Abstract
Objective
To assess undernutrition and associated factors among children aged 6–59 months in Gondar Town, northwest Ethiopia.
Methods
A community-based cross-sectional study was conducted in 2014. Multistage sampling method was used to select study participants. Structured interviewer administered questionnaire and anthropometric measurements were used. Binary logistic regression was fitted to identify associated factors.
Results
The prevalences of wasting and stunting were 6.8% and 45.7%, respectively. Higher odds of wasting were observed among children whose fathers were daily laborers (AOR = 2.63), children who had eating problem (AOR = 2.96), and those who were not exclusively breast-fed for the first six months (AOR = 5.63). Similarly, higher odds of stunting were found among female children (AOR = 1.65), children who lived in households having four to six families (AOR = 2.14), and children who did not start breast-feeding within one hour of birth (AOR = 0.67).
Conclusion
Childhood undernutrition was a significant problem. Child eating problem, paternal occupation, and exclusive breast-feeding were associated with wasting, whereas family size, child sex, and breast-feeding initiation time were associated with stunting. Therefore, strengthening of early initiation and exclusive breast-feeding, promoting healthcare seeking behavior, and designing social support programme for poor family are recommended to reduce undernutrition.
1. Background
Undernutrition encompasses all stunting, wasting, underweight, and micronutrient deficiencies [1, 2]. According to World Health Organization (WHO), undernutrition is defined as Z-scores less than −2 standard deviations, irrespective of the indicators used [3]. Child undernutrition (stunting and wasting) has been a serious global public health problem for the past many decades, especially in the developing countries including Ethiopia [4, 5]. It is considered as a very critical public health problem when the prevalence of stunting and that of wasting among children are higher than 40 and 15%, respectively [6]. The consequences of undernutrition are numerous, such as increased mortality, irreversible brain damage, and negative effect on cognitive ability, which have a cumulative effect on social development [7–9]. In addition, it has been associated with adverse functional consequences, such as overweight, obesity, insulin resistance, hypertension, dyslipidemia, a reduced capacity for manual work, and other chronic noncommunicable diseases during adolescence and adulthood [8, 10, 11]. Furthermore, it has a negative effect on future reproductive outcomes [12].
Globally, around 8 and 26% of children below five years of age are wasted and stunted, respectively. The highest prevalence of childhood undernutrition is found in Africa (38.2%) followed by Southeast Asia (27.6%), while the lowest magnitude is found in Latin America and the Caribbean countries (13.5%) [13, 14].
In Ethiopia, the magnitude of childhood undernutrition has decreased from 58% in 2000 to 40% in 2014 with regional differences ranging from 52% in Amhara to 22% in Addis Ababa [15, 16], but it is sustained as the major public health problem. For instance, 28% of all child mortality, 16% of all repetitions in primary school, and 8% of the workforce lost are associated with undernutrition. In addition, it is also associated with an estimated annual cost loss of 55.5 billion Ethiopian Birr (ETB), which is equivalent to 16.5% of the country's gross domestic product (GDP) [17].
Previous researchers from different corners of the globe confirmed that undernutrition is a complex interaction between different factors. Accordingly, poor child feeding practice [18], being male [19], poor household wealth status [19], prelacteal feeding and large household size [4, 5], and poor paternal educational status and an eating problem [5] were factors associated with undernutrition.
The Government of Ethiopia has planned to reach the zero-level undernutrition by 2030 [20]. Accordingly, it endorsed a National Nutrition Program, prepared infant and young child feeding manual, implemented monthly child growth and monitoring program, and has been working in collaboration with different nongovernmental organizations, such as Micronutrient Initiatives (MI), United Nations Children's Fund (UNICEF), and Save the Children. However, about 40 and 9% of children below five years of age were stunted and wasted, respectively [16].
In addition, level of undernutrition depends on the ecological setting of the country, and there is a need to investigate the magnitude and risk factors to generate comprehensive data for policy makers and governmental and nongovernmental organizations working on child health and nutrition. Therefore, the aim of this study was to assess the magnitude and associated factors of undernutrition among children aged 6–59 months in Gondar Town, northwest Ethiopia.
2. Materials and Methods
2.1. Study Period and Setting
The study was conducted in Gondar Town from 15 October to 30 November 2014. The town is found in North Gondar Zone, Amhara Regional State of Ethiopia, and is located at 750 km from Addis Ababa to the northwest. According to population projection rate, a population of around 300,000 had been estimated to have resided in Gondar Town in 2014. Administratively, the town is divided into 12 administrative areas (subcities) which consist of 21 kebeles (the smallest administrative units in Ethiopia).
2.2. Study Participants, Sample Size, and Sampling Procedure
All children who are aged 6–59 months and had lived in Gondar Town for at least six months were eligible for the study. The sample size of the study was calculated using the OpenEpi software, version 2.3, by assuming 46% of stunting prevalence [4], a 95% Confidence Interval, and a 5% margin of error. Finally, the sample size of 764 was obtained by considering the design effect of 2. A multistage sampling followed by the simple random sampling technique was employed to reach the study participants. Initially, four of the 12 subcities were selected by the lottery method. Then, four kebeles (one kebele from each subcity) were selected. A total number of children in the selected kebeles were obtained from health extension workers, and then the total number of children included in the study was proportionally allocated to each kebele. Finally, a simple random sampling method was used to select the study subjects from the list of children in each kebele.
2.3. Data Collection Instrument and Procedure
A structured interviewer-administered questionnaire was used to collect data. The questionnaire was adopted from EDHS (Ethiopian Demographic and Health survey) 2011 and other similar studies with some modifications to fit the local context. The questionnaire consisted of the sociodemographic characteristics of the children and their parents, child health, dietary practice related factors, and maternal health service utilization.
The questionnaire was initially prepared in English and translated to Amharic and, finally, retranslated to English to maintain consistency. A pretest was done on 5% of the sample outside the study area. Two-day training was given to data collectors and supervisors. The data were collected by clinical nurses and public health experts.
2.4. Assessment of Comorbidity Symptoms and Eating Problem
Assessment of comorbidity symptoms was begun by asking the mothers using a two-week recall period. Finally, the prevalence of undernutrition was compared among children who had the symptoms and those who did not. Similarly, eating problem was assessed by asking the mothers whether or not the child faced difficulty of swallowing, eating things that are not really food, and eating only certain types of food.
2.5. Assessment of Exclusive Breast-Feeding
Children were considered exclusively breast-fed for the first six month when the child had only received breast milk (including breast-feeding by a wet nurse and feeding expressed breast milk and children received ORS, drops, and syrups) [21].
2.6. Anthropometric Measurement
Birth date was determined by asking mothers and by cross-checking against immunization status certificates of the children. The weight of children aged 24–59 months was taken in light clothes and no shoe to the nearest 0.1 kg by a Seca beam balance (Germany, serial number 5755107131646 with graduation of 0.1 kg and measuring up to 160 kg). Similarly, the weight of children aged 6–23 months was measured by the salter scale to the nearest 0.1 kg (Germany, serial number 3541317009 with graduation of 0.1 kg and measuring up to 20 kg). The height of children aged 24–59 months was measured in Frankfurt (the child's head, shoulders, buttocks, knees, and heels touch the vertical board) position using the Seca vertical height scale (Germany, model number 213) to the nearest 0.1 cm, whereas the length of children aged 6–23 months was taken by a measuring board to the nearest 0.1 cm in recumbent position. Instrument calibration was done before measuring the next child. The weight and height of mothers were also measured in standing position using Seca beam balance and Seca vertical height scale, respectively.
The anthropometric data of the children were entered into the ENA/SMART software, version 2011. Z-scores of nutritional indices, such as Weight-for-Age (WAZ), Weight-for-Height (WHZ), and Height-for-Age (HAZ), were calculated using the WHO Multicenter Growth Reference Standard. Finally, children were classified as stunted, underweight, and wasted when the HAZ, WAZ, and WHZ scores were less than −2 standard deviations (SD), respectively [3]. Maternal body mass index (BMI) was calculated.
2.7. Data Processing and Analysis
The collected data were manually checked for completeness and consistency of responses. Then, the data were entered into Epi Info, version 7, and exported to SPSS, version 20, for further analysis. Descriptive statistics were used to summarize variables. Both bivariable and multivariable binary logistic regression analyses were used to identify variables associated with undernutrition. Variables with a p value less than 0.2 in the bivariable analysis were fitted into the multivariable binary logistic regression analysis to control the possible effect of confounding. Both Crude Odds Ratio (COR) and Adjusted Odds Ratio (AOR) with the corresponding 95% Confidence Interval (CI) were calculated to show the strength of association. Finally, in the multivariable analysis, variables with a p value less than 0.05 were considered statistically significant.
3. Results
3.1. Sociodemographic and Socioeconomic Characteristics of Parents
A total of 707 child-mother pairs were included in the study. The median age of the mothers was 27 years (Interquartile Range (IQR): 25–31 years). About 9.6% of the respondents had a family size of greater than seven. Around one-third (31.1%) of the mothers were unable to read and write. Most (70.7%) and nearly one-third (32.9%) of the mothers and their husbands were housewives and government employees, respectively (Table 1).
Table 1.
Sociodemographic and socioeconomic characteristics of parents, Gondar Town, northwest Ethiopia (n = 707).
| Variables | Frequency | Percentage |
|---|---|---|
| Age of mothers (in years) | ||
| <25 | 165 | 23.3 |
| 25–30 | 364 | 51.5 |
| 31–35 | 102 | 14.4 |
| >35 | 76 | 10.7 |
| Mothers' marital status | ||
| Currently married | 619 | 87.6 |
| Currently unmarried$ | 88 | 12.4 |
| Mothers' religion | ||
| Orthodox | 501 | 70.9 |
| Muslim | 201 | 28.4 |
| Others | 5 | 0.7 |
| Mothers' educational status | ||
| Unable to read and write | 220 | 31.1 |
| Primary | 210 | 29.7 |
| Secondary and above | 277 | 39.2 |
| Husbands' education | ||
| Unable to read and write | 85 | 12 |
| Primary | 315 | 44.5 |
| Secondary and above | 307 | 43.4 |
| Mothers' occupation | ||
| Housewife | 500 | 70.7 |
| Government employee | 38 | 5.3 |
| Merchant | 52 | 7.4 |
| Daily laborer | 117 | 16.5 |
| Husbands' occupation | ||
| Government employee | 233 | 32.9 |
| Merchant | 207 | 29.3 |
| Daily laborer | 184 | 26.0 |
| Own private work | 83 | 11.7 |
| Family size | ||
| ≥7 | 68 | 9.6 |
| 4–6 | 389 | 55.0 |
| ≤3 | 250 | 35.4 |
| Number of under-five children | ||
| Two and above | 142 | 20.1 |
| One | 565 | 79.9 |
| Monthly income | ||
| <2175 ETB# | 446 | 63.1 |
| ≥2175 ETB | 261 | 36.9 |
$Single, widowed, or divorced; #Ethiopian Birr (1$ = 26.7 ETB).
3.2. Child Feeding and Health-Related Characteristics
Of the total children, 378 (53.5%) were males. The mean (±SD) age of the children was 30 ± 14.39 months. One-fifth (21.5%) of the children were sick two weeks prior to the date of survey. Four (0.6%) of the children had vitamin A deficiency (Bitot's spot) (Table 2). Fifty-eight (8.2%) of the children were given prelacteal feeding, and 5.8% of the mothers used bottles for complementary feeding. More than two-thirds (81.6%) of children were exclusively breast-fed for the first six months (Table 3).
Table 2.
Selected health-related and sociodemographic characteristics of the children aged 6–59 months, Gondar Town, northwest Ethiopia (n = 707).
| Variables | Frequency | Percentage |
|---|---|---|
| Sex of the child | ||
| Male | 378 | 53.5 |
| Female | 329 | 46.5 |
| Age of the child (in months) | ||
| 6–11 | 74 | 10.5 |
| 12–23 | 181 | 25.6 |
| 24–35 | 174 | 24.6 |
| 36–47 | 155 | 21.9 |
| 48–59 | 123 | 17.4 |
| Birth interval | ||
| 1st child | 289 | 40.9 |
| 1 year | 28 | 4.0 |
| 2 years | 79 | 11.2 |
| 3 years | 84 | 11.9 |
| 4 years and above | 227 | 32.1 |
| Weight changes in the last two weeks | ||
| Yes | 64 | 9.1 |
| No | 643 | 90.9 |
| Illness in the last two weeks | ||
| Yes | 152 | 21.5 |
| No | 555 | 78.5 |
| Morbidity symptoms (n = 152)∗ | ||
| Diarrhea | 83 | 54.6 |
| Cough | 44 | 28.9 |
| Fever | 25 | 16.5 |
| Children had eating problem | ||
| Yes | 146 | 20.7 |
| No | 561 | 79.3 |
| Types of eating problems (n = 146)∗ | ||
| Loss of appetite | 140 | 89.2 |
| Swallowing problem | 2 | 1.3 |
| Vomiting | 15 | 9.5 |
| Children had Bitot's spot | ||
| Yes | 4 | 0.6 |
| No | 703 | 99.4 |
| Vitamin A supplementation | ||
| Yes | 682 | 96.5 |
| No | 25 | 3.5 |
∗Multiple responses.
Table 3.
Feeding practices of children aged 6–59 months, Gondar Town, northwest Ethiopia (n = 707).
| Variables | Frequency | Percentage |
|---|---|---|
| Prelacteal feeding | ||
| Yes | 58 | 8.2 |
| No | 649 | 91.8 |
| Initiation of breast-feeding | ||
| Within one hour | 532 | 75.2 |
| More than one hour | 175 | 24.8 |
| Children being breast-fed | ||
| Yes | 301 | 42.6 |
| No | 406 | 57.4 |
| Exclusively breast-fed | ||
| No | 130 | 18.4 |
| Yes | 577 | 81.6 |
| Reasons not to be exclusively breast-fed (130) | ||
| Work related | 16 | 12.3 |
| Medical problem | 4 | 3.1 |
| Formula feeding has advantage | 5 | 3.8 |
| Maternal perception | 25 | 19.2 |
| Not having enough milk | 42 | 32.3 |
| Lack of family support | 27 | 20.8 |
| Not to breast-feed | 11 | 8.5 |
| Frequency of complementary feeding | ||
| Less than 3 times per day | 27 | 3.8 |
| 3–5 times per day | 541 | 76.5 |
| More than five times per day | 139 | 19.7 |
| Material used for complementary feeding | ||
| Bottle | 41 | 5.8 |
| Cup | 46 | 6.5 |
| Spoon | 218 | 30.8 |
| Fork | 14 | 2.0 |
| Mother hand | 137 | 19.4 |
| His/her hand | 430 | 60.8 |
3.3. Nutritional Status, Health, and Reproduction-Related Characteristics of Mothers
Around two-thirds (66.8%) of the maternal BMI were within the recommended range (BMI = 18.5–24.9 kg/m2). But 13.4% of mothers had BMI less than 18.5 kg/m2. Regarding their antenatal care (ANC) utilization, 85.9% of them had four and above visits during the pregnancy of the selected children. 458 (64.8%) of the mothers were receiving information about infant and child feeding during their ANC visits.
3.4. Nutritional Status of Infants and Young Children
The overall prevalence of stunting was 45.7% (95% CI: 41.9, 49.5%), of which 21.2% were severely stunted, whereas the prevalence of wasting was 6.5% (95% CI: 4.8, 8.2%). Stunting and underweight were higher among females (Figure 1).
Figure 1.
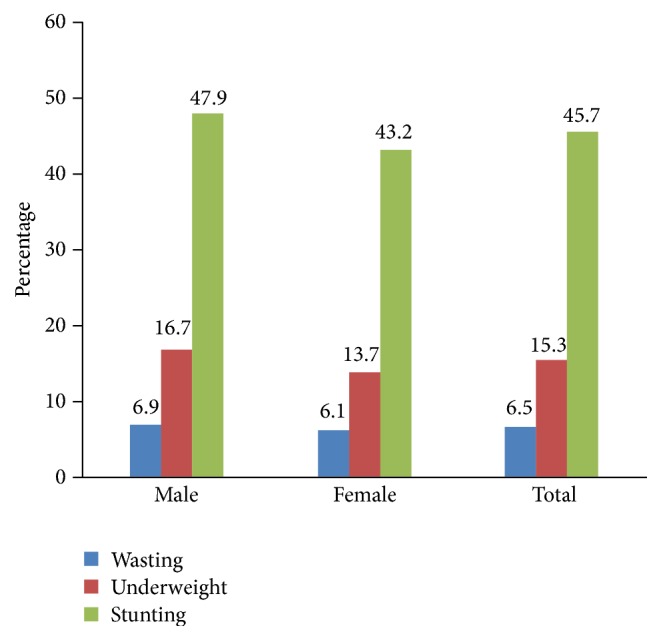
Prevalence of undernutrition by sex of children in Gondar Town, northwest Ethiopia, 2014.
3.5. Factors Associated with Stunting and Wasting
According to the multivariable binary logistic regression, being female (AOR = 1.65; 95% CI: 1.10, 2.48) and household family size of 4–6 (AOR = 2.48; 95% CI: 1.10, 2.15) were positively associated with stunting. However, initiation of breast-feeding within one hour of birth (AOR = 0.67; 95% CI: 0.46, 0.96) was negatively associated with stunting (Table 4).
Table 4.
Factors associated with stunting among children aged 6–59 months, Gondar Town, northwest Ethiopia.
| Variables | Nutritional status (stunting) | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Stunted | Not stunted | |||
| Age of the child | ||||
| 6–11 | 38 | 36 | 1.35 (0.76, 2.41) | 1.37 (0.85, 1.58) |
| 12–23 | 80 | 101 | 1.02 (0.64, 1.61) | 1.00 (0.61, 1.66) |
| 24–35 | 85 | 89 | 1.22 (0.77, 1.94) | 1.15 (0.70, 1.89) |
| 36–47 | 66 | 89 | 0.95 (0.59, 1.53) | 0.90 (0.54, 1.48) |
| 48–59 | 54 | 69 | 1.00 | 1.00 |
| Mothers' educational status | ||||
| Illiterate | 95 | 125 | 0.92 (0.65, 1.32) | 1.04 (0.65, 1.64) |
| Primary | 103 | 107 | 1.17 (0.82, 1.68) | 1.24 (0.83, 1.84) |
| Secondary and above | 125 | 152 | 1.00 | 1.00 |
| Husbands' educational status | ||||
| Illiterate | 38 | 47 | 0.90 (0.56, 1.46) | 0.93 (0.51, 1.69) |
| Primary | 140 | 175 | 0.89 (0.65, 1.23) | 0.88 (0.60, 1.28) |
| Secondary and above | 145 | 162 | 1.00 | 1.00 |
| Mothers' occupation | ||||
| Housewife | 226 | 274 | 1.00 | 1.00 |
| Government employee | 17 | 21 | 0.98 (0.51, 1.91) | 0.91 (0.45, 1.84) |
| Merchant | 24 | 28 | 1.04 (0.59, 1.84) | 1.04 (0.57, 1.91) |
| Daily laborer | 56 | 61 | 1.11 (0.744, 1.67) | 1.41 (0.89, 2.25) |
| Husbands' occupation | ||||
| Government employee | 113 | 120 | 1.00 | 1.00 |
| Merchant | 96 | 111 | 0.92 (0.63, 1.33) | 0.96 (0.64, 1.43) |
| Daily laborer | 75 | 109 | 0.73 (0.49, 1.08) | 0.68 (0.44, 1.07) |
| Own private work | 39 | 44 | 0.94 (0.57, 1.56) | 0.95 (0.56, 1.62) |
| Family size | ||||
| ≥7 | 27 | 41 | 0.88 (0.51, 1.52) | 1.10 (0.58, 2.08) |
| 4–6 | 189 | 200 | 1.26 (0.92, 1.74) | 2.48 (1.10, 2.15) ∗∗ |
| ≤3 | 107 | 143 | 1.00 | 1.00 |
| Number of under-five children | ||||
| Two and above | 62 | 80 | 0.90 (0.62, 1.31) | 0.78 (0.51, 1.18) |
| One | 261 | 304 | 1.00 | 1.00 |
| Sex of the child | ||||
| Male | 167 | 211 | 1.00 | 1.00 |
| Female | 156 | 173 | 1.14 (0.85, 1.53) | 1.65 (1.10, 2.48) ∗∗ |
| BMI of the mothers | ||||
| ≤18.4 | 36 | 59 | 1.00 | 1.00 |
| 18.5–24.9 | 221 | 251 | 1.44 (0.92, 2.27) | 1.50 (0.94, 2.39) |
| 25–29.9 | 54 | 61 | 1.45 (0.83, 2.52) | 1.47 (0.82, 2.62) |
| ≥30 | 12 | 13 | 1.51 (0.62, 3.67) | 1.39 (0.55, 3.54) |
| Number of ANC visits | ||||
| 1–3 times | 43 | 57 | 0.88 (0.58, 1.35) | 0.91 (0.57, 1.45) |
| 4 times and above | 280 | 327 | 1.00 | 1.00 |
| Prelacteal feeding | ||||
| Yes | 27 | 31 | 1.04 (0.61, 1.78) | 0.96 (0.48, 1.94) |
| No | 296 | 353 | 1.00 | 1.00 |
| Exclusive breast-feeding for the first six months | ||||
| No | 61 | 69 | 1.06 (0.726, 1.56) | 1.11 (0.68, 1.83) |
| Yes | 262 | 315 | 1.00 | 1.00 |
| Breast-feeding initiation | ||||
| Within 1 hour of birth | 232 | 300 | 0.71 (0.51, 1.01) | 0.67 (0.46, 0.96) ∗∗ |
| More than 1 hour of birth | 91 | 84 | 1.00 | 1.00 |
∗∗ indicates significance at p value less than 0.05.
Higher odds of wasting were observed among children who were not exclusively breast-fed for the first six months (AOR = 5.63; 95% CI: 1.7, 18.36), faced eating problems in the last two weeks prior to the date of survey (AOR = 2.96; 95% CI: 1.13, 7.78), and had daily laborer fathers (AOR = 2.63; 95% CI: 1.10, 6.27) (Table 5).
Table 5.
Factors associated with wasting among children aged 6–59 months, Gondar Town, northwest Ethiopia.
| Variables | Nutritional status (wasting) | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Wasted | Normal | |||
| Age of the child | ||||
| 6–11 | 6 | 68 | 1.12 (0.38, 3.28) | 2.38 (0.47, 12.0) |
| 12–23 | 8 | 173 | 0.58 (0.22, 1.56) | 0.87 (0.20, 3.63) |
| 24–35 | 11 | 163 | 0.86 (0.34, 2.13) | 0.76 (0.19, 1.09) |
| 36–47 | 12 | 143 | 1.06 (0.43, 2.61) | 2.56 (0.69, 9.41) |
| 48–59 | 9 | 114 | 1.00 | 1.00 |
| Mothers' educational status | ||||
| Illiterate | 14 | 206 | 1.11 (0.53, 2.32) | 1.04 (0.26, 4.08) |
| Primary | 16 | 194 | 1.35 (0.66, 2.76) | 1.32 (0.38, 4.62) |
| Secondary and above | 16 | 261 | 1.00 | 1.00 |
| Husbands' education | ||||
| Illiterate | 5 | 80 | 0.77 (0.28, 2.09) | 1.04 (0.22, 4.97) |
| Primary | 18 | 297 | 0.75 (0.39, 1.42) | 1.08 (0.38, 3.08) |
| Secondary and above | 23 | 284 | 1.00 | 1.00 |
| Husbands' occupation | ||||
| Government employee | 13 | 220 | 1.00 | 1.00 |
| Merchant | 11 | 196 | 0.95 (0.42, 2.17) | 1.12 (0.46, 2.71) |
| Daily laborer | 18 | 166 | 1.84 (0.87, 3.85) | 2.63 (1.10, 6.27) ∗∗ |
| Own private work | 4 | 79 | 0.86 (0.27, 2.71) | 0.97 (0.29, 3.29) |
| Family size | ||||
| ≥7 | 5 | 63 | 0.87 (0.32, 2.35) | 0.69 (0.14, 3.48) |
| 4–6 | 25 | 364 | 0.86 (0.30, 2.44) | 0.44 (0.13, 1.57) |
| ≤3 | 16 | 234 | 1.00 | 1.00 |
| Number of under-five children | ||||
| Two and above | 10 | 132 | 1.11 (0.54, 2.30) | 1.16 (0.39, 3.45) |
| One | 36 | 529 | 1.00 | 1.00 |
| Sex of the child | ||||
| Male | 26 | 352 | 1.00 | 1.00 |
| Female | 20 | 309 | 0.88 (0.48, 1.60) | 0.67 (0.28, 1.58) |
| Number of ANC visits | ||||
| 1–3 times | 8 | 92 | 1.30 (0.59, 2.88) | 0.95 (0.26, 3.38) |
| 4 times and above | 38 | 569 | 1.00 | 1.00 |
| Exclusive breast-feeding for the first six months | ||||
| No | 12 | 118 | 1.62 (0.82, 3.23) | 5.63 (1.7, 18.36) ∗∗ |
| Yes | 34 | 543 | 1.00 | 1.00 |
| Had eating problem | ||||
| Yes | 13 | 133 | 1.56 (0.80, 3.05) | 2.96 (1.13, 7.78) ∗∗ |
| No | 33 | 528 | 1.00 | 1.00 |
∗∗ indicates significance at p value less than 0.05.
4. Discussion
Childhood undernutrition was a significant public health problem in the study area. Eating problem, exclusive breast-feeding status for the first six months, family size, and breast-feeding initiation time were associated with undernutrition.
The prevalence of stunting was 45.7% (95% CI: 41.9, 49.5%), which is comparable to the finding of a study done in Dembiya District, Ethiopia (46%) [4], but it is lower than other districts of Ethiopia: Shire (Inda Selassie) (56.6%) [5] and Belesa District (57.7%) [2]. This may be due to Ethiopian recent nutrition programme that gives special attention to child nutrition and the promotion campaign on infant and young child feeding options. In addition, in this study, most of the mothers were educated: attended secondary and above school. Educated mothers can easily understand the effect of undernutrition on child health and survival. Furthermore, educated mothers are more likely to provide nutrient dense, adequate, and frequent complementary feeding to their children. Previous reports have also documented that boosting maternal knowledge is one of the fundamental instruments to break the intergenerational cycle of malnutrition [22].
Furthermore, the observed prevalence of stunting is also higher than what is reported in Indonesia (28.4%) [19], Bhutan (34.9%) [23], and EDHS (40%) [16]. This could be explained by cultural and food security status differences which may have an effect on the infant and young child feeding practices in the three settings: Ethiopia, Indonesia, and Bhutan.
The prevalence of wasting in the study area is in line with the finding from Ethiopia (9%) [24]. However, the prevalence is higher than the reports in Kenya (2.6%) [25] and Nigeria (3.7%) [26]. This is due to low family income in Ethiopia compared to Kenya and Nigeria. Supporting this argument, paternal occupation was one of the determinant factors of infant and young child wasting, according to the finding of this study [27].
In agreement with another study [28], higher odds of stunting were observed among females compared to males. In contrast, stunting was higher among males compared to females in studies done in Indonesia [19] and China [29]. This might be due to cultures in which parents give special attention and feed male children better than females.
Similar to other studies [30–32], the odds of stunting were higher among children from larger families compared to children from a family with less than three members. This is due to the fact that when families are large and their resources are limited, the available food is shared by all members, reducing the amount individuals get. In addition, low-quality foods and inappropriate feeding practice may contribute to the high prevalence of undernutrition [33–35].
Exclusive breast-feeding for the first six months is one of the strategies employed to decrease the burden of undernutrition [30]. Similarly, the higher odds of wasting were observed among children who were not exclusively breast-fed for the first six months compared to their counterparts. This is due to an early and late introduction of complementary foods. Early initiation of complementary feeding has been strongly associated with infection, which leads to an increased energy demand and loss of appetite and nutrients [36]. In addition, after six months, the energy requirements of children are not satisfied with breast milk alone [37].
In this study, the odds of being wasted were nearly 3 times (AOR = 2.96; 95% CI: 1.13, 7.78) higher among children who had eating problems for the last two weeks prior to the survey compared to children who did not have eating problems. This finding is in line with another finding from Ethiopia [38]. It is evident that recurrent infection is one of the determining factors for the high prevalence of undernutrition among under-five children [39].
Higher odds of wasting (AOR = 2.63; 95% CI: 1.10, 6.27) were found among children whose fathers were daily laborers compared to children whose fathers were government employees. This finding is supported by finding from Bangladesh [40]. In Ethiopia, men are the main source of family income. Family income depends on the type of paternal occupation, as the food purchasing power of the family is dependent on family income. Studies show that poor household wealth status has been strongly associated with inadequate intake of nutrient and poor personal and environmental hygiene, which are likely to increase the frequency of infection and undernutrition [29, 41]. In addition, government employees have a fixed monthly income, and most of them are educated. Educated individuals are more likely to follow the recommended child feeding practices, which improve the nutritional status.
5. Limitation of the Study
Even though adequate training was given to data collectors and supervisors, instruments were calibrated frequently, and respondents were clearly informed about the objectives of the study; still, there might be some limitations. First, there might be some intraobserver bias during weighing and recording. Second, the study may not show the causal relationship between undernutrition (stunting and wasting) and selected variables due to its cross-sectional nature.
6. Conclusion
Despite the fact that Ethiopia has been planning to reach zero level of childhood undernutrition, the study showed a significant public health problem in the study area. The finding alerts the public authorities about the implementation of undernutrition reduction initiatives. Therefore, strengthening early initiation and exclusive breast-feeding, promoting healthcare seeking behavior, and designing social support programme for poor family are recommended to reduce the burden of childhood undernutrition.
Acknowledgments
The authors would like to thank all respondents for their willingness to participate in the study. They are also grateful to North Gondar Zonal Health Department and the University of Gondar for material support.
Abbreviations
- ANC:
Antenatal care
- AOR:
Adjusted Odds Ratio
- BMI:
Body mass index
- CI:
Confidence Interval
- COR:
Crude Odds Ratio
- EDHS:
Ethiopia Demography and Health Survey
- ETB:
Ethiopian Birr
- GDP:
Gross domestic product
- HAZ:
Height-for-Age
- IQR:
Interquartile Range
- MI:
Micronutrient Initiatives
- SD:
Standard deviation
- UNICEF:
United Nations Children's Fund
- WAZ:
Weight-for-Age
- WHO:
World Health Organization
- WHZ:
Weight-for-Height.
Data Access
Data will be available upon request from the corresponding author.
Disclosure
Mulugeta Melku and Zegeye Abebe are co-first authors of the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Mulugeta Melku conceived the study, developed the tool, coordinated the data collection activity, and carried out the statistical analysis. Degefaye Zelalem Anlay participated in the design of the study, tool development, and drafting the manuscript. Belete Biadgo, Asemarie Kebede, Bamlaku Enawgaw, and Tsedalu Melku were involved in data collection supervision and drafting the manuscript. Zegeye Abebe participated in the design of the study and tool development, performing statistical analysis, and reviewing and editing the manuscript. All authors read and approved the final manuscript. Zegeye Abebe and Mulugeta Melku contributed equally to this work.
Supplementary Materials
The dataset, “mat.5367070.v2”, provided as supplementary material includes the anthropometric data of children aged 6–59 months who participated in the study in MS Excel Workbook format (.xlsx).
References
- 1.Vollmer S., Harttgen K., Subramanyam M. A., Finlay J., Klasen S., Subramanian S. V. Association between economic growth and early childhood undernutrition: evidence from 121 Demographic and Health Surveys from 36 low-income and middle-income countries. The Lancet Global Health. 2014;2(4):E225–E234. doi: 10.1016/S2214-109X(14)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Fentahun W., Wubshet M., Tariku A. Undernutrition and associated factors among children aged 6–59 months in East Belesa District, northwest Ethiopia: a community based cross-sectional study. BMC Public Health. 2016;16(1) doi: 10.1186/s12889-016-3180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: joint statement by the World Health Organization and the United NationsChildren’s Fund In.; 2009. [PubMed]
- 4.Tariku A., Woldie H., Fekadu A., Adane A. A., Ferede A. T., Yitayew S. Nearly half of preschool children are stunted in Dembia district, Northwest Ethiopia: a community based cross-sectional study. Archives of Public Health. 2016;74(1, article no. 13) doi: 10.1186/s13690-016-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brhane G., Regassa N. Nutritional status of children under five years of age in Shire Indaselassie, North Ethiopia: examining the prevalence and risk factors. Kontakt. 2014;16(3, article no. 23):e161–e170. doi: 10.1016/j.kontakt.2014.06.003. [DOI] [Google Scholar]
- 6. World Health Organization. Nutrition Landscape Information System country profile indicators interpretation guide. In Geneva: World Health Organization; 2010.
- 7.Black R. E., Allen L. H., Bhutta Z. A., et al. Maternal and child undernutrition: global and regional exposures and health consequences. The Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 8.Victora C. G., Adair L., Fall C., et al. Maternal and child undernutrition: consequences for adult health and human capital. The Lancet. 2008;371(9609):340–357. doi: 10.1016/s0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Bhutta Z. A., Coates M. M., et al. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study. Lancet. 2016;388(10053):1725–1774. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Onis M., Blössner M., Borghi E. Global prevalence and trends of overweight and obesity among preschool children. American Journal of Clinical Nutrition. 2010;92(5):1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 11.Wojcicki J. M. The double burden household in sub-saharan Africa: maternal overweight and obesity and childhood undernutrition from the year 2000:results from world health organization data (WHO) and Demographic Health Surveys (DHS) BMC Public Health. 2014;14(1, article no. 1124) doi: 10.1186/1471-2458-14-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black R. E., Victora C. G., Walker S. P., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 13.Lutter C. K., Daelmans B. M. E. G., De Onis M., et al. Undernutrition, poor feeding practices, and low coverage of key nutrition interventions. Pediatrics. 2011;128(6):e1418–e1427. doi: 10.1542/peds.2011-1392. [DOI] [PubMed] [Google Scholar]
- 14.Kinyoki D. K., Berkley J. A., Moloney G. M., Odundo E. O., Kandala N.-B., Noor A. M. Environmental predictors of stunting among children under-five in Somalia: cross-sectional studies from 2007 to 2010. BMC Public Health. 2016;16(1, article no. 654) doi: 10.1186/s12889-016-3320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Central Statistical Agency [Ethiopia] and ICF International: Ethiopia Demographic and Health Survey 2011, Addis Ababa, Ethiopia and Calverton,Maryland, USA: Central Statistical Agency and ICF Internationa, 2012.
- 16.Kedir H., Berhane Y., Worku A. Subclinical iodine deficiency among pregnant women in haramaya district, Eastern Ethiopia: a community-based study. Journal of Nutrition and Metabolism. 2014;2014:8. doi: 10.1155/2014/878926.878926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Federal Democratic Republic of Ethiopia, The Cost of Hunger in Ethiopia: Implications for the Growth and Transformation of Ethiopia In: State Minister of Health, 2009. [DOI]
- 18. UNHCR, WFP with collaboration of UNSCN and WHO. Guidelines for selective feeding: The management of malnutrition in emergencies, 2009.
- 19.Torlesse H., Cronin A. A., Sebayang S. K., Nandy R. Determinants of stunting in Indonesian children: evidence from a cross-sectional survey indicate a prominent role for the water, sanitation and hygiene sector in stunting reduction. BMC Public Health. 2016;16(1, article no. 3339) doi: 10.1186/s12889-016-3339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Federal Democratic Republic of Ethiopia: Seqota declaration, A commitment to end child undernutition in Ethiopia by the year 2030. In Ethiopia Federal Ministry of Health, 2016. [DOI]
- 21. WHO, Indicators for assessing infant and young child feeding practices. In Washington D.C., USA: WHO, 2008.
- 22.Nambile Cumber S., Ankraleh N., Monju N. Mothers’ knowledge on the effects of malnutrition in children 0–5 years in Muea health area Cameroon. Journal of Family Medicine and Health Care. 2016;2(4):p. 36. doi: 10.11648/j.jfmhc.20160204.13. [DOI] [Google Scholar]
- 23.Zangmo U., de Onis M., Dorji T. The nutritional status of children in Bhutan: results from the 2008 National Nutrition Survey and trends over time. BMC Pediatrics. 2012;12, article 151 doi: 10.1186/1471-2431-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coates J., Swindale A., Bilinsky P. Wash, USA: 2007. Household food insecurity access scale (HFIAS) for measurement of food access: indicator guide. [Google Scholar]
- 25.Olack B., Burke H., Cosmas L., et al. Nutritional status of under-five children living in an informal urban settlement in Nairobi, Kenya. Journal of Health, Population and Nutrition. 2011;29(4):357–363. doi: 10.3329/jhpn.v29i4.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aliyu A. A., Oguntunde O. O., Dahiru T., Raji T. Prevalence and determinants of malnutrition among pre-school children in Northern Nigeria. Pakistan Journal of Nutrition. 2012;11(11):1092–1095. doi: 10.3923/pjn.2012.1092.1095. [DOI] [Google Scholar]
- 27. Cost of living-average monthly disposable salary after tax: Countries Compared Map, Available at: http://www.nationmaster.com/country-info/stats/Cost-of-living/Average-monthly-disposable-salary/After-tax. Accessed on May 20, 2017.
- 28.Shahnawaz M., Singh J. B. Nutritional status among the children living in predominantly tribal block of jhadol in district Udaipur, Rajasthan, India: a cross sectional study. Epidemiology, Biostatistics and Public Health. 2014;11(2) doi: 10.2427/8893.e8893 [DOI] [Google Scholar]
- 29.Pei L., Ren L., Yan H. A survey of undernutrition in children under three years of age in rural Western China. BMC Public Health. 2014;14(1, article 121) doi: 10.1186/1471-2458-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fikadu T., Assegid S., Dube L. Factors associated with stunting among children of age 24 to 59 months in Meskan district, Gurage Zone, South Ethiopia: a case-control study. BMC Public Health. 2014;14(1, article no. 800) doi: 10.1186/1471-2458-14-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degarege A., Hailemeskel E., Erko B. Age-related factors influencing the occurrence of undernutrition in northeastern Ethiopia. BMC Public Health. 2015;15(1, article no. 108) doi: 10.1186/s12889-015-1490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degarege D., Degarege A., Animut A. Undernutrition and associated risk factors among school age children in Addis Ababa, Ethiopia Global health. BMC Public Health. 2015;15(1, article no. 375) doi: 10.1186/s12889-015-1714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bantamen G., Belaynew W., Dube J. Assessment of factors associated with malnutrition among under five years age children at Machakel Woreda, Northwest Ethiopia: a case control study. Journal of Nutrition & Food Sciences. 2014;4(1, article 256) doi: 10.4172/2155-9600.1000256. [DOI] [Google Scholar]
- 34.Macharia C., Kogi-Makau W., Muroki N. A comparative study on the nutritional status of children (6–59 months) in a world vision project area and a non-project area in Kathonzweni division, Makueni District, Kenya. East African Medical Journal. 2004;81(8) doi: 10.4314/eamj.v81i8.9201. [DOI] [PubMed] [Google Scholar]
- 35.Contreras M., Blandón E. Z., Persson L.-Å., Hjern A., Ekström E.-C. Socio-economic resources, young child feeding practices, consumption of highly processed snacks and sugar-sweetened beverages: a population-based survey in rural northwestern Nicaragua. BMC Public Health. 2015;15(1, article no. 25) doi: 10.1186/s12889-015-1374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asfaw M., Wondaferash M., Taha M., Dube L. Prevalence of undernutrition and associated factors among children aged between six to fifty nine months in Bule Hora district, South Ethiopia. BMC Public Health. 2015;15(1):p. 41. doi: 10.1186/s12889-015-1370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daelmans B., Mangasaryan N., Martines J., Saadeh R., Casanovas C., Arabi M. Strengthening actions to improve feeding of infants and young children 6 to 23 months of age: Summary of a recent World Health Organization/UN ICEF technical meeting, Geneva, 6-9 October 2008. Food and Nutrition Bulletin. 2009;30(2):S236–S238. doi: 10.1177/15648265090302S208. [DOI] [PubMed] [Google Scholar]
- 38.Demissie S., Worku A. Magnitude and factors associated with malnutrition in children 6–59 months of age in pastoral community of Dollo Ado district, Somali region, Ethiopia. Science Journal of Public Health. 2013;1(4):175–183. doi: 10.11648/j.sjph.20130104.12. [DOI] [Google Scholar]
- 39.Herrador Z., Sordo L., Gadisa E., et al. Cross-sectional study of malnutrition and associated factors among school aged children in rural and urban settings of fogera and libo kemkem districts, ethiopia. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0105880.e105880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs C., Sultana T., Ahmed T., Iqbal Hossain M. Factors associated with acute malnutrition among children admitted to a diarrhoea treatment facility in Bangladesh. International Journal of Pediatrics. 2014;2014:1–5. doi: 10.1155/2014/267806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinsugi C., Matsumura M., Karama M., Tanaka J., Changoma M., Kaneko S. Factors associated with stunting among children according to the level of food insecurity in the household: A cross-sectional study in a rural community of Southeastern Kenya Global health. BMC Public Health. 2015;15(1, article no. 441) doi: 10.1186/s12889-015-1802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The dataset, “mat.5367070.v2”, provided as supplementary material includes the anthropometric data of children aged 6–59 months who participated in the study in MS Excel Workbook format (.xlsx).


