Abstract
Background
Previous studies show that overexpression of EMMPRIN involved in the malignant biological behavior of tumors. This investigation was to disclose the expression status of EMMPRIN in non-small cell lung cancer (NSCLC) and its clinical value for the diagnosis of NSCLC.
Methods
The expression of EMMPRIN was examined using immunohistochemistry and enzyme-linked immunosorbent assay. The clinical value of EMMPRIN was evaluated by drawing a receiver operating characteristic (ROC) curve.
Results
NSCLC tissues and serum exhibited higher expression levels of EMMPRIN than the normal control (p < 0.05), and the expression of the EMMPRIN was significantly associated with lymphatic invasion and advanced stage of NSCLC (p < 0.05). ROC curve suggested that the threshold level of serum EMMPRIN for distinguishing NSCLC from control group was 80.3 pg/mL, and displayed a sensitivity of 97.22% and a specificity of 95%. And higher EMMPRIN expression in serum and tissues appeared to be risk factors for NSCLC development (risk ratio =1.56 and 1.1).
Conclusion
Overexpression of EMMPRIN was associated with lymphatic metastasis and advanced stage of NSCLC and test of serum EMMPRIN contributes to the NSCLC diagnosis.
Keywords: Non-small cell lung cancer, NSCLC, EMMPRIN, Lymphatic metastasis, Biomarker
Background
Lung cancer is one of the leading causes of deaths globally, especially in developing countries like China. One recent investigation shows that there will be about 733,000 newly diagnosed lung cancer cases in 2015 in China and about 610,000 Chinese will die from this disease [1]. Non-small cell lung cancer (NSCLC) accounts for the majority of lung cancer (75%) in clinic, and most of them are lung squamous cell carcinoma (LSCC) and lung adenocarcinoma (LAC). People believe that early diagnosis of lung cancer play an important role in reducing of morbidity and mortality [2]. This is a consensus that the change levels of some molecules contribute to the diagnosis, treatment and prognosis of cancers [3–5]. And some valuable tumor biomarkers derived from these molecules are considered to be beneficial for molecular typing and individualized therapy [5].
Extracellular matrix metalloproteinase inducer (EMMPRIN), other names now include CD147, basigin, and HAb18G, which is originally isolated from the LX-1 human lung cancer cell line and is now considered to be a transmembrane glycoprotein [6]. EMMPRIN is a 269 aa TM protein with a glycosylated molecular weight with an approximate range of 43–66 kDa [7]. Now EMMPRIN in normal cell function and other disease states has been investigated, especially in malignant tumors. In a series of tumors, including liver, breast, colon, prostate and esophageal cancer, EMMPRIN often exhibits a high expression [7]. Recent reports have indicated that the expressions of EMMPRIN correlate with poor clinical factors and outcomes in lung cancer but some studies have inconsistent conclusions [8]. Therefore, we conducted this study for analyzing the relationship between EMMPRIN and clinical features of NSCLC, and disclosed the clinical significance and diagnostic value of EMMPRIN expression in NSCLC.
Methods
Patients
From January 2015 to December 2016, the blood samples from 72 patients with NSCLC at the Jining NO.1 People’s Hospital, Jining, China were collected for this retrospective study. Over the same period, the blood samples of 60 individuals for control group (receiving strict physical examination) were gathered as normal control (56 ± 3.1 years) from the hospitals mentioned above. All patients were not received radio/chemotherapy before collecting specimens and were grouped according to the tumor-node-metastasis (TNM) classification (IASLC, Eighth edition, 2016) [9]. The lung cancer tissues from 55 of 72 patients who underwent surgical resection were collected for studying on EMMPRIN expression in tissues. Meanwhile the matched adjacent non-malignant tissues were also collected as the normal control tissues, which was at least 3 cm away from the edge of lung cancer mass. The patients were grouped according to different clinical features, which are showed in Table 1.
Table 1.
Clinico-pathological features of patients (n=72)
| Items | Characteristics | Lung cancer patients (N=72) | Healthy individuals (N=60) |
|---|---|---|---|
| Gender | |||
| Male | 46(63.9%) | 38(63.3%) | |
| Female | 26(36.1%) | 22(36.7%) | |
| Ages | |||
| <60 | 31(43.06%) | 36(60%) | |
| ≥60 | 41(56.94%) | 24(40%) | |
| Smoking | |||
| Yes | 28(38.9%) | 19 (31.7%) | |
| No | 44(61.1%) | 41(68.3%) | |
| Classification of pathology | |||
| LAC | 33(45.83%) | ||
| LSCC | 39(54.17%) | ||
| Differentiation degree | |||
| Poorly differentiated | 24(33.3%) | ||
| Moderately differentiated | 19(26.4%) | ||
| Well-differentiated | 29(40.3%) | ||
| Clinical staging | |||
| IIA - IIIA | 35(48.6%) | ||
| IIIB - IV | 37(51.4%) | ||
| Lymphatic metastasis | |||
| N0 - N1 | 26(63.9%) | ||
| N2 - N3 | 46(36.1%) | ||
NSCLC, non-small cell lung cancer; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; N, the grade of lymphatic invasion
The study was approved by Research Ethics Committee of Jining NO.1 People’s Hospital, Jining, China. Each specimen was collected after the patient's permission, while patients signed informed consent. The entire process of collecting the specimen is ethical and regulated by the approved body. We confirmed that all methods were performed in accordance with the relevant guidelines and regulations.
Production and preservation of tissue microarray (TMA)
Before constructing the TMA, we carefully selected the representative tissue areas of lung cancer and marked. We used a tissue array instrument (Beecher Instruments, Manual Tissue Arrayer, USA) to construct the TMA. The tissue cores from donor tissue block were took out using a thin-walled needle of diameter of approximately 2.0 mm, and were precisely inserted a recipient block according to the selection number. TMA tissue blocks were sliced, baked in an oven, and stored at 4 °C.
Treatment of blood samples
Blood samples were collected by a method of conventional venous blood collection. After collection, the blood samples were immediately mixed to avoid the generation of air bubbles. Then, the blood was centrifuged at 3000 rpm for 10 min, and the serum was carefully isolated and frozen at -20°C. The cryopreserved serum is taken out before testing and thawed at room temperature (ensuring that the sample is well thawed evenly).
Immunohistochemistry (IHC)
The expression of EMMPRIN in tissues was determined using a Strept Actividin-Biotin Complex of IHC techniques (SABC kit, Bostere Biotech Company, Wuhan, China) according to the protocol of the SABC kit. A rabbit anti-human EMMPRIN monoclonal antibody was used as primary antibody with a dilution concentration of 1:50. The positive staining sections provided by antibody kit were served as a positive control and the first antibody replaced by the same volume of PBS was considered as a negative control. Immunostaining was blindly evaluated according to previous method reported [9]. The staining was scored separately as follows: 0 for no staining in tumour cells; 1 for moderate intensity; and 2 for intense staining of more than 75% of tumour cells; >1 was considered to be a positive result.
Enzyme-linked immunosorbent assay (ELISA)
The EMMPRIN level of serum was measured by ELISA Kit (Shanghai Senwei Technology Industrial Co., Ltd; Shanghai, China) strictly following the testing procedures designed by the manufacturer. Samples and standards were added to the wells, and then the antibody mixture was added. After the incubation, each well was carefully washed to remove unbound material. Followed by the addition of TMB substrate, and catalyzed by HRP, resulting a blue. The final reaction is terminated by adding a stop solution, resulting in a change from blue to yellow color. The color reaction was measured by a photometer at a wavelength of 450 nm. Standard curves were made according to the concentration of the standard sample and corresponding value of optical density of each well.
Statistical analysis
The data on the EMMPRIN expression in tissues were tested using the χ2, Fisher's exact, and McNemar test. The data on the serum level of EMMPRIN were analyzed using the Student’s T-test (matched samples) and One-WAY ANOVA (single factor analysis). To assess the diagnostic potential of EMMPRIN in serum for NSCLC, a ROC analysis was performed. All tests were two-sided, and p-values <0.05 were considered to be statistically significant. The statistical analysis was finished using the SPSS 19.0 software package (SPSS Institute, Chicago, USA).
Results
Expressions of EMMPRIN was up-regulated in NSCLC
EMMPRIN protein is mainly expressed in the cytoplasm and cell membrane of NSCLC (Fig. 1a–f). The statistical results showed that EMMPRIN was highly expressed in 29 (52.7%) of the 55 NSCLC tissues, whereas it was lowly expressed in 14 (25.5%) of the 55 matched adjacent non-malignant tissues. The up-regulation of EMMPRIN in the NSCLC tissues and the downregulation in the adjacent non-malignant tissues were observed (p = 0.003) (Table 2).
Fig. 1.
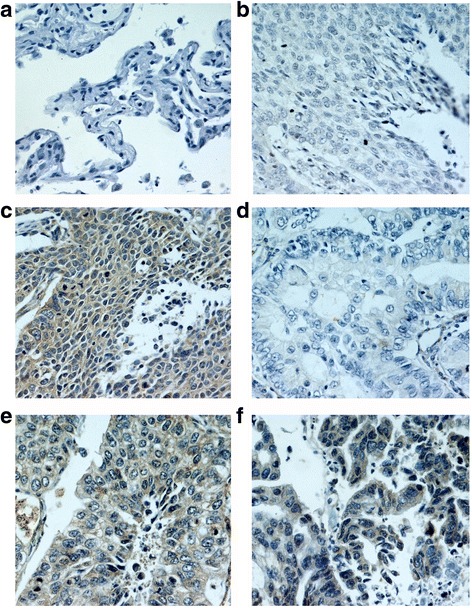
IHC analysis of EMMPRIN in NSCLC and normal tissues (IHC×400) a. No staining of EMMPRIN in normal tissues; b. No staining of EMMPRIN in well differentiated LSCC; c. Intensive staining of EMMPRIN in poorly differentiated LSCC; d. No staining of EMMPRIN in well differentiated LAC; e. Moderate staining of EMMPRIN in moderately differentiated LAC; f. Intensive staining of EMMPRIN in poorly differentiated LAC; NSCLC, non-small cell lung cancer; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma
Table 2.
Correlation between clinico-pathological features and the expressions of EMMPRIN in NSCLC tissues (n=55)
| Items | Groups | N | Expressions of EMMPRIN in lung tissues | |||
|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | χ2 value | P value | |||
| Resourse | ||||||
| Normal | 55 | 41(74.5) | 14(25.5) | 8.591 | 0.003 | |
| NSCLC | 55 | 26(47.3) | 29(52.7) | |||
| Gender | ||||||
| Male | 36 | 19(52.8) | 17(47.2) | 1.267 | 0.260 | |
| Female | 19 | 7(36.8) | 12(63.2) | |||
| Ages | ||||||
| <60 | 27 | 13(48.1) | 14(51.9) | 0.016 | 0.898 | |
| ≥60 | 28 | 13(46.4) | 15(53.6) | |||
| Smoking | ||||||
| Yes | 21 | 13(61.9) | 8(38.1) | 2.918 | 0.088 | |
| No | 34 | 13(38.2) | 21(61.8) | |||
| Histology | ||||||
| LAC | 23 | 7(30.4) | 16(69.6) | 4.496 | 0.034 | |
| LSCC | 32 | 19(59.4) | 13(40.6) | |||
| Pathological grade | ||||||
| Poorly | 17 | 2(11.8) | 15(88.2) | 20.762 | <0.001 | |
| Moderately | 13 | 4(30.8) | 9(69.2) | |||
| Well | 25 | 20(80) | 5(20) | |||
| Lymphatic invasion | ||||||
| N0 - N1 | 26 | 20(76.9) | 6(23.1) | 17.392 | <0.001 | |
| N2 - N3 | 29 | 6(20.7) | 23(79.3) | |||
| pTNM | ||||||
| IIA-IIIA | 35 | 24(68.6) | 11(31.4) | 17.517 | <0.001 | |
| IIIB | 20 | 2(10) | 18(90) | |||
LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; Smoking, pack years of smoking; T stage, tumor size; pTNM, clinical stage of lung cancer
Over-expression of EMMPRIN correlated with histological type, poor differentiation, lymph node metastasis and advanced stage of NSCLC
As shown in Table 2, the expression of EMMPRIN did not relate to gender, age, and smoking status of NSCLC patients (p > 0.05). However, the expression level of EMMPRIN was up-regulated in LAC (16/23, 69.6%) (p = 0.034), poorly differentiated lung cancer tissues (15/17, 88.2%) (p < 0.001), NSCLC cases with N2-N3 of lymph node metastasis (23/29, 79.3%) (p < 0.001) and NSCLC tissues of stages IIIB (18/20, 90%) (p < 0.001), as compared with LSCC (13/32, 40.6%), well-differentiated tissues (5/25, 20%), lung cancer cases with N0-N1 of lymph node metastasis (6/26, 23.1%) and those of stages IIA-IIIA (11/35, 31.4%).
Higher serum level of EMMPRIN was showed in NSCLC patients
In a series of 72 serum specimens of NSCLC patients and a series of 60 serum specimens of control individuals, the statistical analysis of Kolmogorov-Smirov indicated that two sets of data from test value of serum EMMPRIN showed an approximately normal distribution (p=0.122). Student's t test showed that high serum level of EMMPRIN was found in NSCLC patients (101.93±15.01 pg/mL), whereas it was lowly expressed in control group (59.04±10.97 pg/mL), indicating that the serum level of EMMPRIN was higher in NSCLC patients than that in control individuals (p < 0.001) (Table 3).
Table 3.
Correlation between clinico-pathological parameters and the expressions of EMMPRIN in serum of NSCLC (n=72)
| Parameter | Group | N | Expressions of EMMPRIN in serum of NSCLC | |||
|---|---|---|---|---|---|---|
| Value (pg/ml) | Degree of freedom | Statistical value | P value | |||
| Resource | ||||||
| Normal | 60 | 59.04±10.97 | 130 | 18.411 | <0.001 | |
| NSCLC | 72 | 101.93±15.01 | ||||
| Gender | ||||||
| Male | 46 | 100.86±15.09 | 70 | -0.800 | 0.43 | |
| Female | 26 | 103.81±14.98 | ||||
| Ages | ||||||
| <60 | 31 | 101.12±12.06 | 70 | -0.396 | 0.69 | |
| ≥60 | 41 | 102.56±17.03 | ||||
| Smoking | ||||||
| Yes | 28 | 103.23±16.06 | 70 | 0.585 | 0.56 | |
| No | 44 | 101.09±14.13 | ||||
| Histology | ||||||
| LAC | 33 | 104.02±13.13 | 70 | 1.087 | 0.28 | |
| LSCC | 39 | 100.16±16.39 | ||||
| Pathological grade | ||||||
| Poorly | 24 | 105.33±11.46★ | 2 | 3.719 | 0.029 | |
| Moderately | 19 | 106.24±14.92★ | ||||
| Well | 29 | 96.28±16.27 | ||||
| Lymphatic invasion | ||||||
| N0 - N1 | 26 | 91.78±9.97 | 70 | -4.983 | <0.001 | |
| N2 - N3 | 46 | 107.66±14.39 | ||||
| pTNM | ||||||
| IIA - IIIA | 35 | 93.92±10.45 | 70 | -5.125 | <0.001 | |
| IIIB - IV | 37 | 109.49±14.83 | ||||
M±SD, mean±standard deviation; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; ★, poor and moderate differentiation compared with well differentiation; N, lymphatic metastasis; pTNM, clinical stage of lung cancer
Serum level of EMMPRIN correlated with poor differentiation, lymph node metastasis and advanced stage of NSCLC
As shown in Table 3, High serum level of EMMPRIN was observed in poorly differentiated lung cancer patients (105.33±11.46 pg/mL) and moderately differentiated lung cancer patients (106.24±14.92 pg/mL), as compared with well differentiated lung cancer patients (96.28±16.27 pg/mL) (p=0.029). In addition, the serum level of EMMPRIN in NSCLC patients with N0-N1 of lymph node metastasis was 91.78±9.97 pg/mL, which is lower than those lung cancer patients with N2-N3 of lymph node metastasis (107.66±14.39 pg/mL) (p < 0.001). Moreover, the serum level of EMMPRIN was highly expressed in NSCLC patients of stages IIIB-IV (109.49±14.83 pg/mL) compared with those of stages IIA-IIIA (93.92±10.45 pg/mL) (p < 0.001). However, the serum level of EMMPRIN did not correlate to gender, age, smoking status and histology of NSCLC patients (p > 0.05).
Threshold value and diagnostic accuracy of serum EMMPRIN for discerning NSCLC from control group
The cut-off value of serum EMMPRIN for discerning NSCLC was determined via ROC curve analysis [10]. As shown in Table 4, the threshold of serum EMMPRIN discerning NSCLC from control group intersected near the zone of 70 to 91.4 pg/mL, and the 95% CI (confidence interval) was from 77.4 to 99.6. The cut-off value of EMMPRIN for distinguishing NSCLC patients from control group was finally defined as 80.3 pg/mL (Fig. 2a). The minimum serum value of EMMPRIN was 47.11 pg/mL, which was in the control group while the maximum was found in the NSCLC group (146.23 pg/mL) (Fig. 2b). When 80.3 pg/mL is used as a threshold, the sensitivity of serum EMMPRIN for discerning NSCLC from normal individuals was 97.22% and specificity was 95% (Fig. 3a). ROC curve analysis showed that the AUC (area under the curve) of EMMPRIN arrived at 0.984 with 0.00867 of standard error, while 95% CI was from 0.945 to 0.998, and then Z value was 55.817 (P < 0.0001) (Fig. 3b).
Table 4.
Criterion values and coordinates of the ROC curve for EMMPRIN
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | 95% CI | -LR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| >68.6 | 100.00 | 95.0 - 100.0 | 88.33 | 77.4 - 95.2 | 8.57 | 7.8 - 9.4 | 0.00 | - |
| >70 | 97.22 | 90.3 - 99.7 | 88.33 | 77.4 - 95.2 | 8.33 | 7.5 - 9.2 | 0.031 | 0.007 - 0.1 |
| >80.1 | 97.22 | 90.3 - 99.7 | 91.67 | 81.6 - 97.2 | 11.67 | 10.7 - 12.7 | 0.030 | 0.006 - 0.2 |
| >80.3 * | 97.22 | 90.3 - 99.7 | 95.00 | 86.1 - 99.0 | 19.44 | 18.1 - 20.9 | 0.029 | 0.005 - 0.2 |
| >87.6 | 84.72 | 74.3 - 92.1 | 95.00 | 86.1 - 99.0 | 16.94 | 15.1 - 19.0 | 0.16 | 0.05 - 0.6 |
| >91.4 | 76.39 | 64.9 - 85.6 | 96.67 | 88.5 - 99.6 | 22.92 | 20.0 - 26.3 | 0.24 | 0.06 - 1.0 |
| >93.9 | 69.44 | 57.5 - 79.8 | 100.00 | 94.0 - 100.0 | - | - | 0.31 | - |
95% CI, 95% confidence; +LR, positive likelihood ratio; -LR, negative likelihood ratio; *cut-off value of EMMPRIN for distinguishing NSCLC patients from control group
Fig. 2.
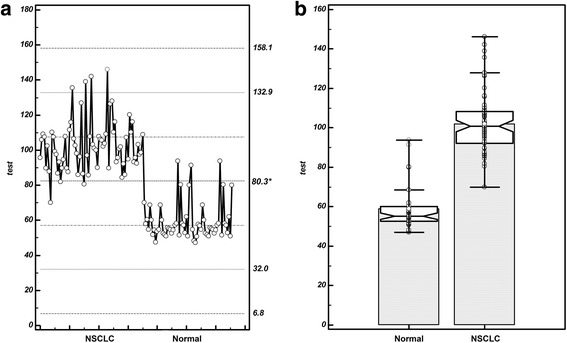
Threshold value of serum EMMPRIN for discerning NSCLC from control group a. Selection of cut-off value (80.3 pg/mL) of serum EMMPRIN for discerning NSCLC patients from control group, responding a sensitivity of 97.22% and specificity of 95%; b. The minimum value of EMMPRIN was 47.11 pg/ mL, which was observed in the control group while the maximum was 146.23 pg/mL, located in the disease group with NSCLC; NSCLC, non-small cell lung cancer
Fig. 3.
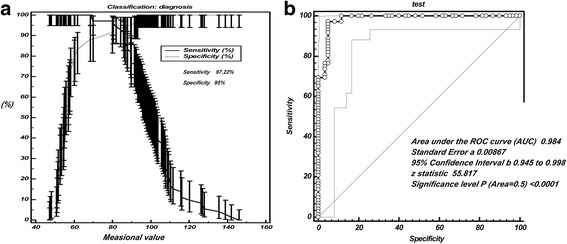
Diagnostic accuracy of serum EMMPRIN for discerning NSCLC from control group a. The cut-off value of EMMPRIN for distinguishing NSCLC patients from control group was finally defined as 80.3 pg/mL, responding a sensitivity of 97.22% and specificity of 95%; b. ROC curve analysis showed that the AUC (area under the curve) of EMMPRIN arrived at 0.984 and Z value was 55.817 (P<0.001); ROC, receiver operating characteristic curve; AUC, area under the curve
EMMPRIN expression in lung cancer tissue and serum of NSCLC patients displayed a positive correlation
When a serum value of EMMPRIN that is greater than a threshold of 80.3 pg/mL was defined as positive and a value of less than 80.3 pg/mL was defined as negative. The results showed that the positive co-expression rate of serum and tissues of EMMPRIN in NSCLC was 50.9% (28/55) and the negative co-expression rate was 3.6% (2/55). Fisher’s Exact Test suggested that the difference of EMMPRIN positive rate in cancer tissue and serum was not statistically significant (P=0.001) (Table 5). However, the P value of McNemar Test was less than 0.001, suggesting that EMMPRIN expression in lung cancer tissue and serum of NSCLC patients had a correlation.
Table 5.
Correlation of EMMPRIN expression in lung tissue and serum of NSCLC patients (n=55)
| Items | 55 cases | Tissues | Pearson Chi square | Fisher’s Exact Test★ | P value | McNemar Test # | |
|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||||
| Serum | Positive (%) | 28(50.9) | 25(45.5) | 0.432 | 0.605 | 0.515 | P<0.001 |
| Negative (%) | 1(1.8) | 2(3.6) | |||||
★Statistical results suggest: 2 cells (50.0%) have expected count less than 5. The minimum expected count is 1.45; ♯McNemar Test's P value is less than 0.001, indicating that EMMPRIN positive rate in cancer tissue and serum were statistically correlated
The relative risk (RR) for the over-expression of EMMPRIN in NSCLC
Pearson’s Chi square-test was employed to evaluate the risk ratio on the expressions of EMMPRIN in serum and tissues of NSCLC. The results indicated that the risk ratio value of over-expression of EMMPRIN in serum was 1.56 (P < 0.001) with a 95% confidence interval (CI) of 1.301 to 1.84. In addition, the risk ratio value in tissues was 1.1 (P < 0.001), and the 95% CI was 0.68 to 1.35. The results showed that subjects with higher EMMPRIN expression in serum and tissues implied a higher risk for NSCLC possibility (risk ratio =1.56 and 1.1) compared with subjects with lower EMMPRIN expression, and the RR of EMMPRIN expression in serum is greater than in tissues.
Discussion
The clinical management decisions of lung cancer patients are increasingly dependent on the guidance of by prognostic and predictive markers. At present, some valuable molecular markers play an increasingly important role in the individualized treatment of tumors [11]. Most of the NSCLC are usually diagnosed before the disease reaches a late stage, resulting in a low 5-year survival rate of 20% [12]. The occurrence and development of NSCLC is involved a wide range of molecular biological changes. With the development of molecular technologies, increasingly more tumor markers have been applied in clinic [13]. Carcinoembryonic antigen (CEA), cytokeratin 19 fragments (CYFRA 21-1) and squamous cell carcinoma antigen are commonly recommended in NSCLC management [14]. EMMPRIN is encoded by a gene localized to 19p13.3, which has recently been recognized as an important modulator of tumor-stromal communication and mediates a wide range of tumor-promoting molecular events [15]. EMMPRIN is mainly known for its protease inducing function but a role in promoting tumor angiogenesis has also been demonstrated [16]. So far, the exploration on EMMPRIN has focused on basic research in vitro, and the number of studies on its expression in lung cancer is limited. In our study, the clinical significance of EMMPRIN expression in serum and tissues of NSCLC patients were evaluated.
In tissues level, our findings showed that EMMPRIN exhibited a higher expression in NSCLC than in adjacent non-malignant tissues. Therefore, we have reason to believe that there is relation between the high expression of EMMPRIN and the occurrence and development of lung cancer. We found that EMMPRIN expression was higher in LAC than in LSCC. In addition, the EMMPRIN was also highly expressed in poorly differentiated lung cancer tissues. In clinic, LAC is more aggressive and often early metastasizes to liver and brain, compared with LSCC and poorly differentiated lung cancer progresses faster. Thus, the results may suggest that there is a correlation between EMMPRIN over-expression and these malignant biological behaviors of NSCLC. According to our study, over-expression of EMMPRIN is closely related to lymph node invasion and advanced TNM staging of NSCLC. Studies have shown that metastasis of tumor cells and lymph node invasion are key factors in the progression of NSCLC. Therefore our study suggests that EMMPRIN over-expression may promote the development and progression of NSCLC. Our findings is consistent with the previous reports, which showed that compared with patients with low expression level of EMMPRIN, higher level of EMMPRIN in cancer tissues were associated with poor prognosis [15–18].
In serum level, our results showed that the serum EMMPRIN showed a remarkable elevation in NSCLC patients, as compared with control group, thus meaning a possible correlation between the increase of EMMPRIN level and an upward risk of NSCLC. Previous studies show that EMMPRIN can induce cancer aggressiveness and angiogenesis via up-regulating the expressions of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR), and promote invasion and metastasis by the up-regulation of matrix metalloprotease (MMP) [19]. In addition, elevated EMMPRIN expression in tumor tissues was correlated with shorter overall survival and disease free survival [19]. In our study, NSCLC with the increased EMMPRIN level in serum of NSCLC patients seems to be correlated with malignant phenotype of NSCLC such as lymph node metastasis, poorly differentiated tissues and advanced stage of NSCLC patients. Our study showed that the higher the degree of tumor progression is developed, the higher the serum expression of EMMPRIN is showed, suggesting that EMMPRIN increased with tumor invasion and invasion. When the serum test of EMMPRIN was used to discern NSCLC patients from control group, it responded a better screening ability with a sensitivity of 97.22% and specificity of 95%, suggesting that highly expressed EMMPRIN may be an indicator of NSCLC diagnosis and judgment of invasive degree. We found that a threshold of 80.3 pg/mL of EMMPRIN could discern the NSCLC patients theoretically from control group, which indicated that EMMPRIN can be applied to distinguish NSCLC. Previous research reports that the high expression of EMMPRIN in primary colorectal adenocarcinomas is an important prognostic factor, and patients with EMMPRIN-negative tumours had a relatively good prognosis [20]. In addition, increased expression of EMMPRIN may enhance gastric cancer growth, invasion and angiogenesis by up-regulating MMP expression, and EMMPRIN is considered to be an objective and effective marker for predicting invasion and prognosis [18].
In our study, serum EMMPRIN level was significantly higher in NSCLC patients than in control group and serum EMMPRIN level reflects the development and metastasis of NSCLC. Especially, the statistical analysis suggested that there was a positive expression correlation in NSCLC tissues and serum. The risk ratio analysis also indicated that the up-regulation of EMMPRIN might be an unfavorable factor in NSCLC. The risk ratio values of EMMPRIN higher expression for NSCLC in serum and tissues were 1.56× and 1.1×, respectively. These results suggest that EMMPRIN expression levels are of significance in the diagnosis and prediction of NSCLC. However, before the EMMPRIN is considered as a tumor marker for NSCLC, a larger sample of clinical studies should be required.
Although we have done our best, several deficiencies existed in this study. Firstly, the selection of surgical specimens may result in a selection bias of patients because surgery is involved only in patients with stage IIIB and below. Secondly, the number of research samples was not large, thus larger samples research of NSCLC patients is needed to get a high level of evidence. Thirdly, we did not explore the signal mechanism of EMMPRIN. Hence, further molecular biology experiments on EMMPRIN in NSCLC should be performed to explain the mutual regulation-mechanism regarding lung cancer cell lines.
Conclusions
We demonstrated that EMMPRIN was upregulated in tissues and serum of NSCLC, and the upregulation of EMMPRIN in NSCLC was associated with poorly differentiated NSCLC, lymph node metastasis and advanced stage of NSCLC patients. In addition, the serum EMMPRIN could serve as a potential biomarker for discerning NSCLC patients. These results indicated that the up-regulation of EMMPRIN was potentially involved in the progression and prognosis of NSCLC.
Acknowledgements
We are grateful for the technical advice provided by Dr. R BX (Chaoying Biotech Company, Xi’an, China) and L J (The Fourth Military Medical University, Xi’an, China).
Funding
This work was supported by Jining science and Technology Bureau of Shandong Province Funded Projects, China (No. JN1211).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- CD147
Cluster of differentiation 147
- CEA
Carcinoembryonic antigen
- CYFRA 21-1
Cytokeratin 19 fragments
- EGFR
Epidermal growth factor receptor
- ELISA
Enzyme-linked immunosorbent assay
- HRP
Horseradish peroxidase
- IHC
Immunohistochemistry
- LAC
Lung adenocarcinoma
- LSCC
Lung squamous cell carcinoma
- MMP
Matrix metalloprotease
- NSCLC
Non-small cell lung cancer
- PBS
Phosphate buffer saline
- ROC
Receiver operating characteristic
- TMA
Tissue microarray
- TMB
3,3′,5,5′-Tetramethylbenzidine
- TNM
Tumor-node-metastasis
- VEGF
Vascular endothelial growth factor
Authors' contributions
Y L, T F, QD Z and CC Q participated in the study design, discussed the results, and helped draft the manuscript. B L, ZH W and BW S participated in the study design, performed experiments and data statistics, and wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by Research Ethics Committee of Jining NO.1 People’s Hospital, Jining, China. We confirmed that all methods were performed in accordance with the relevant guidelines and regulations. All specimens were approved by the patient prior to collection and received written informed consent. The collection of specimens conforms to the ethical guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bing Liu, Email: 115422979@qq.com.
Zhaohui Wan, Email: 770423943@qq.com.
Baowei Sheng, Phone: +86 15864550097, Email: sbw3363930@aliyun.com.
Yong Lin, Email: linzhou78@163.com.
Tian Fu, Email: 20536446@qq.com.
Qingdi Zeng, Email: 20536446@qq.com.
Congcong Qi, Email: qlsh02@163.com.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Biaoxue R, Xiguang C, Hua L, Tian F, Wenlong G, Chongchong Z, et al. Increased level of Hsp90-beta in bronchoalveolar lavage fluid correlates with lymphatic invasion and advanced stage of lung cancer patients. Am J Transl Res. 2016;8(10):4147–4159. [PMC free article] [PubMed] [Google Scholar]
- 3.Biaoxue R, Hua L, Wenlong G, Shuanying Y. Overexpression of stathmin promotes metastasis and growth of malignant solid tumors: a systemic review and meta-analysis. Oncotarget. 2016;7(48):78994–79007. doi: 10.18632/oncotarget.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ujiie H, Kato T, Lee D, Hu HP, Fujino K, Kaji M, et al. Overexpression of MAGEA2 has a prognostic significance and is a potential therapeutic target for patients with lung cancer. Int J Oncol. 2017;50(6):2154–2170. doi: 10.3892/ijo.2017.3984. [DOI] [PubMed] [Google Scholar]
- 5.Nogimori K, Hori T, Kawaguchi K, Fukui T, Mii S, Nakada H, et al. Increased expression levels of ppGalNAc-T13 in lung cancers: Significance in the prognostic diagnosis. Int J Oncol. 2016;49(4):1369–1376. doi: 10.3892/ijo.2016.3638. [DOI] [PubMed] [Google Scholar]
- 6.Kong LM, Liao CG, Fei F, Guo X, Xing JL, Chen ZN. Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 2010;101(6):1463–1470. doi: 10.1111/j.1349-7006.2010.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn JN, Kaushik DK, Yong VW. The role of EMMPRIN in T cell biology and immunological diseases. J Leukoc Biol. 2015;98(1):33–48. doi: 10.1189/jlb.3RU0215-045R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Tian T, Liu C, Fang X. Elevated CD147 expression is associated with shorter overall survival in non-small cell lung cancer. Oncotarget. 2017; [DOI] [PMC free article] [PubMed]
- 9.Biaoxue R, Xiguang C, Hua L, Hui M, Shuanying Y, Wei Z, et al. Decreased expression of decorin and p57(KIP2) correlates with poor survival and lymphatic metastasis in lung cancer patients. Int J Biol Markers. 2011;26(1):9–21. doi: 10.5301/JBM.2011.6372. [DOI] [PubMed] [Google Scholar]
- 10.Biaoxue R, Hua L, Tian F, Wenlong G. Increased stathmin in serum as a potential tumor marker for lung adenocarcinoma. Jpn J Clin Oncol. 2017;47(4):342–349. doi: 10.1093/jjco/hyx005. [DOI] [PubMed] [Google Scholar]
- 11.McShane LM, Hayes DF. Publication of tumor marker research results: the necessity for complete and transparent reporting. J Clin Oncol. 2012;30(34):4223–4232. doi: 10.1200/JCO.2012.42.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Wang X, He H, Liu Z, Hu JF, Li W. Combination of circulating tumor cells with serum carcinoembryonic antigen enhances clinical prediction of non-small cell lung cancer. PLoS One. 2015;10(5):e0126276. doi: 10.1371/journal.pone.0126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Yu H, Han Z, Gao N, Xue J, Wang Y. Clinical significance of joint detection of serum CEA, SCCA, and bFGF in the diagnosis of lung cancer. Int J Clin Exp Pathol. 2015;8(8):9506–9511. [PMC free article] [PubMed] [Google Scholar]
- 14.Chen F, Wang XY, Han XH, Wang H, Qi J. Diagnostic value of Cyfra21-1, SCC and CEA for differentiation of early-stage NSCLC from benign lung disease. Int J Clin Exp Med. 2015;8(7):11295–11300. [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Kodama J, Hongo A, Hiramatsu Y. Role of emmprin in endometrial cancer. BMC Cancer. 2012;12:191. doi: 10.1186/1471-2407-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khayati F, Perez-Cano L, Maouche K, Sadoux A, Boutalbi Z, Podgorniak MP, et al. EMMPRIN/CD147 is a novel coreceptor of VEGFR-2 mediating its activation by VEGF. Oncotarget. 2015;6(12):9766–9780. doi: 10.18632/oncotarget.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue YJ, Lu Q, Sun ZX. CD147 overexpression is a prognostic factor and a potential therapeutic target in bladder cancer. Med Oncol. 2011;28(4):1363–1372. doi: 10.1007/s12032-010-9582-4. [DOI] [PubMed] [Google Scholar]
- 18.Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006;95(10):1371–1378. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bovenzi CD, Hamilton J, Tassone P, Johnson J, Cognetti DM, Luginbuhl A, et al. Prognostic Indications of Elevated MCT4 and CD147 across Cancer Types: A Meta-Analysis. Biomed Res Int. 2015;2015:242437. doi: 10.1155/2015/242437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boye K, Nesland JM, Sandstad B, Haugland Haugen M, Maelandsmo GM, Flatmark K. EMMPRIN is associated with S100A4 and predicts patient outcome in colorectal cancer. Br J Cancer. 2012;107(4):667–674. doi: 10.1038/bjc.2012.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


