Abstract
Background
Obesity induced brain inflammation is associated with cognitive disorders. We aimed to investigate the influence of vitamin D on hypothalamus and hippocampus inflammatory response in high-fat diet induced obese rats.
Methods
In the beginning of the study, 40 rats were divided into two groups: control diet and high fat diet (HFD) for 16 weeks; then each group subdivided into two groups including: N, ND + vitamin D, HFD and HFD + vitamin D. Vitamin D supplementation was done for 5 weeks at 500 IU/kg dosage. IL-6, IL-1β, NF-Kβ and acetylcholine (ACH) and brain derived neurotropic factor (BDNF) concentrations in hippocampus and hypothalamus homogenate samples were measured by commercial ELISA kits.
Results
Vitamin D administration, reduced food intake and weight gain in studied groups (P < 0.001). Vitamin D reduced hippocampus acetylcholine concentrations in ND + vitamin D group (P < 0.001). High fat diet increased hippocampus IL-6 concentrations significantly (P < 0.05) compared with normal diet receiving groups. Vitamin D could not have significant effects on IL-6 concentrations. Vitamin D administrations reduced IL-1β, NF-Kβ and acetylcholine concentration and BDNF concentrations in ND + vitamin D compared with ND group. These reductions were not significant in HFD + vitamin D versus HFD group.
Conclusion
According to our results, vitamin D reduced food intake and weight gain and modulated the HFD induced inflammatory response in hippocampus and hypothalamus of high fat diet induced obesity. Therefore, this neurosteroid, can be suggested as a supplemental therapeutic tool in prevention of obesity related cognitive and neurodegenerative problems.
Keywords: NF-Kβ, IL-6, IL-1β, Acetylcholine, Vitamin D, Obesity, HFD
Background
Obesity is a major health problem worldwide and is associated with numerous chronic disease including cardiovascular disease, diabetes, metabolic syndrome and even some types of cancers [1]. The prevalence of obesity is growing rapidly and according to the WHO estimation, in 2014, more than 1.9 billion adults, 18 years and older, were overweight. Of these over 600 million were obese [2]. High-fat diet has been shown to influence eating behavior and is make susceptibility in both human and animal models in developing obesity [3]. Moreover, because of the great similarity between the genomes of rodents and humans, this animal model is a very useful tool for studying obesity [4].
Obesity has been regarded as a chronic low-grade inflammatory condition and this increased peripheral inflammatory tone, will also increase the occurrence of central nervous system (CNS) inflammation [5]. The increased inflammation in CNS, involves greatly in the pathogenesis of CNS-related disease including dementia, Alzheimer disease (AD) and stroke [6]. More important regions of CNS extensively studied to be susceptible in HFD-induced damages are hypothalamus and hippocampus [7–9]. Hypothalamus is especially attracted much attention because of its pivotal role in food intake regulation and availability of nutrients [10]. Hypothalamus inflammatory response after dietary fat-induced obesity is an important contributor in developing insulin and leptin resistance and defective food intake [11, 12]. High fat diet feeding is associated with a disruption of the homeostasis in the hypothalamus and increased inflammatory response due to glial cell activation [13]. The inflammatory response to dietary fat and especially dietary saturated fatty acids in the hypothalamus is mediated by toll-like receptors (TLRs) which their activation and signaling by dietary fat leads to activation of nuclear factor kappa-β (NF-Kβ) and production of inflammatory cytokines including interleukin (IL)-1β and IL-6 [14]. Accordingly, another important brain region, hippocampus, is also susceptible to inflammation in obesity and numerous studies have revealed that neural systems of hippocampus involved in memory and cognition are also negatively affected in obesity [15]. Although numerous mechanisms such as change in gut peptides and reduced neurotrophic factors are suggested, increased pro-inflammatory cytokines like IL-6 might also be important contributor in hippocampal injury in obesity [16]. Moreover, clinical evidence suggests that inflammation in the brain and hippocampus of HFD-fed mice is regulated by IL-6 that has a main function in cognitive performance like learning and memory [17].
Recently it has been proposed that there is a link between acetylcholine and obesity or insulin resistance [18, 19] and the potential of muscarinic acetylcholine receptors as a therapeutic target of obesity has been proposed [20, 21]. This is probably because of altered acetylcholine turn-over in high fat diet induced obesity; previous studies suggest that choline deficient diets prevents high fat induced obesity in rats [18]. The suggested mechanism is the conversion of choline to acetylcholine in neurons. Under normal conditions the availability of choline for acetylcholine production in cholinergic neurons is controlled by a high affinity choline transporter, which is normally saturated with choline [22, 23]. However, when acetylcholine turnover is high, choline supply appears to become limiting for the production of acetylcholine [24]. Furthermore, brain choline and acetylcholine levels are lower in rats fed diet choline-deficient diets than in rats fed a choline-rich diet [25]. The M3 muscarinic acetylcholine subtype receptor is important in regulating energy metabolism [26]. Mice that lacked the M3 receptor displayed increased energy expenditure, were protected from obesity, and showed increased insulin sensitivity. Because the M3 receptor is expressed primarily in the central nervous system, and not in tissues (such as muscle, adipose and liver) that play major roles in glucose and lipid metabolism, it was suggested that activation of the M3 receptor in the central nervous system by acetylcholine is required for obesity to develop on a HF diet [26]. Taken together, these data indicate that altered acetyl choline metabolism plays a central role in the high fat diet induced obesity and its related disorders.
Considering the above-mentioned introduction, the role of obesity and high-fat diet in development of brain inflammation and triggering the obesity-related neural disorders are well elucidated; however, much less is known about the responsiveness of this pathogenic phenomenon to therapeutic agents especially brain-influential nutrients. Vitamin D classically is known as a steroid hormone responsible in regulating calcium homeostasis, in addition, it can also involve in brain functions because of its capability in passing through blood brain barrier (BBB) and acting on CNS via its receptors. Vitamin D can preserve cognition including attention, memory orientation, executive function [27, 28] and inhibits brain dysfunction in disease models of multiple sclerosis [29] and AD [30]. It also acts as an anti-inflammatory, antioxidant and neuro-protective steroid hormone [31]. However, no study is available evaluating the role of this vitamin in obesity-induced brain inflammation; therefore, the primary hypothesis of the current experimental study was to investigate the influence of vitamin D on brain derived neurotropic factor (BDNF) and neuro-inflammatory factors including IL-6, IL-1β, NF-Kβ and acetylcholine (ACH) with especial regard to hypothalamus and hippocampus in high-fat diet induced obese rats. The secondary hypothesis was to investigate the effects of vitamin D on food intake and weight gain in these animals.
Methods
The design of study has been mentioned in our previous report [32, 33]. Therefore, the animals and procedures are reported here briefly.
Animals, diets and experimental protocol
Forty male Wistar rats weighted 200–220 g were purchased from the Pasteur institute animal care center (Karaj, Iran) and were housed five in each cage under standard conditions (light on from 07:00 AM to 07:00 PM and constant temperature of 25 ± 2 °C) with ad libitum access to standard laboratory chow diet and water. After a week, animals were randomly assigned into 2 groups (n = 20, each group) of normal diet (ND) or high fat diet (HFD). ND included 10% fat, 30% protein and 60% carbohydrate and HFD with 59% fat, 11% protein and 30% carbohydrate. After 4 months of receiving these diets, groups were randomized into two sub-groups according to vitamin D or vehicle administration as follows: ND, ND + vitamin D, HFD and HFD + vitamin D. Migliol (Sigma Adrich, USA) was used as vehicle and vitamin D dosage was 500 IU/kg/d administered by oral gavage. The duration of this phase of study was 5 weeks.
The rationale of vitamin D dose and duration was according to the previous studies confirming the neuro-protective role of this dosage of vitamin in animal models [34].
Body weight was weekly measured by scale (PAND Industries, px3000, 5 kg ± 1 g) and food intake was monitored 3 times a week. Daily food intake was recorded in a metabolic cage until sacrifice. Briefly, five rats from each group were housed per cage and amount of remaining food from past 24 h was weighed every day. All of the experiments were conducted in accordance with the National Institutes of Health (NIH) ethical guidelines for the care and use of laboratory animals (NIH; Publication No. 85-23, revised 1985) and was approved by the veterinary ethics committee of the Tabriz university of medical sciences (Registration number: TBZMED.REC.1395.532).
Preparation of blood, hippocampus and hypothalamus samples
After an overnight fasting, the rats were anesthetized with Ketamin (6.6 mg/kg) and Xylazine (0.3 mg/kg) intra peritoneally. Blood samples were obtained from cardiac puncture and centrifuged at 10,000 g at 4 °C for 20 min; sera were separated and stored in an ultra-low temp freezer (Jal Tajhiz Production, Iran) at − 80 °C until assaying. After rats were sacrificed by decapitation, their brains were removed and the hippocampus was dissected. Accordingly, hypothalamus was also located and isolated according to brain control planes and the hippocampus hemispheres and hypothalamus samples were immediately stored at − 80 °C until further use. For assay, the hippocampal and hypothalamus tissues were homogenized in phosphate buffered saline (PBS) and centrifuged at 10,000 g at 4 °C for 20 min, and clear supernatants were collected for assessment of inflammatory parameters by ELISA assay.
ELISA
Before and after vitamin D supplementation, initial and terminal serum vitamin D concentrations was measured by individual enzyme-linked immunosorbent assay kit (ELISA) (Eastbiopharm, Zhejiang, China) according to the manufacturer’s instructions. The inter and intra assay coefficient of variation for vitamin D was < 10% and sensitivity was 0.95 ng/ml. Accordingly, measurements of inflammatory cytokines including IL-6, IL-1β, NF-Kβ and acetylcholine (ACH) and brain derived neurotropic factor (BDNF) in hippocampus and hypothalamus homogenate samples were performed by commercial ELISA kit (Hangzhou Eastbiopharm, Zhejiang, China). The inter and intra assay coefficient of variation for ACH were < 12% and sensitivity was 0.52 u/ml. The corresponding values for BDNF were < 10, < 12% and 0.01 ng/ml, for IL-6, < 12% and 2.49 ng/L, for IL-1β < 12% and 10.23 pg/L and for NF-Kβ were < 10, < 12% and 0.023 ng/ml.
Statistical analysis
All statistical analyses were performed using SPSS software, version 16. Kolmogorov–Smirnov test was performed for normality of the distributions of variables. Data are expressed as the mean ± SD. The data were analyzed using one-way analysis of variance (ANOVA) with 4 levels followed by post hoc Tukey test for comparisons between multiple groups. Paired sample t test was used for before and after comparison of parameters. Repeated measures test was used for comparison on intra-group changes in body weight and food intake. P < 0.05 was considered as statistically significant.
Results
Changes in food intake, body weight and serum vitamin D concentrations during the study period
Changes in food intake of animals during the study period have been shown in Fig. 1. Food intake of animals was gradually increased until the administration of vitamin D and this increase was meaningful in all of study groups. However, after vitamin D administration, in ND and HFD groups, food intake decreased significantly (P < 0.001). Accordingly, body weight of all of studied groups increased significantly and HFD led to a significant weight gain compared with ND as published in our previous report (Table 1). However, after vitamin D administration, a gradual reduction in body weight has been observed. The results of the trend of body weight have been published elsewhere [23, 24]. As shown in Table 1, the results of repeated measure analysis showed that in each group significant changes in the weight has been occurred. By the way, the results of one way ANOVA and post hoc Tuky test in between group comparisons showed that the significant difference in comparison of the weight of 16th week was between ND versus HFD and HFD + Vit D, ND + Vit D versus HFD and HFD + Vit D and HFD versus ND. Whereas in comparison of weight of 21st week between groups all of the inter group comparisons were significant. Moreover, vitamin D administrations led to a marked increase in serum vitamin D concentrations in ND + vitamin D and HFD + vitamin D groups (Table 2). According to the results of one way—ANOVA and its followed post hoc Tukey test, the significant difference in inter-group comparisons of serum vitamin D concentrations between ND versus ND + Vit D and HFD + Vit D, ND + Vit D versus HFD and HFD versus HFD + Vit D. [23, 24].
Fig. 1.
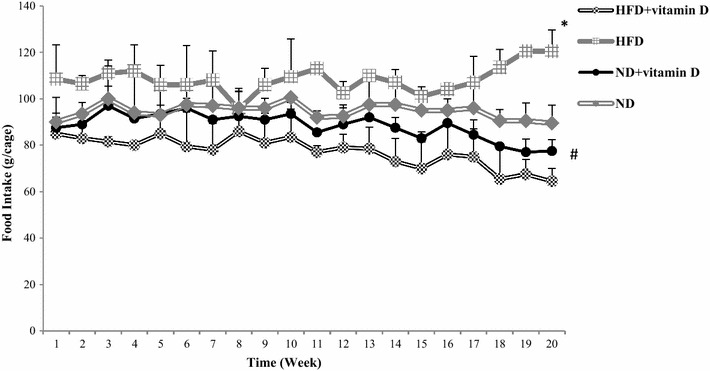
Vitamin D and food intake of rats with normal diet (ND), normal diet supplemented with vitamin D (ND + vitamin D), high fat diet (HFD) and high fat diet supplemented with vitamin D (HFD + vitamin D). Data are expressed as mean ± SD. (*P = 0.11 vs. ND; # P = 0.008 vs. HFD)
Table 1.
Changes in body weight of rats
| Groups | 1st week | 16th week | 21st week | P value† |
|---|---|---|---|---|
| ND | 219 ± 10.83 | 276 ± 26.72 | 289 ± 29.80 | 0.001 |
| ND + vitamin D | 225 ± 22.09 | 278 ± 27.38 | 256 ± 26.90 | 0.001 |
| HFD | 219 ± 13.27 | 403 ± 4.13 | 425 ± 3.71 | 0.001 |
| HFD + vitamin D | 225 ± 13.77 | 340 ± 8.7 | 381 ± 7.80 | 0.001 |
| F values | 0.170 | 135.097 | 147.026 | |
| ‡ P value | 0.69 | 0.001 | 0.001 |
Data are expressed as mean ± SD. Statistical differences between groups were assessed by one-way ANOVA followed by Tukey’s test for Post Hoc analysis. Intra group comparisons of body weight were performed by repeated measure analysis
HFD high fat diet, ND normal diet
† P value and ‡ P value indicated intra group and inter group differences respectively. ‡The significant difference in comparison of the weight of 16th week was between ND versus HFD and HFD + Vit D, ND + Vit D versus HFD and HFD + Vit D and HFD versus ND. Whereas in comparison of weight of 21st week between groups all of the inter group comparisons were significant. P < 0.05 was considered as statistically significant. N = 20 in each group
Table 2.
Vitamin D concentrations in study groups
| Groups | 16th week | 21th week | Mean difference* | P value† |
|---|---|---|---|---|
| ND | 47.5 ± 7.32 | 36.3 ± 7.74 | − 11.20 ± 1 | 0.001 |
| ND + vitamin D | 54.9 ± 11.53 | 119 ± 26.01 | 64.2 ± 7.8 | 0.001 |
| HFD | 51.2 ± 11.16 | 37.7 ± 11.53 | − 13.5 ± 4.25 | 0.01 |
| HFD + vitamin D | 53.2 ± 14.11 | 124 ± 39.39 | 70.5 ± 12.85 | 0.001 |
| F values | 0.792 | 39.272 | – | – |
| ‡ P value | 0.50 | 0.001 | – | – |
Data are expressed as mean ± SD. Statistical differences between groups were assessed by one-way ANOVA followed by Tukey’s test for Post Hoc analysis. Intra group comparisons of vitamin D concentration were performed by paired t-test analysis
HFD high fat diet, ND normal diet
† P value and ‡ P value indicated intra group and inter group differences, respectively. ‡The significant difference is between ND versus ND + Vit D and HFD + Vit D, ND + Vit D versus HFD and HFD versus HFD + Vit D
*Mean difference was calculated by subtracting the weight of 16th from weight of 21st weeks. P < 0.05 was considered as statistically significant. n = 20 in each group
Vitamin D administration and neuro-inflammatory parameters in the hippocampus
The effects of vitamin D administration on hippocampus acetylcholine and IL-6 concentrations are presented in Fig. 2. Vitamin D reduced hippocampus acetylcholine concentrations in ND + vitamin D group compared with ND group (P < 0.001). Whereas, reduced acetylcholine concentrations in HFD + vitamin D group compared with HFD group was not significant. Moreover, high fat diet increased hippocampus IL-6 concentrations significantly in HFD and HFD + Vit D groups (P < 0.05) compared with ND and ND + Vit D groups. Vitamin D could not have significant effects on IL-6 concentrations.
Fig. 2.
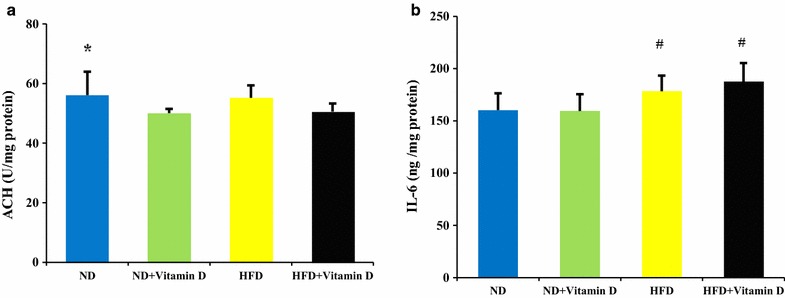
Acetylcholine (ACH) and IL-6 concentration in hippocampus of studied groups. ND, normal diet; HFD, high fat diet. Data are expressed as mean ± SD. Statistical differences between groups were assessed by one-way ANOVA followed by post hoc Tukey’s test for post hoc analysis.*P < 0.05 versus ND + Vit D. # P < 0.05 versus ND and ND + Vit D. F values of one way ANOVA for a and b were 3.34 and 4.099 respectively. P < 0.05 was considered as statistically significant. n = 20 in each group
Vitamin D administration and neuro-inflammatory parameters in the hypothalamus
Among inflammatory parameters in hypothalamus, vitamin D administrations reduced IL-1β, NF-Kβ and acetylcholine concentration and BDNF concentrations in ND group compared with ND + vitamin D, HFD and HFD +Vit D (P < 0.05). These reductions were not significant in HFD + vitamin D versus HFD group (Fig. 3).
Fig. 3.
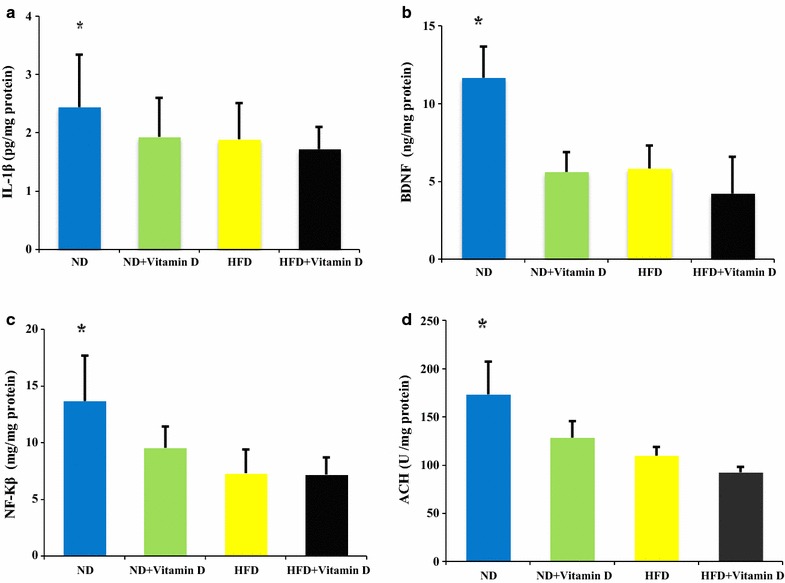
IL-1β, BDNF, NF-Kβ and acetylcholine (ACH) concentration in hypothalamus of studied groups. ND, normal diet; HFD, high fat diet. Data are expressed as mean ± SD. Statistical differences between groups were assessed by one-way ANOVA followed by post hoc Tukey’s test for post hoc analysis.*P < 0.05 versus ND + Vit D, HFD and HFD + Vit D. F values of one way ANOVA for a, b, c and d were 4.12, 3.57, 4.57 and 3.44 respectively. P < 0.05 was considered as statistically significant; n = 20 in each group
Discussion
In the current study, vitamin D reduced food intake and weight in ND +vitamin D and HFD + vitamin D compared with ND and HFD rats; moreover, vitamin D reduced ACH concentrations in hippocampus of ND + vitamin D compared with ND group. Hippocampus IL-6 in HFD receiving groups was significantly higher than ND receiving groups while vitamin D had no significant effect on its concentrations. Also, the IL-1β, BDNF, NF-Kβ and ACH in hypothalamus of ND groups was higher than other groups. Vitamin D reduced IL-1β, BDNF, NF-Kβ and ACH concentrations in hypothalamus of ND compared with ND + vitamin D group. The reduction of these inflammatory parameters in HFD + vitamin D group were not statistically significant.
Our hypothesis about the effects of vitamin D on food intake and weight has been accepted; reduced food intake after vitamin D administration which also led to reduced weight in HFD + vitamin D and ND + vitamin D compared with HFD and ND groups was also confirmed in previous animal or human studies [35, 36]. In the study by Major GC [36] vitamin D supplementation reduced weight and fat mass and lipid intake in females. The suggested underlying mechanisms is that increased calcium absorption leads to reduced intra-adipocyte lipogenic gene expression and stimulation of lipolysis and adipocytes uncoupling protein 2 expression [37, 38]. It has been suggested that vitamin D deficiency increases appetite and decreases energy consumption by stimulating Agouti Related Protein/Neuropeptide Y (AgRP/NPY) and suppressing the pro-Opiomelanocortin/Cocaine-Amphetamine-Regulated Transcription (POMC/CART) pathway [39].
According to our results, vitamin D administrations led to a significant reduction in neuro-inflammatory factors including IL-1β, BDNF, NF-Kβ and ACH in hypothalamus and ACH in the hippocampus of ND +vitamin D group compared with ND group. Whereas, this reduction, was not significant in HFD + vitamin D. In other word, reduced neuro-inflammatory factors after vitamin D administrations in normal diet receiving rats was more pronounced compared with high fat receiving rats. The possible underlying mechanism is that obesity leads to decreased vitamin D bioavailability via several possible mechanisms including trapping vitamin D in fat mass, decreased vitamin D biosynthesis by reduced physical activity and reduced exposure to sunlight and hepatic steatosis and reduced 25-hydroxy vitamin D synthesis in obese individuals [40].
The effects of vitamin D in reducing neuro-inflammatory parameters in hypothalamus like IL-6, IL-1β and NF-Kβ further confirms the anti-inflammatory effects of this vitamin. Recent studies showed that vitamin D and its analogues—1, 25(OH)2D3 and 25(OH)D3—inhibits lipopolysaccharide-induced p38 phosphorylation, IL-6, and TNFα production by human monocytes in a dose-dependent manner; moreover, 1,25(OH)2D3 or its analogs reduce monocyte chemoattractant protein (MCP)-1 and IL-6 expression via inhibiting NF-κB activation in macrophages [41, 42]. Accordingly, the potential ability of vitamin D for crossing through the BBB and activation of its receptors in brain cells exerts its direct impact in the CNS [43]. The involvement of vitamin D in the function of the central nervous system is supported by the presence of the enzyme 25(OH)D3-1α-hydroxylase, responsible for the formation of the active form of vitamin D, as well as the presence of vitamin D receptors in the brain, mainly in the hypothalamus and dopaminergic neurons of the substantia nigra [44]. The anti-inflammatory actions of vitamin D against neuro-degenerative disease like multiple sclerosis and schizophrenia has been studied before [45, 46].
Reduced acetylcholine synthesis in hippocampus and hypothalamus of rats in the current study after vitamin D supplementation was also approved in previous reports; vitamin D has been introduced as an important factor modifying the synthesis of several neuro-mediators like acetylcholine via increased gene expression of the enzyme choline acetyl-transferase (CAT) [47, 48]. This action all confirms the neurosteroid actions of vitamin D as previously suggested by Kalueff [49]. As previously suggested, the activation of the M3 muscarinic acetylcholine subtype receptor in the central nervous system by acetylcholine is required for obesity to develop on a high fat diet [26]. In choline deficient diets obesity did not develop because of lower CNS acetylcholine supply [25]. Choline deficient animals are protected against obesity and vitamin D also regulated this pathway by lowering acetyl choline supply in the brain [25].
Brain derived neurotropic factor (BDNF), is a neurotropic hormone that plays a fundamental role in development and plasticity of the central nervous system (CNS). It is currently recognized as a major participant in appetite control and food intake [50]. Previous studies reported the increased BDNF concentration in obesity and its major role in disturbed glucose metabolism [50–52] and increased BDNF gene expression in adipose tissue of high-calorie diet induced obese mice [53]. In the current study, vitamin D administrations also reduced BDNF concentrations in ND + vitamin D group. Same as our results, in a study by Pozzi et al. [27] vitamin D supplementation, diminished BDNF expression and its plasma concentrations in postmenopausal women. In other study, consistently, vitamin D administrations decreased exercise-induced BDNF in rat hippocampus and abolished the BDNF downstream signal transduction cascade important for learning and memory. The authors suggested that BDNF is a vitamin D receptor (VDR) regulated protein affected by VDR suppression; this results can be explained by the fact that vitamin D supplementation in this issue plays a crucial role in the modulation of BDNF in a compensatory mechanism [27, 54–56].
In conclusion, the current study revealed the potential anti-inflammatory effects of vitamin D administration in hippocampus and hypothalamus and its modulating effects on BDNF and acetyl choline in high fat-diet induced obese rats. Therefore, this neurosteroid, can be suggested as a supplemental therapeutic tool to prevent obesity induced CNS-related neurodegenerative problems and inflammation-related cognitive disorders in obesity. Although, because of the animal model of the study, more studies in human models are warranted for further confirming the findings.
Authors’ contributions
MAF designed the project, wrote the manuscript and performed the statistical analysis, revised the manuscript and supervised the project. MMA was involved in laboratory works and experimental design of the work. GH and GN were involved in data collection and lab assessments and PSH was involved in brain analysis procedures and study designing. All authors read and approved the final manuscript.
Acknowledgements
We thank all of the study participants.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data are available for any scientific use with kind permission.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Animal experiments were conducted in conformity with the National Institutes of Health ethical guidelines for the care and use of laboratory animals (NIH; Publication No. 85-23, revised 1985) and approved by the veterinary ethics committee of the Tabriz university of medical sciences (Registration Number: TBZMED.REC.1395.532).
Funding
This research has been performed by a grant from Tabriz University of Medical Sciences.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahdieh Abbasalizad Farhangi, Phone: +9809146466411, Email: abbasalizad_m@yahoo.com.
Mehran Mesgari-Abbasi, Email: mesgarim@tbzmed.ac.ir.
Ghazaleh Nameni, Email: Gh.nameni@gmail.com.
Ghazaleh Hajiluian, Email: lghazalehl@yahoo.com.
Parviz Shahabi, Email: parvizshahabi@gmail.com.
References
- 1.Barry VB, Raiff BR. Weight management preferences in a non-treatment seeking sample. Health Promot Perspect. 2013;3(2):147–153. doi: 10.5681/hpp.2013.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Obesity and overweight: Fact Sheets. 2017. Cited 2017; Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 3.Chisuwa-Hayami N, Haruki T. Associations of body-related teasing with weight status, body image, and dieting behavior among Japanese adolescents. Health Promot Perspect. 2017;7(2):80–87. doi: 10.15171/hpp.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Diemen V, Trindade EN, Trindade MRM. Experimental model to induce obesity in rats. Acta Cir Bras. 2006;21(6):425–429. doi: 10.1590/S0102-86502006000600013. [DOI] [PubMed] [Google Scholar]
- 5.Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease—the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89(3):144–149. doi: 10.1016/j.brainresbull.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG. High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J Neuroinflamm. 2016;13(1):206. doi: 10.1186/s12974-016-0666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams LM. Hypothalamic dysfunction in obesity. Proc Nutr Soc. 2012;71(04):521–533. doi: 10.1017/S002966511200078X. [DOI] [PubMed] [Google Scholar]
- 8.Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62(8):2629–2634. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 11.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 12.Carvalheira JBC, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, et al. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia. 2003;46(12):1629–1640. doi: 10.1007/s00125-003-1246-x. [DOI] [PubMed] [Google Scholar]
- 13.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Investig. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobesky JL, Barrientos RM, Henning S, Thompson BM, Weber MD, Watkins LR. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immu. 2014;42:22–32. doi: 10.1016/j.bbi.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, et al. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34(7):2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs RL, Zhao Y, Koonen DP, Sletten T, Su B, Lingrell S, et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem. 2010;285(29):22403–22413. doi: 10.1074/jbc.M110.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio-Aliaga I, Roos B, Sailer M, McLoughlin GA, Boekschoten MV, van Erk M, et al. Alterations in hepatic one-carbon metabolism and related pathways following a high-fat dietary intervention. Physiol Genom. 2011;43(8):408–416. doi: 10.1152/physiolgenomics.00179.2010. [DOI] [PubMed] [Google Scholar]
- 20.Maresca A, Supuran CT. Muscarinic acetylcholine receptors as therapeutic targets for obesity. Expert Opin Ther Targets. 2008;12(9):1167–1175. doi: 10.1517/14728222.12.9.1167. [DOI] [PubMed] [Google Scholar]
- 21.Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, Bencherif M. An α7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332(1):173–180. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 22.Klein J, Köppen A, Löffelholz K. Regulation of free choline in rat brain: dietary and pharmacological manipulations. Neurochem Int. 1988;32:479–485. doi: 10.1016/S0197-0186(97)00127-7. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann J, Kiewert C, Duysen EG, Lockridge O, Klein J. Choline availability and acetylcholine synthesis in the hippocampus of acetylcholinesterase-deficient mice. Neurochem Int. 2008;52:972–978. doi: 10.1016/j.neuint.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Köppen A, Klein J, Erb C, Löffelholz K. Acetylcholine release and choline availability in rat hippocampus: effects of exogenous choline and nicotinamide. J Pharmacol Exp Ther. 1997;282:1139–1145. [PubMed] [Google Scholar]
- 25.Cohen EL, Wurtman RJ. Brain acetylcholine: control by dietary choline. Science. 1976;191:561–562. doi: 10.1126/science.1251187. [DOI] [PubMed] [Google Scholar]
- 26.Gautam D, Gavrilova O, Jeon J, Pack S, Jou W, Cui Y, Li JH, Wess J. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 2006;4:363–375. doi: 10.1016/j.cmet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Pozzi F, Frajese GV, Frajese G. Vitamin D (Calcifediol) supplementation modulates NGF and BDNF and improves memory function in postmenopausal women: a Pilot study. Endocrinology. 2013 [Google Scholar]
- 28.Latimer CS, Brewer LD, Searcy JL, Chen K-C, Popovic J, Kraner SD. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci. 2014;111(41):E4359–E4366. doi: 10.1073/pnas.1404477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol. 2008;194(1):7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Soni M, Kos K, Lang IA, Jones K, Melzer D, Llewellyn DJ. Vitamin D and cognitive function. Scand J Clin Lab Investig. 2012;72(sup243):79–82. doi: 10.3109/00365513.2012.681969. [DOI] [PubMed] [Google Scholar]
- 31.Moore M, Piazza A, McCartney Y, Lynch M. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33(4):573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- 32.Hajiluian G, Nameni G, Shahabi P, Mesgari-Abbasi M, Sadigh-Eteghad S, Farhangi MA. Vitamin D administration, cognitive function, BBB permeability and neuroinflammatory factors in high-fat diet-induced obese rats. Int J Obes (Lond) 2017;41(4):639–644. doi: 10.1038/ijo.2017.10. [DOI] [PubMed] [Google Scholar]
- 33.Nameni G, Hajiluian G, Shahabi P, Farhangi MA, Mesgari-Abbasi M, Hemmati MR, et al. The impact of vitamin D supplementation on neurodegeneration, TNF-α concentration in hypothalamus, and CSF-to-plasma ratio of insulin in high-fat-diet-induced obese rats. J Mol Neurosci. 2017;61(2):247–255. doi: 10.1007/s12031-016-0864-y. [DOI] [PubMed] [Google Scholar]
- 34.Alrefaie Z. Vitamin D 3 improves decline in cognitive function and cholinergic. transmission in prefrontal cortex of streptozotocin-induced diabetic rats. Behav Brain Res. 2015;287:156–162. doi: 10.1016/j.bbr.2015.03.050. [DOI] [PubMed] [Google Scholar]
- 35.Marcotorchino J, Tourniaire F, Astier J. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem. 2014;25:1077–1083. doi: 10.1016/j.jnutbio.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Major GC, Alarie FP, Dore J, Tremblay A. Calcium plus vitamin D supplementation and fat mass loss in female very low-calcium consumers: potential link with a calcium-specific appetite control. Br J Nutr. 2009;101(5):659–663. doi: 10.1017/S0007114508030808. [DOI] [PubMed] [Google Scholar]
- 37.Shi H, Dirienzo D, Zemel MB. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001;15:291–293. doi: 10.1096/fj.00-0584fje. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W, Cai D, Wang Y, Lin N, Hu Q, Qi Y, et al. Calcium plus vitamin D3 supplementation facilitated Fat loss in overweight and obese college students with very-low calcium consumption: a randomized controlled trial. Nutr J. 2013;12:1–8. doi: 10.1186/1475-2891-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, Dehghani S, et al. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. doi: 10.1186/1475-2891-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallahi E. Vitamin D and obesity: Which one is affected by the other? Nut Food Sci Res. 2015;2(3):1–3. [Google Scholar]
- 41.Zhang Y, Leung DY, Richers BN. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez-Niño MD, Bozic M, Córdoba-Lanús E, Valcheva P, Gracia O, Ibarz M, et al. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2012;302(6):F647–F657. doi: 10.1152/ajprenal.00090.2011. [DOI] [PubMed] [Google Scholar]
- 43.Gode S, Aksu T, Demirel A, Sunbul M, Gul M, Bakır I, et al. Effect of vitamin D deficiency on the development of postoperative atrial fibrillation in coronary artery bypass patients. J Cardiovasc Thorac Res. 2016;8(4):140–146. doi: 10.15171/jcvtr.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 a-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Alharbi Fatimah M. Update in vitamin D and multiple sclerosis. Neurosciences (Riyadh) 2015;20(4):329–335. doi: 10.17712/nsj.2015.4.20150357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PMH, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/S1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 48.Sonnenberg J, Luine VN, Krey LC, Christakos S. 1,25-Dihydroxyvitamin D3 treatment results in increased cho-line acetyltransferase activity in specific brain nuclei. Endocrinology. 1986;118:1433–1439. doi: 10.1210/endo-118-4-1433. [DOI] [PubMed] [Google Scholar]
- 49.Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care. 2007;10:12–19. doi: 10.1097/MCO.0b013e328010ca18. [DOI] [PubMed] [Google Scholar]
- 50.Rosas-Vargas H, Martínez-Ezquerro JD, Bienvenu T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch Med Res. 2011;42(6):482–494. doi: 10.1016/j.arcmed.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Ma XY, Qiu WQ, Smith CE, Parnell LD, Jiang ZY, Ordovas JM et al. Association between BDNF rs6265 and obesity in the Boston puerto rican health study. J Obes. 2012. [DOI] [PMC free article] [PubMed]
- 52.Suwa M, Kishimoto H, Nofuji Y, Nakano H, Sasaki H, Radak Z, et al. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism. 2006;55(7):852–857. doi: 10.1016/j.metabol.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Nakagomi A, Okada Sh, Yokoyama M, Yoshida Y, Shimizu I, Miki T, et al. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. Aging Mech Dis. 2015;1:15009. doi: 10.1038/npjamd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyon DE, Mohanraj L, Kelly DL, Elswick RK., Jr Health promoting life-style behaviors and systemic inflammation in African American and Caucasian women prior to chemotherapy for breast cancer. Health Promot Perspect. 2014;4(1):18–26. doi: 10.5681/hpp.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaynman S, Ying ZWA, Gomez- Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated plasticity. Neuroscience. 2006;139(4):1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 56.Saeedian-Kia A, Amani R, Cheraghian B. The association between the risk of premenstrual syndrome and vitamin D, calcium, and magnesium status among university students: a case control study. Health Promot Perspect. 2015;5(3):225–230. doi: 10.15171/hpp.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available for any scientific use with kind permission.


