced by superoxide production in cultured renal epithelial tubular and mesangial cells, cardiomyocytes, myofibroblasts, endothelial cells, and endothelial progenitor cells (EPCs) [21, 27, 29, 37, 40]. Moreover, kallistatin exhibits antioxidant activity in cultured pterygium epithelial cells through inhibition of ROS formation [41]. Kallistatin's heparin-binding site is crucial for blocking tumor necrosis factor- (TNF-) α-induced NADPH oxidase activity and expression, and its active site is a key for stimulating the activity and expression of the antioxidant enzymes, eNOS, sirtuin 1 (SIRT1), and catalase in endothelial cells and EPCs [9, 10, 29]. Collectively, kallistatin protects against multiorgan damage through its antioxidative actions.
4. High Oxygen Downregulates Kallistatin Expression
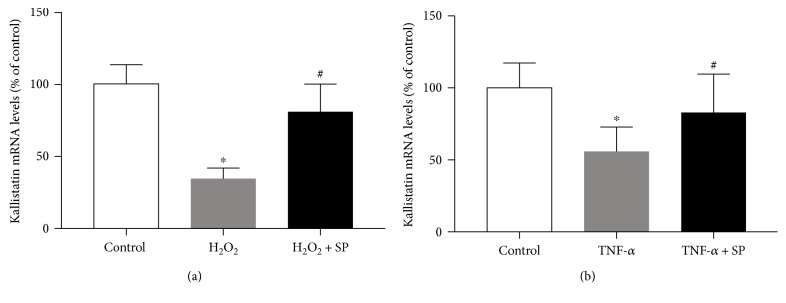
Oxidative stress effectively suppresses kallistatin expression in vivo and in vitro. Kallistatin synthesis is rapidly diminished in the liver of rats after endotoxin shock [26]. In cultured endothelial cells, high H2O2 concentration at 100 or 200 μM significantly reduces kallistatin mRNA and protein levels by reverse transcription polymerase chain reaction and Western blot analysis [27]. Likewise, high H2O2 concentration (100 μM, 24 hours) or TNF-α (10 ng/ml, 24 hours) markedly inhibits kallistatin expression, and the inhibitory effect of high H2O2 or TNF-α on kallistatin expression is abolished by SP, a JNK inhibitor in endothelial cells (Figures 2(a) and 2(b)). Moreover, knockout of FOXO1 with antisense RNA has been shown to block oxidative stress-mediated suppression of kallistatin synthesis in endothelial cells [27]. Therefore, oxidative stress activates the JNK-dependent FOXO1 signaling pathway leading to the inhibition of kallistatin expression.
Figure 2.
High H2O2 concentration (100 μM, 24 hr) or TNF-α (10 ng/ml, 24 hr) inhibits kallistatin expression in human umbilical vein endothelial cells (HUVECs), which is blocked by SP, a JNK inhibitor. ∗P < 0.05 versus control group; #P < 0.05 versus H2O2 or TNF-α group.
5. Mild Oxygen or Hyperoxia Upregulates Kallistatin, HIF-1, and eNOS Expression
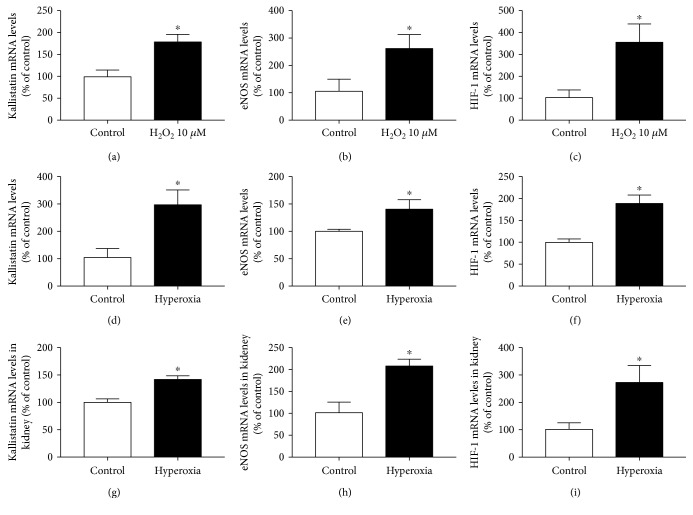
The physiology oxygen levels differ under in vitro and in vivo conditions. High H2O2 concentration (100 μM) inhibits kallistatin expression (Figure 2). In contrast to high H2O2, low or mild H2O2 concentrations (1 to 30 μM for 12 hours) dose dependently stimulate kallistatin synthesis in endothelial cells (data not shown). The stimulatory effect of H2O2 (10 μM) on kallistatin expression is associated with increased eNOS and HIF-1 synthesis (Figures 3(a), 3(b), and 3(c)). Likewise, hyperoxia (95% O2/5% CO2 for 6 hours) stimulates the expression of kallistatin, eNOS, and HIF-1 in endothelial cells (Figures 3(d), 3(e), and 3(f)). In the in vivo hyperoxia model, mice were placed in a 95% O2/5% CO2 chamber for 90 minutes as described [42]. Hyperoxia treatment increases kallistatin, eNOS, and HIF-1 mRNA levels in the mouse kidney (Figures 3(g), 3(h), and 3(i)). Hyperbaric oxygen therapy has been shown to prolong survival of mice with systemic metastatic cancer, reduces the growth, and induces apoptosis in rat mammary tumors [43]. Indeed, our previous study showed that hyperoxia markedly induces kallistatin expression in MDA-MB-231 and MCF-7 breast cancer cells and kallistatin treatment inhibits tumor progression by inducing cancer cell apoptosis and autophagy [11, 28]. Therefore, mild oxygen or hyperbaric oxygen upregulates kallistatin, HIF-1, and eNOS expression and thus NO formation.
Figure 3.
Mild H2O2 (10 μM, 12 hr) or hyperoxia (95% O2/5% CO2, 6 hr for endothelial cells and 90 min for mice) stimulates kallistatin, eNOS, and HIF-1 synthesis in endothelial cells and in the kidney of mice. ∗P < 0.05 versus control group.
6. Kallistatin Mediates Mild Oxygen-Induced HIF-1 and eNOS Synthesis
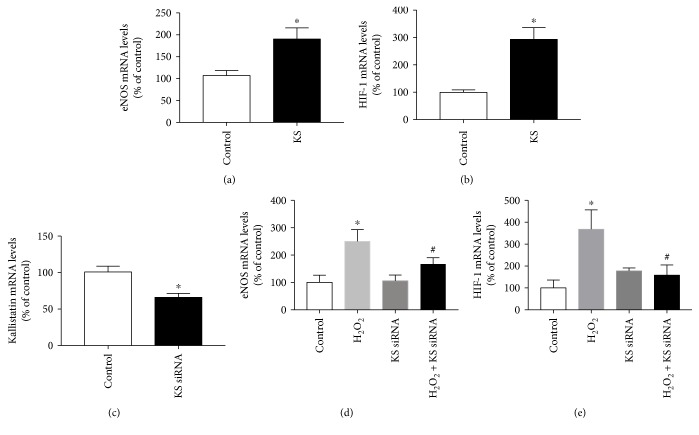
In parallel with mild oxygen/hyperoxia, kallistatin is capable of stimulating eNOS and HIF-1 synthesis in endothelial cells (Figures 4(a) and 4(b)). Importantly, kallistatin not only elevates eNOS mRNA level but also increases eNOS activity and immunostaining, as well as NO formation in EPCs and endothelial cells [25, 40]. Moreover, kallistatin antisense RNA abolishes H2O2-induced eNOS and HIF-1 synthesis, indicating a role of kallistatin in mediating mild oxygen-induced eNOS and HIF-1 synthesis (Figures 4(c), 4(d), and 4(e)). Mild hyperoxia via protein kinase C (PKC) activation increases HIF-1 synthesis, and eccentric exercise induces HIF-1-mediated-eNOS expression [44–48]. Taken together, these findings indicate that kallistatin is a novel mediator of mild oxygen-induced HIF-1 and eNOS synthesis, and mild oxygen through PKC activation stimulates kallistatin-mediated HIF-1-eNOS pathway.
Figure 4.
Kallistatin stimulates eNOS and HIF-1 expression and mediates mild oxygen-induced eNOS and HIF-1 synthesis in endothelial cells. ∗P < 0.05 versus control group; #P < 0.05 versus H2O2 group.
7. Kallistatin Protects against Vascular and Organ Injury by Mediating Oxygen/Exercise-Induced HIF-1-eNOS-NO Pathway
Physical activity and exercise training lower blood pressure in individuals with hypertension [49]. Moreover, exercise training exerts beneficial effects in diabetes and attenuates the decline of immune function associated with aging [50, 51]. Regular exercise prevents oxidative stress-related diseases, while acute exercise increases free-radical generation and oxidative injury in the elderly [52–54]. Older men who exercise on a regular basis do not demonstrate age-associated vascular oxidative stress [54]. Indeed, exercise prevents aging-induced decline of eNOS/NO in the aorta [55], and regular physical activity improves endothelial function in patients with coronary artery disease by increasing eNOS phosphorylation and in animals with elevated NO levels [56]. Likewise, regular aerobic exercise restores endothelial function in arteries of aged mice by reducing oxidative stress, increasing superoxide dismutase activity, and downregulating NADPH oxidase activity [57]. Kallistatin treatment attenuates vascular senescence and aging by suppression of oxidative stress and stimulation of antioxidant gene expression [29]. Moreover, kallistatin by stimulation of eNOS expression and activity and NO formation leads to blood pressure lowering, antioxidant and anti-inflammatory actions, and antiaging effect [9, 19, 21, 27, 29]. Therefore, regular exercise/mild oxygen increases kallistatin expression, leading to activation of HIF-1-eNOS-NO signaling. Collectively, kallistatin plays a protective role in vascular and organ injury by mediating oxygen/exercise-induced HIF-1-eNOS-NO pathway.
8. Conclusion
Oxidative stress exerts opposite effects in regulating kallistatin expression. High H2O2 concentration (100 μM) inhibits kallistatin expression via activating the JNK-dependent signaling pathway, while low or mild oxygen (H2O2 at 1 to 30 μM) and mild hyperoxia via PKC activation stimulate kallistatin-mediated HIF-1 and eNOS synthesis (Figure 5). Moreover, kallistatin possesses both antioxidant and prooxidant actions. Reduced kallistatin levels in the circulation or tissues are inversely correlated with elevated superoxide levels, and kallistatin treatment attenuates oxidative stress and organ damage. In contrast, kallistatin stimulates ROS formation in immune cells and causes marked bacterial killing activity in septic mice. Kallistatin stimulates eNOS and HIF-1 synthesis, and depletion of kallistatin by kallistatin antisense RNA blocks mild oxygen-induced eNOS and HIF-1 synthesis. HIF-1 modulates eNOS expression induced by exercise training. Therefore, kallistatin acts as a novel mediator of oxygen/exercise-induced HIF-1-eNOS-NO pathway and protects against oxidative vascular and organ injury. In summary, these findings reveal two important messages: (1) oxygen at high or low concentration exerts opposing effects on kallistatin expression and (2) kallistatin, by its dual role in regulation of oxidative stress, displays beneficial effects in pathological conditions such as hypertension, cardiovascular and renal injury, sepsis, and cancer development.
Figure 5.
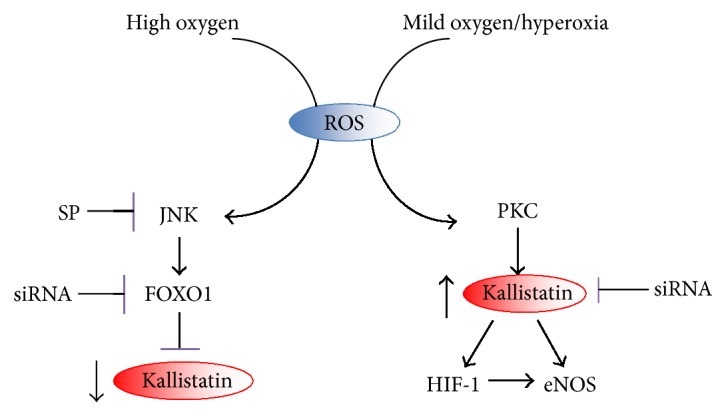
Signaling pathways mediated by high or mild oxygen on kallistatin regulation. High oxygen through ROS generation stimulates JNK/FOXO1 signaling activation, leading to kallistatin inhibition; mild oxygen/hyperoxia through PKC activation stimulates kallistatin-mediated HIF-1 and eNOS synthesis in endothelial cells.
Acknowledgments
This work was supported by the National Institutes of Health Grants HL118516 and HL44083.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Julie Chao contributes to the writing of the paper, and Youming Guo, Pengfei Li, and Lee Chao contribute to the revision and citation of the paper.
References
- 1.Chao J., Tillman D. M., Wang M. Y., Margolius H. S., Chao L. Identification of a new tissue-kallikrein-binding protein. The Biochemical Journal. 1986;239(2):325–331. doi: 10.1042/bj2390325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao J., Chai K. X., Chen L. M., et al. Tissue kallikrein-binding protein is a serpin. I. Purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. The Journal of Biological Chemistry. 1990;265(27):16394–16401. [PubMed] [Google Scholar]
- 3.Zhou G. X., Chao L., Chao J. Kallistatin: a novel human tissue kallikrein inhibitor. Purification, characterization, and reactive center sequence. The Journal of Biological Chemistry. 1992;267(36):25873–25880. [PubMed] [Google Scholar]
- 4.Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacological Reviews. 1992;44(1):1–80. [PubMed] [Google Scholar]
- 5.Chen V. C., Chao L., Chao J. Reactive-site specificity of human kallistatin toward tissue kallikrein probed by site-directed mutagenesis. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 2000;1479(1-2):237–246. doi: 10.1016/S0167-4838(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen V. C., Chao L., Pimenta D. C., Bledsoe G., Juliano L., Chao J. Identification of a major heparin-binding site in kallistatin. The Journal of Biological Chemistry. 2001;276(2):1276–1284. doi: 10.1074/jbc.M005791200. [DOI] [PubMed] [Google Scholar]
- 7.Chen V. C., Chao L., Chao J. Roles of the P1, P2, and P3 residues in determining inhibitory specificity of kallistatin toward human tissue kallikrein. The Journal of Biological Chemistry. 2000;275(49):38457–38466. doi: 10.1074/jbc.M005605200. [DOI] [PubMed] [Google Scholar]
- 8.Miao R. Q., Chen V., Chao L., Chao J. Structural elements of kallistatin required for inhibition of angiogenesis. American Journal of Physiology - Cell Physiology. 2003;284(6):C1604–C1613. doi: 10.1152/ajpgi.00524.2002. [DOI] [PubMed] [Google Scholar]
- 9.Yin H., Gao L., Shen B., Chao L., Chao J. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-α–induced nuclear factor κB activation. Hypertension. 2010;56(2):260–267. doi: 10.1161/HYPERTENSIONAHA.110.152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y., Li P., Bledsoe G., Yang Z. R., Chao L., Chao J. Kallistatin inhibits TGF-β-induced endothelial–mesenchymal transition by differential regulation of microRNA-21 and eNOS expression. Experimental Cell Research. 2015;337(1):103–110. doi: 10.1016/j.yexcr.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Guo Y., Bledsoe G., Yang Z., Chao L., Chao J. Kallistatin induces breast cancer cell apoptosis and autophagy by modulating Wnt signaling and microRNA synthesis. Experimental Cell Research. 2016;340(2):305–314. doi: 10.1016/j.yexcr.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao J., Stallone J. N., Liang Y. M., Chen L. M., Wang D. Z., Chao L. Kallistatin is a potent new vasodilator. The Journal of Clinical Investigation. 1997;100(1):11–17. doi: 10.1172/JCI119502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L. M., Ma J., Liang Y. M., Chao L., Chao J. Tissue kallikrein-binding protein reduces blood pressure in transgenic mice. The Journal of Biological Chemistry. 1996;271(44):27590–27594. doi: 10.1074/jbc.271.44.27590. [DOI] [PubMed] [Google Scholar]
- 14.Chen L. M., Chao L., Chao J. Beneficial effects of kallikrein-binding protein in transgenic mice during endotoxic shock. Life Sciences. 1997;60(17):1431–1435. doi: 10.1016/S0024-3205(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 15.Chao J., Chao L. Biochemistry, regulation and potential function of kallistatin. Biological Chemistry Hoppe-Seyler. 1995;376(12):705–713. [PubMed] [Google Scholar]
- 16.Chen L. M., Song Q., Chao L., Chao J. Cellular localization of tissue kallikrein and kallistatin mRNAs in human kidney. Kidney International. 1995;48(3):690–697. doi: 10.1038/ki.1995.339. [DOI] [PubMed] [Google Scholar]
- 17.Wolf W. C., Harley R. A., Sluce D., Chao L., Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. Journal of Histochemistry & Cytochemistry. 1999;47(2):221–228. doi: 10.1177/002215549904700210. [DOI] [PubMed] [Google Scholar]
- 18.Chao J., Schmaier A., Chen L. M., Yang Z., Chao L. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. The Journal of Laboratory and Clinical Medicine. 1996;127(6):612–620. doi: 10.1016/S0022-2143(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 19.Chao J., Bledsoe G., Chao L. Protective role of kallistatin in vascular and organ injury. Hypertension. 2016;68(3):533–541. doi: 10.1161/HYPERTENSIONAHA.116.07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao R. Q., Agata J., Chao L., Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100(9):3245–3252. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 21.Shen B., Hagiwara M., Yao Y. Y., Chao L., Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51(5):1358–1365. doi: 10.1161/HYPERTENSIONAHA.107.108514. [DOI] [PubMed] [Google Scholar]
- 22.Li P., Bledsoe G., Yang Z. R., Fan H., Chao L., Chao J. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology. 2014;142(2):216–226. doi: 10.1111/imm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P., Guo Y., Bledsoe G., et al. Kallistatin treatment attenuates lethality and organ injury in mouse models of established sepsis. Critical Care. 2015;19(1):p. 200. doi: 10.1186/s13054-015-0919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L., Yang Z., Zhu B., et al. Kallikrein-binding protein suppresses growth of hepatocellular carcinoma by anti-angiogenic activity. Cancer Letters. 2007;257(1):97–106. doi: 10.1016/j.canlet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Shen B., Gao L., Hsu Y. T., et al. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. American Journal of Physiology - Heart and Circulatory Physiology. 2010;299(5):H1419–H1427. doi: 10.1152/ajpheart.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao J., Chen L. M., Chai K. X., Chao L. Expression of kallikrein-binding protein and alpha 1-antitrypsin genes in response to sex hormones, growth, inflammation and hypertension. Agents and Actions Supplements. 1992;38(Part 1):174–181. doi: 10.1007/978-3-0348-7321-5_23. [DOI] [PubMed] [Google Scholar]
- 27.Shen B., Chao L., Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. American Journal of Physiology - Heart and Circulatory Physiology. 2010;298(3):H1048–H1054. doi: 10.1152/ajpheart.00826.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao J., Li P., Chao L. Kallistatin suppresses cancer development by multi-factorial actions. Critical Reviews in Oncology/Hematology. 2017;113:71–78. doi: 10.1016/j.critrevonc.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y., Li P., Gao L., et al. Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell. 2017;16(4):837–846. doi: 10.1111/acel.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu S. L., Tsai C. Y., Luo Y. H., et al. Kallistatin modulates immune cells and confers anti-inflammatory response to protect mice from group A streptococcal infection. Antimicrobial Agents and Chemotherapy. 2013;57(11):5366–5372. doi: 10.1128/AAC.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao J., Chao L. A major difference of kallikrein-binding protein in spontaneously hypertensive versus normotensive rats. Journal of Hypertension. 1988;6(7):551–558. doi: 10.1097/00004872-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Chao C., Madeddu P., Wang C., Liang Y., Chao L., Chao J. Differential regulation of kallikrein, kininogen, and kallikrein-binding protein in arterial hypertensive rats. American Journal of Physiology - Renal Physiology. 1996;271(1):F78–F86. doi: 10.1152/ajprenal.1996.271.1.F78. [DOI] [PubMed] [Google Scholar]
- 33.Vaziri N. D. Causal link between oxidative stress, inflammation, and hypertension. Iranian Journal of Kidney Diseases. 2008;2(1):1–10. [PubMed] [Google Scholar]
- 34.Modaresi A., Nafar M., Sahraei Z. Oxidative stress in chronic kidney disease. Iranian Journal of Kidney Diseases. 2015;9(3):165–179. [PubMed] [Google Scholar]
- 35.Pashkow F. J. Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? International Journal of Inflammation. 2011;2011:9. doi: 10.4061/2011/514623.514623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao J., Yin H., Yao Y. Y., Shen B., Smith R. S., Jr., Chao L. Novel role of kallistatin in protection against myocardial ischemia–reperfusion injury by preventing apoptosis and inflammation. Human Gene Therapy. 2006;17(12):1201–1213. doi: 10.1089/hum.2006.17.1201. [DOI] [PubMed] [Google Scholar]
- 37.Gao L., Yin H., Smith R. S., Chao L., Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Laboratory Investigation. 2008;88(11):1157–1166. doi: 10.1038/labinvest.2008.85. [DOI] [PubMed] [Google Scholar]
- 38.Huang X., Wang X., Lv Y., Xu L., Lin J., Diao Y. Protection effect of kallistatin on carbon tetrachloride-induced liver fibrosis in rats via antioxidative stress. PLoS One. 2014;9(2, article e88498) doi: 10.1371/journal.pone.0088498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Bledsoe G., Hagiwara M., Shen B., Chao L., Chao J. Depletion of endogenous kallistatin exacerbates renal and cardiovascular oxidative stress, inflammation, and organ remodeling. American Journal of Physiology - Renal Physiology. 2012;303(8):F1230–F1238. doi: 10.1152/ajprenal.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L., Li P., Zhang J., et al. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. Journal of the American Heart Association. 2014;3(5, article e001194) doi: 10.1161/JAHA.114.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu C., Pan F., Ge L., et al. SERPINA3K plays antioxidant roles in cultured pterygial epithelial cells through regulating ROS system. PLoS One. 2014;9(10, article e108859) doi: 10.1371/journal.pone.0108859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W., Chen Q., Liu J., Liu K. Normobaric hyperoxia protects the blood brain barrier through inhibiting Nox2 containing NADPH oxidase in ischemic stroke. Medical Gas Research. 2011;1(1):p. 22. doi: 10.1186/2045-9912-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poff A. M., Ari C., Seyfried T. N., D'Agostino D. P. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS One. 2013;8(6, article e65522) doi: 10.1371/journal.pone.0065522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Miguelez P., Lima-Cabello E., Martinez-Florez S., Almar M., Cuevas M. J., Gonzalez-Gallego J. Hypoxia-inducible factor-1 modulates the expression of vascular endothelial growth factor and endothelial nitric oxide synthase induced by eccentric exercise. Journal of Applied Physiology. 2015;118(8):1075–1083. doi: 10.1152/japplphysiol.00780.2014. [DOI] [PubMed] [Google Scholar]
- 45.Page E. L., Robitaille G. A., Pouyssegur J., Richard D. E. Induction of hypoxia-inducible factor-1α by transcriptional and translational mechanisms. The Journal of Biological Chemistry. 2002;277(50):48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 46.Alavian F., Hajizadeh S., Bigdeli M. R., Javan M. The role of protein kinase C in ischemic tolerance induced by hyperoxia in rats with stroke. EXCLI Journal. 2012;11:188–197. [PMC free article] [PubMed] [Google Scholar]
- 47.Zara S., Macchi V., De Caro R., Rapino M., Cataldi A., Porzionato A. pPKCα mediated-HIF-1α activation related to the morphological modifications occurring in neonatal myocardial tissue in response to severe and mild hyperoxia. European Journal of Histochemistry. 2012;56(1):p. 2. doi: 10.4081/ejh.2012.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Movafagh S., Crook S., Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species : new developments in an old debate. Journal of Cellular Biochemistry. 2015;116(5):696–703. doi: 10.1002/jcb.25074. [DOI] [PubMed] [Google Scholar]
- 49.Borjesson M., Onerup A., Lundqvist S., Dahlof B. Physical activity and exercise lower blood pressure in individuals with hypertension: narrative review of 27 RCTs. British Journal of Sports Medicine. 2016;50(6):356–361. doi: 10.1136/bjsports-2015-095786. [DOI] [PubMed] [Google Scholar]
- 50.Ryan A. S. Exercise in aging: its important role in mortality, obesity and insulin resistance. Aging Health. 2010;6(5):551–563. doi: 10.2217/ahe.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson R. J., Lowder T. W., Spielmann G., Bigley A. B., LaVoy E. C., Kunz H. Exercise and the aging immune system. Ageing Research Reviews. 2012;11(3):404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Nordin T. C., Done A. J., Traustadottir T. Acute exercise increases resistance to oxidative stress in young but not older adults. Age. 2014;36(6):p. 9727. doi: 10.1007/s11357-014-9727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sallam N., Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxidative Medicine and Cellular Longevity. 2016;2016:32. doi: 10.1155/2016/7239639.7239639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierce G. L., Donato A. J., LaRocca T. J., Eskurza I., Silver A. E., Seals D. R. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10(6):1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanabe T., Maeda S., Miyauchi T., et al. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiologica Scandinavica. 2003;178(1):3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 56.Hambrecht R., Adams V., Erbs S., et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107(25):3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 57.Durrant J. R., Seals D. R., Connell M. L., et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. The Journal of Physiology. 2009;587(13):3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]