Abstract
Anthropogenic stressors, including pollutants, are key evolutionary drivers. It is hypothesized that rapid evolution to anthropogenic changes may alter fundamental physiological processes (e.g., energy metabolism), compromising an organism’s capacity to respond to additional stressors. The Elizabeth River (ER) Superfund site represents a “natural-experiment” to explore this hypothesis in several subpopulations of Atlantic killifish that have evolved a gradation of resistance to a ubiquitous pollutant—polycyclic aromatic hydrocarbons (PAH). We examined bioenergetic shifts and associated consequences in PAH-resistant killifish by integrating genomic, physiological, and modeling approaches. Population genomics data revealed that genomic regions encoding bioenergetic processes are under selection in PAH-adapted fish from the most contaminated ER site and ex vivo studies confirmed altered mitochondrial function in these fish. Further analyses extending to differentially PAH-resistant subpopulations showed organismal level bioenergetic shifts in ER fish that are associated with increased cost of living, decreased performance, and altered metabolic response to temperature stress—an indication of reduced thermal plasticity. A movement model predicted a higher energetic cost for PAH-resistant subpopulations when seeking an optimum habitat. Collectively, we demonstrate that pollution adaption and inhabiting contaminated environments may result in physiological shifts leading to compromised organismal capacity to respond to additional stressors.
Graphical Abstract
INTRODUCTION
Anthropogenic activities have contributed to rapid environmental change, altering the trajectory and rate of biological evolution.1 Adaptive changes to abiotic stressors are likely to alter physiological and biochemical processes to maintain homeostasis, but may have trade-offs. Notably, abiotic stressors often affect energy metabolism via effects on reaction rates and macromolecular structures, and due to the increased energy demand to mount an adequate response. Thus, stress responses over evolutionary time may pose a selection pressure on organismal metabolic phenotype, potentially altering the capacity of organisms to respond to additional stressors.2 Here, we examined this hypothesis in subpopulations of the estuarine fish, Atlantic killifish (Fundulus heteroclitus), inhabiting the Elizabeth River (ER), Virginia, U.S, that have evolved resistance to a highly toxic class of ubiquitous pollutants, polycyclic aromatic hydrocarbons (PAHs).3
Evolutionary adaptation to anthropogenic contaminants is a key element of biological pollution response. Pollution adaptation has occurred across taxa4–6 including several teleost species such as Atlantic killifish that show evolved resistance to complex hydrocarbon compounds and mixtures.3,7–10 Despite a number of studies examining the mechanisms of resistance in these fish, potential trade-offs, including the capacity to mount an adequate response to additional stressors, are poorly understood.7,11 In fact, consequences of contemporary evolutionary shifts in physiological processes across vertebrates are largely unknown, but remain an important consideration in determining “winners” and “losers” of global environmental change.12
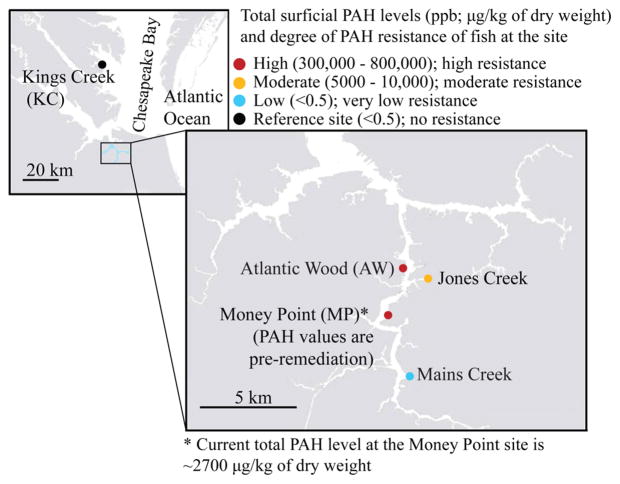
Killifish are remarkably eurytolerant, withstanding fluctuations in temperature, salinity, and oxygen in estuaries along the North American Atlantic Coast.13 Eurytolerance to physical stressors and evolved resistance to PAHs, enable the use of ER killifish as a unique model to investigate trade-offs of pollution adaptation. Several sites of the ER are differentially PAH contaminated and the degree of resistance to developmental toxicity of PAHs in fish inhabiting these sites directly corresponds to the level of contaminants (Figure 1),3,14 and are genetically different compared to fish from clean sites.8,15,16 Therefore, these differentially resistant ER subpopulations provide a “natural experiment” to investigate consequences of evolved resistance and inhabiting a polluted environment, and the impact of adaptive or ancillary shifts in physiological and biochemical processes on capacity of organisms to maintain optimum performance in a multistressor context.
Figure 1.
Collection sites in the Elizabeth River (Mains Creek, Jones Creek, Money Point (MP) and Atlantic Wood) and York River (Kings Creek) with total sediment PAH levels and the relative resistance to developmental toxicity of PAHs compared to Kings Creek. Data are adapted from Clark et al. (2013) Table 1 and Figure 2F.14 *MP data reflects preremediation PAH levels as reported in 2006.
To this end, a comparative physiological approach and an analysis of an existing population genomic data set was taken to examine bioenergetic consequences of PAH-resistance and inhabiting a chronically polluted environment in ER fish,14 relative to a clean reference subpopulation, Kings Creek (KC) (Figure 1). We focused on energy metabolic processes since they may be under significant selection pressure due to (i) increased ATP demand for PAH detoxification and to maintain cellular homeostasis; (ii) effects of PAHs on mitochondrial integrity17 as well as cardiac development and function18–20 potentially altering oxygen and nutrient circulation; and (iii) presumed role of the aryl hydrocarbon receptor, a key protein involved in PAH metabolism, as a mitochondrial regulator.21
First, we examined a restriction site-associated DNA (RAD) genomic data set comparing fish from a highly contaminated site (Atlantic Wood (AW) Superfund site; Figure 1) with Mains Creek (MC, very low PAH site in the ER) and KC to test if genes associated with energy metabolism are under selection. We then examined cardiac mitochondrial function utilizing an ex vivo oxygen consumption rate (OCR) assay in AW and KC fish. Considering the role of cardiac bioenergetics in defining thermal limits of teleosts,22–24 we evaluated cardiac mitochondrial function at 24 and 34 °C as an index for thermal plasticity. Subsequently, we extended our analysis to include four ER subpopulations (Figure 1) and characterized shifts in organismal aerobic metabolism at different temperatures and also measured thermal tolerance. To characterize potential ecological significance of altered bioenergetics, we measured swimming performance and the costs of finding an optimum environment based on a novel probabilistic movement model. Collectively, the current study provides a comprehensive analysis of bioenergetic consequences and potential ecological significances of rapid evolution to anthropogenic contaminants in inhabiting a chronically polluted environment in a vertebrate.
MATERIALS AND METHODS
Fish Collection and Care
Fish were collected from the reference (KC) and ER sites (AW-highly contaminated; Money Point (MP)-contaminated and under remediation; Jones Creek (JC)-moderately contaminated; MC-low levels of contaminants) (Figure 1, Supporting Information (SI)). MP remediation started in 2009 and fish were collected from a fully remediated section. Upon capture, fish were maintained in static glass tanks (30 × 30 × 75 cm, at 24–25 °C, 14:10 h light-dark cycle, and13–15 ppt artificial seawater (ASW; Instant Ocean, Foster & Smith, Rhinelander, WI) and were used within 2–3 months of acclimation. Fish were fed ad libitum a mix of Cyclop-eeze (Argent Chemical Laboratories, Inc., WA) and Zeigler’s Adult Zebrafish Complete Diet (Zeigler Bros., Inc., Gardners, PA). Water changes were conducted once a week. Fish were carefully chosen to represent a similar size range; weights and lengths are in SI Table S1. Only males were chosen, but gender identification was difficult at times in small juveniles. We specifically focused on wild-caught lab acclimated fish, as opposed to lab reared F1 individuals, to characterize the metabolic phenotype of each subpopulation that is most representative of their physiological function at each ER site. Detailed methods for each experiment are available in SI.
RAD Analysis
A set of genomic scaffolds that were previously characterized as exhibiting genetic differentiation across multiple comparisons of fish from polluted and unpolluted sites (Osterberg and colleagues, unpublished data) were queried for genes related to energy metabolism and maintaining mitochondrial function and integrity based on user defined categories determined by Gene Ontology information and previous literature (SI Table S2). Data were drawn from a double digest RAD sequencing25,26 analysis of 83 144 loci and 12 071 single nucleotide polymorphisms (SNPs) that compared genome-wide variation among AW, KC, and MC fish (n = 32 per population) (SI). RAD loci with smoothed pairwise FST values27 that were significantly greater than the average across the genome (P < 0.001) in both pairwise comparisons of polluted and unpolluted sites (AW-KC and AW-MC) were identified as loci potentially linked to genes under selection in PAH-adapted fish; surrounding candidate genes within 300 kb (the range was determined as described previously27 to include a broad list of potential candidate genes, including their associated cis-regulatory regions) were identified using the annotated F. heteroclitus genome (assembly 3.0.2; GCF_000826765.1).
Cardiac Mitochondrial Function
XFe Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA) was used for heart OCR measurements in KC and AW fish (collected in 2014) as previously described28 at 24 °C (n = 33) and 34 °C (n = 19–21). Basal OCR measurements were followed by an injection of 5 μM carbonyl cyanide-p-trifluoromethoxyphenyl-hydrazone (FCCP) (Sigma-Aldrich, St. Louis, MO) to obtain maximal respiration. Data are presented as OCR per 1 mg of heart by correcting for heart size according to Y ∞ Mb, where Y is metabolic rate, M is heart mass, and b is the heart specific scaling exponent for basal cardiac OCR; b = 0.6588 for F. heteroclitus.28 The same exponent was applied for normalizing FCCP-induced maximal rates, although it is possible this value is lower considering previous data on zebrafish Danio rerio.28 If the exponent is lower it would lead to even higher estimation of maximal rates for AW fish, thus the current approach is likely to be more conservative. Spare capacity (i.e., mitochondrial scope) is the difference between maximal and basal OCR.
Respirometry
KC, MC, JC, MP, and AW fish collected in 2013 and 2014 were used. Experimental protocol was determined based on previous killifish studies.29 Routine (RMO2) and maximum (MMO2) metabolic rate measurements were obtained at 24 °C (n = 13–20) and 34 °C (n = 14–16) using two swim tunnel (170 mL) respirometers (Loligo Systems, Tjele, Denmark). Each fish was tested at only one temperature. Background OCR was <0.001 μmol L−1 min−1. The respirometers were cleaned daily with bleach. Fish were starved for 24 h prior to respirometry and acclimated in the swim tunnel for 1 h prior to measurements. Previous studies in killifish and preliminary experiments conducted over 24 h of acclimation (SI Table S3) confirmed that 1 h was sufficient for RMO2 measurements and that these measurements can be used for aerobic scope calculations. Following acclimation four measurements were taken every 15 min by sealing the tunnel for 5 min and measuring the change in O2 concentration (mg L−1). The lowest OCR value of the four measurements was determined to be RMO2. To obtain MMO2, each fish was transferred to a 15 × 15 × 45 cm3 tank (at 24 or 34 °C) and was chased to exhaustion by hand (minimum 15 min chase) until the fish stopped responding to a stimulus (slight tapping with the finger)29 and then was immediately returned to the respirometer. OCR was determined for the first 3, 4, and 5 min intervals following introduction and the highest OCR reported during this time was considered MMO2. Aerobic Scope (AS) was calculated as MMO2 - RMO2. Q10 values were determined by dividing RMO2 at 34 °C by RMO2 at 24 °C. When normalized based on metabolic scaling RMO2 trends remained the same (data not shown).
Critical Swimming Speeds (Ucrit)
KC, MC, JC, MP, and AW fish were collected in 2014 and Ucrit (body lengths per second (BL s−1)) was measured as described previously.30 Briefly, fish from each site (n = 8) were introduced to the same swim tunnel system as above and acclimated (~20–30 min) to 0.5 BL s−1 water velocity at 24 °C. Fish that failed to orient themselves to the current were excluded. A pretrial was conducted with a velocity increase of 0.3 BL s−1 every 2 min up to 3 BL s−1. After a 1.5-h recovery, starting at a velocity of 1 BL s−1 , Ucrit was determined based on the formula , (Ui—the maximum speed fish swam for the full time period (cm s−1); Uii—incremental increase in speed (0.3 BL s−1); ti - time the fish swam at the final speed (min); tii –10 min) and were corrected for solid blocking effects.31 If the fish failed to swim, a 3 V electrical stimulus was turned on and off immediately and after three consecutive failures, the fish was considered fatigued.
Thermal Tolerance
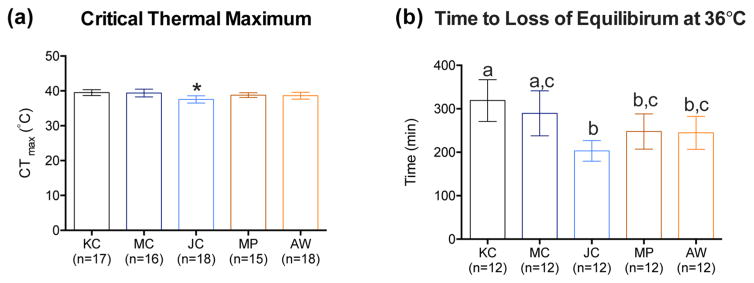
Critical thermal maximum (CTmax), a measure of acute thermal tolerance, was determined by introducing individual fish (n = 15–18 per subpopulation in KC, MC, JC, MP, and AW fish collected in 2014) into a recirculating three-chambered tank connected to a temperature controlled secondary tank. After 1 h of acclimation, temperature was increased at 4 °C h−1 until fish could not maintain dorso-ventral orientation (loss of equilibrium).22 To assess tolerance to an extended elevated temperature exposure, temperature was increased to 36 °C and the amount of time (min) to loss of equilibrium was recorded as tLOE (n = 12).
Cost of Finding an Optimum Environment
To assess the potential ecological significance of bioenergetic differences across populations, the energetic cost of finding an optimum environment (CFOE) was determined. CFOE is defined as cost of transport (COT) × distance swam (d), where d was predicted based on a probabilistic movement model developed according to Random Tour Motion (RTM)32 and .33 To determine COT, OCR was measured at 24 °C for each subpopulation when swimming at 4 cm s−1 (~1 BL s−1) as described above. OCRs at 25 °C –34 °C were computed based on the Q10 relationships (SI Table S4). Oxycaloric value of 0.451 J μmol−1 O2 was used.33 Since a fish is unlikely to swim in a straight line when reaching an optimum destination, the RTM model was utilized to estimate the distance when seeking for a new environment. RTM describes a stochastic process32 in which a fish would move in a random direction, for a random distance turning at a random angle iteratively until the optimum environment is found. Assuming that a fish is in a center of a circular region (radius R) and starts swimming at a speed V ms−1, searching for the optimum environment that is located just outside, we computed the probability (p) that the fish reaches outside the circular region after time t seconds, thus distance d (SI for the derivation). Essentially, d is the distance traveled when seeking an optimum environment, where the probability of finding this environment is 0 < p < 1. Using d at 0 < p < 1, we computed CFOE for 24 °C –34 °C, when R = 1 m. The model predicts that the CFOE exponentially increases when p > 0.9 (SI Figure S1). We then computed the CFOE when seeking an optimum environment when p = 0.9 (p fixed) and R = 1–8 m for each subpopulation. The capacity to sense a threat, the decision to alter the existing position, and the capacity to sense an optimum location were assumed to be equal across subpopulations.
Statistical Analyses
Organismal RMO2, MMO2, AS, Ucrit, CTmax, and tLOE values for each subpopulation were tested for significance using One-Way ANOVA followed by Tukey’s test (significant if P < 0.05) using Graph Pad Prism 6.0 (San Diego, CA). RMO2 and MMO2 measurements were treated independently and were not compared against each other. Cardiac Two-tailed unpaired t test was used for cardiac OCR data. All the data met the assumption of normality (one data point from MC metabolic rate measurements was removed from the analysis to meet this criteria). Homogeneity of variance was tested using Levene’s median test and no significant variance was found.
RESULTS
Genomic Analysis Comparing Fish from Highly Polluted and Unpolluted Sites
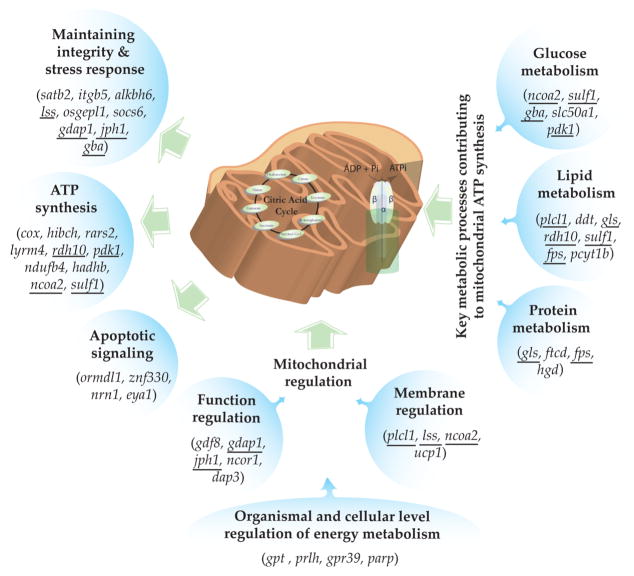
This analysis identified 39 candidate genes (from a total of 193 genes, ~20%) that are associated with energy metabolic processes and maintaining mitochondrial function and integrity (Figure 2; SI Table S2). In particular 10 genes that are involved in mitochondrial ATP synthesis, including cytochrome oxidase and carbon donor steps (glucose, lipid, and protein metabolism) for ATP generation, are found in regions of the genome that are predicted to be under selection in AW. Several of the candidate genes are involved in mitochondrial signaling and stress response including apoptosis, suggesting that mitochondrial integrity is targeted by PAHs. Genes that are involved in mitochondrial function regulation, and organismal and cellular control of energy metabolism (e.g., poly(ADP-Ribose) polymerase family (parp)) were also identified as candidates of selection. parp may be particularly important, considering its role under increased DNA damage conditions (a known toxic effect of PAHs) where it can lead to severe impairment of energy metabolism with almost complete depletion of NAD and ATP pools resulting in mitochondrial dysfunction.34 Collectively, genomic data indicate that metabolic phenotype is likely under a significant selective pressure in fish inhabiting a highly PAH-contaminated site.
Figure 2.
A conceptual figure depicting nine author-defined categories associated with energy metabolism and mitochondrial processes and the genes involved in each of these categories that are candidates for selection as discovered by the restriction site associated DNA sequencing analysis comparing polluted and unpolluted sites. Underlined genes are pleiotropic and are represented in more than one category (Gene functions are in SI Table S2).
Cardiac Energy Metabolism
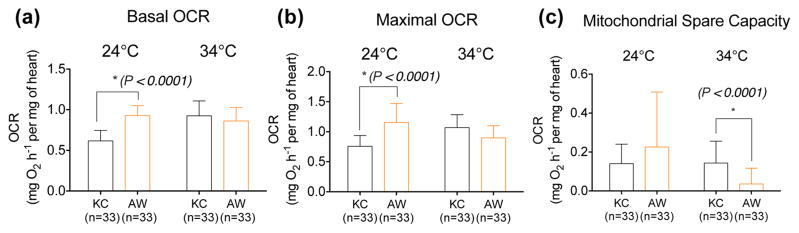
Cardiac bioenergetic assay showed altered mitochondrial function in AW fish compared to KC. Basal OCR and FCCP induced maximal OCR for whole hearts showed a 50% increase in AW fish compared to KC fish at 24 °C (Figure 3a and b). However, this difference was not maintained at 34 °C, where maximal OCR was in fact slightly decreased in AW (0.897 ± 0.201 mg O2 h−1 mg−1) compared to KC fish (1.069 ± 0.216 mg O2 h−1 mg−1). Mitochondrial spare capacity was not different between AW and KC fish at 24 °C (Figure 3c), but was significantly reduced in AW (0.036 ± 0.081 mg O2 h−1 mg−1) compared to KC (0.143 ± 0.113 mg O2 h−1 mg−1) at 34 °C.
Figure 3.
(a) Basal oxygen consumption rates (OCR), (b) maximal OCR and (c) mitochondrial spare capacity per 1 mg of heart in Kings Creek (KC) and Atlantic Wood (AW) fish at 24 and 34 °C. Data are presented as mean ± SD, * denotes statistically significant differences based on two-tailed unpaired t test.
RMO2 and MMO2 at 24 and 34 °C
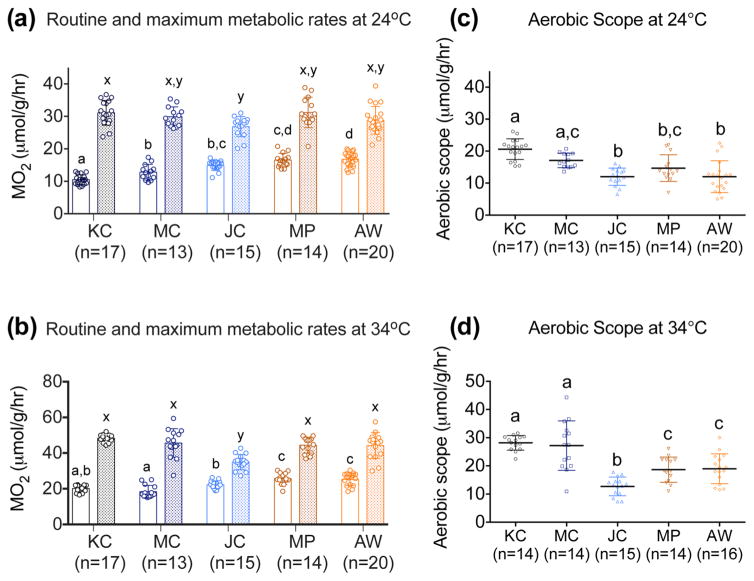
Altered metabolic phenotype detected in ex vivo analysis was reflected in whole organismal studies in AW fish. Further, RMO2 and MMO2 data revealed site-specific metabolic phenotypes across ER fish (Figure 4). A key observation is the increased RMO2 at 24 °C in all ER fish (Figure 4a), where RMO2 was highest in the most PAH-resistant MP (16.52 ± 2.11 μmol g−1 hr−1) and AW fish (16.78 ± 2.22 μmol g−1 hr−1) compared to KC specimens (10.49 ± 1.55). RMO2 was 40% higher in moderately PAH-resistant JC fish (14.88 ± 1.54 μmol g−1 hr−1) and 20% higher in MC fish from the least contaminated site (12.7 ± 2.34 μmol g−1 hr−1) compared to KC. Differences in RMO2 detected at 24 °C were not as pronounced at 34 °C (Figure 4b); however, MP (25.76 ± 3.24 μmol g−1 hr−1) and AW (25.28 ± 3.54 μmol g−1 hr−1) still had higher RMO2 compared to KC (20.15 ± 1.99 μmol g−1 hr−1). Q10, values, calculated based on RMO2 at 24 and 34 °C, were highest for KC compared to ER fish (SI Table S4). MMO2 measurements following exhaustive exercise were similar across all groups except for JC (Figure 4a and 4b). MMO2 in JC fish was lower at 24 °C (26.89 ± 3.09 μmol g−1 hr−1) compared to KC (31.11 ± 3.81 μmol g−1 hr−1) and at 34 °C was 30% lower compared to all groups. Aerobic scope was decreased in JC, MP, and AW compared to KC at 24 and 34 °C (Figure 4c and d). The decrease in AS was most striking in JC fish at 34 °C. AS for MC fish is similar to KC, but demonstrated a high degree of individual variation (standard deviation > ~ 30%) at 34 °C.
Figure 4.
Routine metabolic rates (RMO2; μmol g−1 hr−1) (clear bars), maximum metabolic rates (MMO2; μmol g−1 hr−1) (filled bars), and aerobic scope (MMO2 – RMO2; μmol g−1 hr−1) for Kings Creek (KC), Mains Creek (MC), Jones Creek (JC), Money Point (MP) and Atlantic Wood (AW) fish at 24 °C (a and c) and 34 °C (b and d). Data are presented as mean ± SD. Different letters above the bars (a, b, c, and d for RMO2 and aerobic scope; x and y for MMO2) denote statistically significant differences across populations for a given measurement (One-way ANOVA followed by Tukey’s test; P < 0.05).
Thermal Tolerance
Acute thermal tolerance (CTmax), ranged between 37.5 and 39.5 °C for all fish (Figure 5a). JC significantly differed from other populations (the lowest CTmax of 37.57 ± 1.04 °C). The differences in upper thermal tolerance across populations were more pronounced when considering the capacity to maintain equilibrium (tLOE) at 36 °C (Figure 5b). In comparison to KC (319 ± 48.18 min), tLOE was significantly reduced in PAH-resistant MP (247.67 ± 40.57 min) and AW (244.67 ± 37.75 min) fish. Notably JC fish have the lowest tLOE (203.25 ± 23.75 min), though not significantly different from MP and AW.
Figure 5.
(a) Critical thermal maximum (CTmax) and (b) time to loss of equilibrium (tLOE) for fish from Kings Creek (KC), Mains Creek (MC), Jones Creek (JC), Money Point (MP), and Atlantic Wood (AW). Data are presented as mean ± SD. Different letters above the bars (a, b, and c) denote statistically significant differences across populations and * denotes significant difference compared to all the populations (One-way ANOVA followed by Tukey’s test; P < 0.05).
Swimming Performance at 24 °C
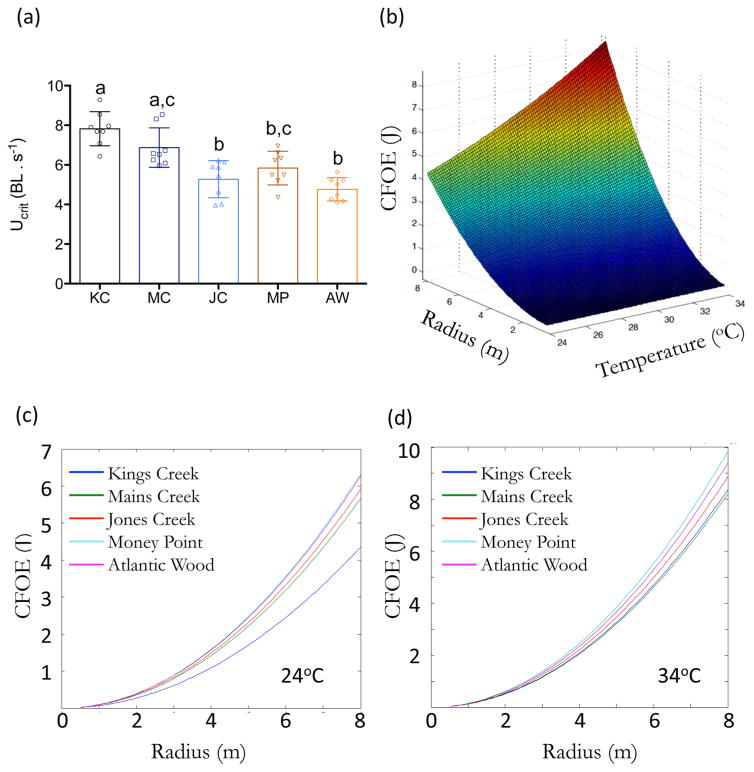
Critical swimming speeds (Ucrit), a standard measurement of overall swimming capabilities measured as body lengths per second (BL s−1), were assessed in all populations. Ucrit values were 50% lower in JC (5.27 ± 0.94 BL s−1), MP (5.84 ± 0.85 BL s−1), and AW (4.77 ± 0.59 BL s−1) compared to KC (7.8 ± 0.86 BL s−1) (Figure 6a). Ucrit of MC (6.87 ± 1.00 BL s−1) was moderately decreased.
Figure 6.
(a) Critical swimming speeds (Ucrit; BL s−1) for Kings Creek (KC), Mains Creek (MC), Jones Creek (JC), Money Point (MP), and Atlantic Wood (AW) fish at 24 °C. Data are presented as mean ± SD. Different letters (a, b, and c) denote statistically significant differences across populations (One-way ANOVA followed by Tukey’s test; P < 0.05). (b) CFOE for Kings Creek fish at different temperatures at different radii (1–8 m) when p = 0.9. (c) CFOE at 24 °C, and (d) at 34 °C at different radii (1–8 m) of the circular region when p = 0.9.
Cost of Finding an Optimum Environment
The RTM model predicted that a fish in a circular environment has ~20–90% (0.2 < p < 0.9, p-probability) likelihood of finding an optimum environment without significantly increasing the total swimming distance (SI Figure S1). However, to achieve over 90% likelihood of finding an optimum environment, distance, thus CFOE, would have to increase exponentially. Thus, subtle differences in COT among subpopulations are magnified when p > 0.9. CFOE also increased with expanding radius of the circular region (at p = 0.9) (Figure 6b). Additionally, due to temperature effects on metabolic rates (Q10 effects), CFOE is elevated at warmer temperatures (Figure 6b; SI Figure S2). Accordingly, CFOE increases exponentially with increasing radius in a temperature specific manner (Figure 6c and 6d). At 24 °C, CFOE for ER fish is similar to KC within a smaller radius (Figure 6c). However, with increasing radius, CFOE significantly differs between populations. For example, when the radius is 8m, CFOE is ~50% higher for MP and AW fish and ~30% higher for JC and MC compared to KC. The difference in CFOE between ER fish and KC is narrower at 34 °C (Figure 6d), however, is 25% higher for MP and AW fish compared to KC. Essentially, this analysis demonstrates that a minor increase in routine metabolic rates can potentially result in significant increases in energy expenditure when searching for an optimum location, but this increase may depend on the capacity for fish to alter their swimming efficiency.
DISCUSSION
Understanding trade-offs of rapid evolution to abiotic stressors, especially in the context of bioenergetic homeostasis is critical in predicting species’ response to anthropogenic change.35 Here, we demonstrate that in fish adapted to inhabit a chronically polluted aquatic ecosystem, energy metabolic processes are under selection and altered at the organismal and cellular level. Furthermore, despite the likely advantages of bioenergetic shifts, we demonstrate that they are linked to reduced swimming performance, altered thermal plasticity and increased cost of finding an optimum environment, potentiating significant ecological consequences, particularly in multistressor habitats.
Altered Bioenergetics: A Consequence of PAHA-daptation or Chronic Exposure?
Current genomic data coupled with increased basal and maximal OCR in AW cardiac mitochondria compared to KC suggest that mitochondrial function is likely under selection in PAH-adapted fish. Previous cellular36–38 and transcriptomic39–41 studies in AW fish also confirm an altered bioenergetic phenotype associated with PAH-adaptation. While this AW mitochondrial phenotype is potentially advantageous with chronic PAH exposure, measurements at 34 °C indicate that it leads to reduced mitochondrial scope under additional stressors. This altered mitochondrial function is also potentially linked to reduced aerobic scope detected in these fish. Previous studies in fish confirm that cardiac metabolism is directly linked to cardiac scope and output,42 which are associated with metabolic rates. Nonetheless, further studies on cardiac mitochondrial density, copy number, structure, and efficiency as well as cardiac performance are necessary in ER killifish to explore this link.
Overall, a number of factors can contribute to the observed cardiac and organismal bioenergetic shifts. For example, increased resting metabolic rates detected in ER fish might be a compensatory response to reduced oxygen circulation resulting from impaired cardiac structure and function detected in acutely PAH exposed fish.20,43,44 This is unlikely, however, since morphological differences between AW and KC hearts were not detected.45 Other factors including (i) increased ATP synthesis to sustain stress responses including xenobiotic metabolism;46,47 (ii) compensatory response to an adaptive shift in another physiological process; and (iii) a compensatory response for mitochondrial toxicity of PAHs17,36 could alter organismal bioenergetics. Theory would suggest that metabolic depression is a more advantageous strategy to compensate for higher metabolic rates, unless ATP requirement is increased in these fish. Overall, these observed shifts can be adaptive, developmental, or acclimatory. Thus, genetic changes as well as epigenetic, acclimatory, or toxic responses to chronic PAH exposure may play a role in altered bioenergetics detected for ER fish. Nonetheless, current and previous data suggest a genetic component, at least in part, underpinning altered metabolic phenotype in ER fish.
Studies showed that metabolic shifts in ER fish at the cellular37,38 and whole organismal level45 are heritable and persistent when they are reared under clean conditions, indicating a strong genetic component. This supposition is further supported by the current genomic data and the physiological data from highly PAH-resistant fish at AW and MP. Notably, similar to AW, MP maintained the highest RMO2, especially at 34 °C. However, MP is under remediation and MP fish, despite being highly PAH-resistant, are exposed to low levels of PAHs. Additionally, previous studies in fish exposed to PAHs generally suggest that PAH exposure does not affect resting or standard metabolic rates. Developmental exposure of mahi–mahi (Coryphaena hippurus) to a crude oil mixture43 and acute exposure of common sole (Solea solea) to fuel48 did not affect standard metabolic rates. Additionally, exposure to the PAH beta-naphthoflavone also had no effect on standard metabolic rate in zebrafish (Danio rerio), although benzo-a-pyrene did affect metabolic rates.49,50 A genetic basis for altered RMO2 was further supported by a parallel study in our laboratory demonstrating elevated RMO2 in F1 AW juveniles reared under lab conditions when compared to KC.45 These results suggest that elevated RMO2 has genetic underpinnings and is unlikely to be due to acute or chronic PAH exposure.
Reduced Aerobic Scope in ER Fish
Elevated RMO2 leads to reduced AS, unless MMO2 is also accordingly increased. Data showed MMO2 was not increased in ER fish compared to KC, resulting in reduced AS in PAH-resistant fish (JC, MP and AW). Surprisingly, JC, a moderately PAH-resistant subpopulation from a moderately contaminated site, maintained the lowest AS. This was a result of increased RMO2 and decreased MMO2. Although PAH exposure can lead to reduced maximum rates in fish,48 MMO2 was similar to KC in highly PAH-resistant AW and MP. It is possible that in contrast to JC, AW, and MP subpopulations were able compensate for PAH-effects on maximum rates. We speculate that the high PAH levels may have accelerated the AW and MP fish evolutionary response, selecting for individuals with higher MMO2, thus greater AS.
In contrast, the rate of evolutionary change may be slower in JC fish with moderate contamination and may not have compensated for PAH effects on MMO2. Alternatively, as previously hypothesized, it is possible that AW emigrants drive the moderate PAH-resistant phenotype at JC.14 Thus, despite not having the complete genetic tool kit to completely compensate for PAH effects on bioenergetics, fish at JC can survive developmental toxicity of PAHs to inhabit this locale. The high degree of intrapopulation variation in RMO2 and AS data for least PAH-resistant MC fish may suggest that this is may also be a mixed subpopulation with individuals from more contaminated regions migrating south, a possibility discussed in detail previously.14 A nongenetic explanation is also plausible, since altered life history strategies at these locations can also lead to reduced AS. Irrespective of the mechanisms, reduced AS in ER fish suggests that aerobic performance and other biological processes (e.g., reproduction and stress responses) might be compromised.
Ecological Implications of Reduced Aerobic Scope
Findings clearly demonstrate that ER fish have reduced capacity to maintain adequate metabolic function at warmer temperatures and have reduced thermal tolerance. This might be a direct result of cardiac and organismal bioenergetic shifts. It is not uncommon for fish inhabiting an estuarine tidal pools to experience brief episodes of temperatures above 35 °C in the summer.13 Our thermal tolerance data indicate that while most ER fish are able to survive a temperature increase up to ~40 °C, they may not be able to sustain optimum performance at warmer temperatures compared to KC fish. As described by the theory “oxygen and capacity limited thermal tolerance (OCLTT)”, reduced AS at warmer temperature may underpin upper thermal limits of teleosts.51 Thus, the capacity to maintain an elevated AS at warmer temperatures may contribute to improved thermal tolerance. All ER and KC AS values were higher at 34 °C compared to 24 °C. An increase in AS was detected with an acute increase in temperature was also previously reported for F. heteroclitus, postulating that elevated AS at warmer temperatures may contribute to the high-degree of thermal plasticity detected in these fish.29 Accordingly reduced AS in ER fish compared to KC at 34 °C may affect their capacity to survive thermally fluctuating habitats. Furthermore, cardiac energy metabolism is thought to be causally linked to cardiac output and organismal metabolism, 28,42 and cardiac mitochondrial dysfunction at warmer temperatures is associated with teleost thermal limits.24 Accordingly, reduced cardiac mitochondrial spare capacity and organismal aerobic capacity observed at 34 °C in AW fish may significantly contribute to the decrease in upper thermal tolerance of AW and other ER fish.
Elevated metabolic rates and altered thermal response also have ecological implications as they affect swimming performance and cost of finding an optimum location. Indeed, Ucrit was lower for ER fish. While further studies are necessary to evaluate underlying mechanisms,30,43,44,52 reduced AS detected in ER fish may contribute to their decreased Ucrit. Reduced Ucrit and elevated CFOE at ~1 BL s−1 as predicted by RTM, is likely to affect the ability of ER fish to seek an optimum environment. The increased CFOE at 34 °C for ER and KC fish suggests that at warmer temperatures all fish have limited capacity to find an optimum environment—an important consideration for teleosts in general in the context of climate change. Notably, even though CFOE at 34 °C of ER fish is similar to KC, ER fish may be susceptible to other stressors at warmer temperatures due to lower AS detected. Notably, given the neurophysiological effects detected in pollution-adapted killifish,53,54 it is likely that cognitive capacity find an optimum environment is impaired in ER fish. Thus, the difference in CFOE between ER fish and KC fish are likely to be higher. However, CFOE in an ecological setting would be lower than values predicted here, since a complete random motion is unlikely. Importantly the model presented here provides a framework to incorporate these parameters for future studies.
In conclusion, findings revealed that shifts in fundamental physiological processes in PAH-adapted fish might compromise their capacity to respond to a thermal stress (i.e., thermal plasticity) and find an optimum environment. The observation that fish inhabiting a moderately contaminated site might be more susceptible to a secondary stressor compared to fish from highly contaminated sites provides intriguing future directions. Importantly, our findings demonstrate that while species may rapidly evolve in response to abiotic stressors, populations may become more vulnerable to secondary stressors during this process. Further understanding of effects of competing selection pressures on fitness of affected and neighboring populations will provide important insights into predicting impact of global environmental change.
Supplementary Material
Acknowledgments
We thank Dr. Andrey Massarsky, Dr. Bryan Clark, Ms Jordan Kozal, and Ms Tess Leuthner at Duke University, and Mr. T. A. Randrianasolo at University of Leoben for their help during project implementation. The Di Giulio lab is supported by National Institutes of Environmental Health award P42- ES010356 (Duke University Superfund Research Center). PWF is funded by Austrian Science Fund (FWF): P23591. Duke University Institutional Animal Care and Use Committee (A184-13-07) approved the protocols.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b01913.
List of acronyms; supplementary methods; Table S1: Average weights or lengths for fish utilized for metabolic rate, critical swimming performance and cost of transport analyses; Table S2: List of genes predicted by the restriction site associated DNA analysis; Table S3. Preliminary metabolic rate experimental data to determine the optimal acclimation time for routine metabolic rates; Table S4. Experimental data utilized in calculating cost of finding an optimum environment for Kings Creek, Mains Creek, Jones Creek, Money Point, and Atlantic Wood fish; Figure S1. Cost of finding an optimum environment (CFOE) for Fundulus heteroclitus from Kings Creek (KC), Mains Creek (MC), Jones Creek (JC), Money Point (MP) and Atlantic Wood (AW) at different temperatures when considering a circular region with a radius of 1m at 0 < p < 1 (p is the probability of finding the optimum location); Figure S2. Cost of finding an optimum environment (CFOE) for Fundulus heteroclitus from Kings Creek (KC), Mains Creek (MC), Jones Creek (JC), Money Point (MP) and Atlantic Wood (AW) at different temperatures at different radii (1–8m) of the circular region when p = 0.9 (PDF)
References
- 1.Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293(5536):1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro R, Lopes I. Contaminant driven genetic erosion and associated hypotheses on alleles loss, reduced population growth rate and increased susceptibility to future stressors: an essay. Ecotoxicology. 2013;22(5):889–899. doi: 10.1007/s10646-013-1070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Giulio RT, Clark BW. The Elizabeth River story: a case study in evolutionary toxicology. J Toxicol Environ Health, Part B. 2015;18(6):259–298. doi: 10.1080/15320383.2015.1074841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amiard-Triquet C, Rainbow PS, Roméo M. Tolerance to Environmental Contaminants. CRC Press; Boca Raton, FL: 2011. [Google Scholar]
- 5.Bickham JW. The four cornerstones of evolutionary toxicology. Ecotoxicology. 2011;20(3):497–502. doi: 10.1007/s10646-011-0636-y. [DOI] [PubMed] [Google Scholar]
- 6.Schlebusch CM, Gattepaille LM, Engström K, Vahter M, Jakobsson M, Broberg K. Human adaptation to arsenic-rich environments. Mol Biol Evol. 2015;32:msv046. doi: 10.1093/molbev/msv046. [DOI] [PubMed] [Google Scholar]
- 7.Weis JS. Tolerance to environmental contaminants in the mummichog. Hum Ecol Risk Assess. 2002;8(5):933–953. [Google Scholar]
- 8.Reid NM, Proestou DA, Clark BW, Warren WC, Colbourne JK, Shaw JR, Karchner SI, Hahn ME, Nacci D, Oleksiak MF, Crawford DL, Whitehead A. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science. 2016;354(6317):1305–1308. doi: 10.1126/science.aah4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason T, Munns W, Jr, Specker J, Cooper K. Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar Biol. 1999;134(1):9–17. [Google Scholar]
- 10.Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ Toxicol Chem. 2002;21(9):1897–1902. [PubMed] [Google Scholar]
- 11.Meyer JN, Di Giulio RT. Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol Appl. 2003;13(2):490–503. [Google Scholar]
- 12.Somero G. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol. 2010;213(6):912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- 13.Schulte PM. Responses to environmental stressors in an estuarine fish: Interacting stressors and the impacts of local adaptation. J Therm Biol. 2007;32(3):152–161. [Google Scholar]
- 14.Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound-and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River Estuary (Virginia, USA) Environ Sci Technol. 2013;47(18):10556–10566. doi: 10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulvey M, Newman MC, Vogelbein W, Unger MA. Genetic structure of Fundulus heteroclitus from PAH-contaminated and neighboring sites in the Elizabeth and York Rivers. Aquat Toxicol. 2002;61(3):195–209. doi: 10.1016/s0166-445x(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 16.Mulvey M, Newman MC, Vogelbein WK, Unger MA, Ownby DR. Genetic structure and mtDNA diversity of Fundulus heteroclitus populations from polycyclic aromatic hydrocarbon contaminated sites. Environ Toxicol Chem. 2003;22(3):671–677. [PubMed] [Google Scholar]
- 17.Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134(1):1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Non-additive effects of PAHs on early vertebrate development: mechanisms and implications for risk assessment. Toxicol Sci. 2008;105(1):5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayasundara N, Garner LVT, Meyer JN, Erwin KN, Di Giulio RT. AHR2-mediated transcriptomic responses underlying the synergistic cardiac developmental toxicity of PAHs. Toxicol Sci. 2015;143(2):469–481. doi: 10.1093/toxsci/kfu245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Incardona JP, Carls MG, Day HL, Sloan CA, Bolton JL, Collier TK, Scholz NL. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol. 2008;43(1):201–207. [Google Scholar]
- 21.Tappenden DM, Lynn SG, Crawford RB, Lee K, Vengellur A, Kaminski NE, Thomas RS, LaPres JJ. The aryl hydrocarbon receptor interacts with ATP5α1, a subunit of the ATP synthase complex, and modulates mitochondrial function. Toxicol Appl Pharmacol. 2011;254(3):299–310. doi: 10.1016/j.taap.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayasundara N, Somero GN. Physiological plasticity of cardiorespiratory function in a eurythermal marine teleost, the longjaw mudsucker. Gillichthys mirabilis J Exp Biol. 2013;216(11):2111–2121. doi: 10.1242/jeb.083873. [DOI] [PubMed] [Google Scholar]
- 23.Farrell AP, Eliason E, Sandblom E, Clark T. Fish cardiorespiratory physiology in an era of climate change. Can J Zool. 2009;87(10):835–851. [Google Scholar]
- 24.Iftikar FI, Hickey AJ. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in. Notolabrus celidotus PLoS One. 2013;8(5):e64120. doi: 10.1371/journal.pone.0064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One. 2008;3(10):e3376. doi: 10.1371/journal.pone.0003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS One. 2012;7(5):e37135. doi: 10.1371/journal.pone.0037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 2010;6(2):e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayasundara N, Kozal JS, Arnold MC, Chan SS, Di Giulio RT. High-throughput tissue bioenergetics analysis reveals identical metabolic allometric scaling for teleost hearts and whole organisms. PLoS One. 2015;10(9):e0137710. doi: 10.1371/journal.pone.0137710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Healy TM, Schulte PM. Thermal acclimation is not necessary to maintain a wide thermal breadth of aerobic scope in the common killifish (Fundulus heteroclitus) Physiol Biochem Zool. 2012;85(2):107–119. doi: 10.1086/664584. [DOI] [PubMed] [Google Scholar]
- 30.Fangue NA, Mandic M, Richards JG, Schulte PM. Swimming performance and energetics as a function of temperature in killifish. Fundulus heteroclitus Physiol Biochem Zool. 2008;81(4):389–401. doi: 10.1086/589109. [DOI] [PubMed] [Google Scholar]
- 31.Bell W, Terhune L. Water Tunnel Design for Fisheries Research. Queen’S Printer; Ottawa: 1970. [Google Scholar]
- 32.Washburn A. Probability density of a moving particle. Oper Res. 1969;17(5):861–871. [Google Scholar]
- 33.Hein AM, Keirsted KJ. The rising cost of warming waters: effects of temperature on the cost of swimming in fishes. Biol Lett. 2012;8(2):266–269. doi: 10.1098/rsbl.2011.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Formentini L, Macchiarulo A, Cipriani G, Camaioni E, Rapizzi E, Pellicciari R, Moroni F, Chiarugi A. Poly (ADP-ribose) catabolism triggers AMP-dependent mitochondrial energy failure. J Biol Chem. 2009;284(26):17668–17676. doi: 10.1074/jbc.M109.002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res. 2012;79:1–15. doi: 10.1016/j.marenvres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Jung D, Cho Y, Collins LB, Swenberg JA, Di Giulio RT. Effects of benzo[a]pyrene on mitochondrial and nuclear DNA damage in Atlantic killifish (Fundulus heteroclitus) from a creosote-contaminated and reference site. Aquat Toxicol. 2009;95(1):44–51. doi: 10.1016/j.aquatox.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du X, Crawford DL, Nacci DE, Oleksiak MF. Heritable oxidative phosphorylation differences in a pollutant resistant Fundulus heteroclitus population. Aquat Toxicol. 2016;177:44–50. doi: 10.1016/j.aquatox.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Du X, Crawford DL, Oleksiak MF. Effects of anthropogenic pollution on the oxidative phosphorylation pathway of hepatocytes from natural populations of Fundulus heteroclitus. Aquat Toxicol. 2015;165:231–240. doi: 10.1016/j.aquatox.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Bozinovic G, Sit TL, Di Giulio R, Wills LF, Oleksiak MF. Genomic and physiological responses to strong selective pressure during late organogenesis: few gene expression changes found despite striking morphological differences. BMC Genomics. 2013;14(1):779. doi: 10.1186/1471-2164-14-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher MA, Oleksiak MF. Convergence and divergence in gene expression among natural populations exposed to pollution. BMC Genomics. 2007;8(1):108. doi: 10.1186/1471-2164-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oleksiak MF. Changes in gene expression due to chronic exposure to environmental pollutants. Aquat Toxicol. 2008;90(3):161–171. doi: 10.1016/j.aquatox.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Driedzic WR. Cardiac energy metabolism. Fish Physiology. 1992;12(part A):219–267. [Google Scholar]
- 43.Mager EM, Esbaugh AJ, Stieglitz JD, Hoenig R, Bodinier C, Incardona JP, Scholz NL, Benetti DD, Grosell M. Acute embryonic or juvenile exposure to Deepwater Horizon crude oil impairs the swimming performance of mahi-mahi (Coryphaena hippurus) Environ Sci Technol. 2014;48(12):7053–7061. doi: 10.1021/es501628k. [DOI] [PubMed] [Google Scholar]
- 44.Hicken CE, Linbo TL, Baldwin DH, Willis ML, Myers MS, Holland L, Larsen M, Stekoll MS, Rice SD, Collier TK. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc Natl Acad Sci U S A. 2011;108(17):7086–7090. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown DR, Thompson Jasmine A, Chernick Melissa A, Hinton David E, Di Giulio Richard T. Later life swimming performance and persistent heart damage following subteratogenic PAH mixture exposure in the Atlantic killifish (Fundulus heteroclitus) Environ Toxicol Chem. 2016 doi: 10.1002/etc.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aluru N, Vijayan MM. β-Naphthoflavone disrupts cortisol production and liver glucocorticoid responsiveness in rainbow trout. Aquat Toxicol. 2004;67(3):273–285. doi: 10.1016/j.aquatox.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Tintos A, Gesto M, Míguez JM, Soengas JL. β-Naphthoflavone and benzo(a)pyrene treatment affect liver intermediary metabolism and plasma cortisol levels in rainbow trout. Oncorhynchus mykiss Ecotoxicol Environ Saf. 2008;69(2):180–186. doi: 10.1016/j.ecoenv.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Davoodi F, Claireaux G. Effects of exposure to petroleum hydrocarbons upon the metabolism of the common sole. Solea solea Mar Pollut Bull. 2007;54(7):928–934. doi: 10.1016/j.marpolbul.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Gerger CJ, Thomas JK, Janz DM, Weber LP. Acute effects of β-naphthoflavone on cardiorespiratory function and metabolism in adult zebrafish (Danio rerio) Fish Physiol Biochem. 2015;41(1):289–298. doi: 10.1007/s10695-014-9982-z. [DOI] [PubMed] [Google Scholar]
- 50.Gerger CJ, Weber LP. Comparison of the acute effects of benzo-a-pyrene on adult zebrafish (Danio rerio) cardiorespiratory function following intraperitoneal injection versus aqueous exposure. Aquat Toxicol. 2015;165:19–30. doi: 10.1016/j.aquatox.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Pörtner HO, Farrell AP. Physiology and climate change. Science. 2008;322(5902):690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- 52.Plaut I. Critical swimming speed: its ecological relevance. Comp Biochem Physiol, Part A: Mol Integr Physiol. 2001;131(1):41–50. doi: 10.1016/s1095-6433(01)00462-7. [DOI] [PubMed] [Google Scholar]
- 53.Weis JS, Smith G, Zhou T, Santiago-Bass C, Weis P. Effects of Contaminants on Behavior: Biochemical Mechanisms and Ecological Consequences Killifish from a contaminated site are slow to capture prey and escape predators; altered neurotransmitters and thyroid may be responsible for this behavior, which may produce population changes in the fish and their major prey, the grass shrimp. BioScience. 2001;51(3):209–217. [Google Scholar]
- 54.Brown D, Bailey J, Oliveri A, Levin E, Di Giulio R. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol Teratol. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.