Abstract
Laboratory tests can play an important role in assessment of alcoholic patients, including for evaluation of liver damage and as markers of alcohol intake. Evidence on test performance should lead to better selection of appropriate tests and improved interpretation of results. We compared laboratory test results from 1578 patients between cases (with alcoholic cirrhosis; 753 men, 243 women) and controls (with equivalent lifetime alcohol intake but no liver disease; 439 men, 143 women). Comparisons were also made between 631 cases who had reportedly been abstinent from alcohol for over 60 days and 364 who had not. ROC curve analysis was used to estimate and compare tests’ ability to distinguish patients with and without cirrhosis, and abstinent and drinking cases. The best tests for presence of cirrhosis were INR and bilirubin, with areas under the ROC curve (AUCs) of 0.91 ± 0.01 and 0.88 ± 0.01, respectively. Confining analysis to patients with no current or previous ascites gave AUCs of 0.88 ± 0.01 for INR and 0.85 ± 0.01 for bilirubin. GGT and AST showed discrimination between abstinence and recent drinking in patients with cirrhosis, including those without ascites, when appropriate (and for GGT, sex-specific) limits were used. For AST, a cut-off limit of 85 units/L gave 90% specificity and 37% sensitivity. For GGT, cut-off limits of 288 units/L in men and 138 units/L in women gave 90% specificity for both and 40% sensitivity in men, 63% sensitivity in women. INR and bilirubin show the best separation between patients with alcoholic cirrhosis (with or without ascites) and control patients with similar lifetime alcohol exposure. Although AST and GGT are substantially increased by liver disease, they can give useful information on recent alcohol intake in patients with alcoholic cirrhosis when appropriate cut-off limits are used.
Keywords: Alcohol, Cirrhosis, Abstinence, Aspartate aminotransferase, Gamma glutamyl transferase
Background
Laboratory tests play an important role in the diagnosis and monitoring of patients with alcoholic cirrhosis, both for assessing the degree of impairment of liver function from cirrhosis and for detecting ongoing alcohol intake. It is important to share information on test performance, to optimize test selection and diagnostic accuracy.
Many aspects of liver function are impaired in cirrhosis, and form the basis of diagnostic or prognostic tests. These include excretory, synthetic, and metabolic functions, reflected in abnormal results for bilirubin, albumin and clotting factors, and glucose and ammonia, respectively. Damage to liver cells, perhaps combined with increased enzyme expression, leads to increases in plasma or serum activity of ‘liver enzymes’ (gamma-glutamyl transferase, GGT; aspartate aminotransferase, AST; alanine aminotransferase, ALT). Although it is recognized that advanced cirrhosis may occur with normal liver function test results (Johnston, 1999; Mueller, Seitz, & Rausch, 2014; Stewart & Day, 2004), and there are recent papers comparing test results in drinking versus abstinent alcoholics (Li, He, et al., 2017) and in ‘heavy-drinking controls’ versus patients with alcoholic hepatitis (Li, Amet, et al., 2017), there is little published evidence on the comparative performance of widely available tests in distinguishing between the presence or absence of cirrhosis in heavy alcohol drinkers. Such evidence would be valuable in its own right, and is valuable because novel tests or algorithms should be judged against the performance of currently available tests.
A related issue is the value of biochemical tests as markers of alcohol use in patients with liver disease, particularly alcoholic liver disease. Because the prognosis in alcoholic cirrhosis is greatly improved by abstinence and treatment decisions may be affected, objective and reliable measures of patients’ alcohol use can be helpful. Measurement of ethanol metabolites shows promise (Niemelä, 2016; Staufer & Yegles, 2016; Wurst et al., 2015), but most either require frequent testing because of short half-lives (ethyl glucuronide and ethyl sulfate in urine) or they are not widely available (ethyl glucuronide or fatty acid ethyl esters in hair, phosphatidylethanol in blood cell membranes). There are mixed reports on whether serum disialotransferrin (carbohydrate-deficient transferrin, CDT) is affected by liver disease (Anttila, Järvi, Latvala, Blake, & Niemelä, 2003; Bell et al., 1993; DiMartini et al., 2001; Fagan et al., 2013; Niemelä, Sorvajärvi, Blake, & Israel, 1995; Scouller, Conigrave, Macaskill, Irwig, & Whitfield, 2000). A number of technical issues, depending on the method used, can affect the validity of CDT results in cirrhosis (Chrostek, Cylwik, Gruszewska, Panasiuk, & Szmitkowski, 2012; Gonzalo et al., 2012; Stewart, Reuben, & Anton, 2017). Serum GGT, which is cheap and widely available, is a rather non-specific marker of liver damage as well as an index of alcohol intake, and it is increased in a high proportion of people with liver disease. GGT has therefore been discounted for this situation, though there is little information on its potential as an alcohol biomarker in the presence of liver disease. Nor is information readily available about the ability of other commonly available tests to distinguish abstinent from non-abstinent patients.
We have collected blood samples and clinical information, including alcohol intake history and laboratory test results, from 1578 patients either with liver cirrhosis due to alcohol or with similar alcohol intake but no history or symptoms of liver disease (Whitfield et al., 2015). These data allow us to address the two questions outlined above. First, which tests (including biochemical liver function tests and hematology tests affected by cirrhosis) are best at distinguishing between those who do or do not have cirrhosis as a result of long-term excessive alcohol intake? Second, can any of these commonly available tests assist in identifying continuing alcohol use among patients with alcoholic liver disease?
Methods
Information was gathered from patients recruited for the GenomALC Study (Whitfield et al., 2015) up to the end of April 2016. Recruitment occurred in Australia, France, Germany, Switzerland, UK, and USA, mainly from hepatology clinics (for cases, as defined below) and from psychiatric or detoxification facilities for the controls. All participants gave informed consent and the study was approved by appropriate Research Ethics Committees.
To be eligible, participants had to have high-risk alcohol intake (greater than 80 g per day for men, or 50 g per day for women) for 10 years or more. Cases had alcohol-related cirrhosis, with the diagnosis based on one or more of the following clinical, histological, or FibroScan criteria as reported (Whitfield et al., 2015) and detailed here. Clinical cirrhosis required documented evidence of one or more of the following: clinically detectable ascites (confirmed by imaging or by paracentesis); spontaneous hepatic encephalopathy (grade 2 or higher); or moderate or large esophageal varices on upper endoscopy. Histological cirrhosis required Metavir fibrosis stage F4 or Ishak fibrosis stage 5 or 6. Fibroscan diagnosis required an adequately performed FibroScan with F4 stiffness; the cut off was ≥22 kPa (if AST <100 IU/L within 2 weeks of FibroScan), or ≥30 kPa (if AST between 100 and 200 IU/L within 2 weeks of FibroScan). Exclusion criteria included liver transplantation for liver disease other than alcoholic cirrhosis, hepatitis B or C (by hepatitis C antibody and hepatitis B surface antigen tests), known HIV, hemochromatosis (by transferrin saturation >45% or 2+ iron on liver biopsy if performed), Wilson’s disease (by ceruloplasmin), or autoimmune hepatitis (by ANA titer).
Control subjects had to meet the alcohol intake criteria with no history or current evidence of liver disease (history of jaundice, ascites, variceal bleeding, upper gastrointestinal bleeding of uncertain etiology, or blood tests that suggest impaired liver function or acute/chronic alcoholic liver injury).
Characteristics of 1578 participants who met the eligibility criteria are summarized in Table 1.
Table 1.
Descriptive data on the 1578 GenomALC Cases and Controls included in the analysis. High-risk drinking is defined as equal to or greater than 80 g of alcohol per day for men or 50 g/day for women, for 10 years or more.
| Cases (N = 997)
|
Controls (N = 581)
|
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Number of subjects | 754 | 243 | 438 | 143 |
| Age (Mean ± SD, in years) | 52.6 ± 8.7 | 50.1 ± 9.6 | 50.2 ± 10.0 | 50.3 ± 10.1 |
| Usual alcohol intake, g/day | 211 ± 148 | 162 ± 118 | 243 ± 135 | 197 ± 109 |
| Years of high-risk drinking | 25.3 ± 11.2 | 19.7 ± 9.8 | 22.2 ± 9.6 | 18.4 ± 7.5 |
| Lifetime alcohol intake, kg | 1953 ± 1754 | 1148 ± 1022 | 2002 ± 1582 | 1346 ± 1039 |
| Number with ascites (ever) | 573 (76%) | 193 (79%) | 0 | 0 |
| Number with esophageal varices (ever) | 404 (54%) | 126 (52%) | 0 | 0 |
| Number with encephalopathy (ever) | 247 (33%) | 89 (37%) | 0 | 0 |
| Number abstinent for ≥60 days | 476 (63%) | 155 (64%) | 13 (3%) | 4 (3%) |
Lifetime alcohol intake estimates were based on participants’ recall of habitual daily use of beer, wine, spirits, or other alcoholic beverages (converted to grams of alcohol), and of the number of years of high-risk drinking. Current abstinence was assessed by whether the patient reported they had been abstinent from alcohol for 60 days or more before recruitment.
Data collection was planned before test and reference standard data were collected. Laboratory test results, as listed in Table 2, were gathered from those done for clinical reasons at the time of recruitment or performed for this study where necessary. We also calculated AST/ALT and AST/platelet ratios. Model for End-Stage Liver Disease (MELD) scores were calculated from INR, bilirubin, and creatinine results (Kamath & Kim, 2007) using the formula MELD = 3.78 [Ln serum bilirubin (mg/dL)] + 11.2 [Ln INR] + 9.57 [Ln serum creatinine (mg/dL)] + 6.43. Results for bilirubin, INR, and creatinine of less than 1.0 (in their respective units) were taken as 1.0, and results for creatinine of greater than 4.0 were taken as 4.0, as recommended by the United Network for Organ Sharing (UNOS) (https://www.unos.org/wp-content/uploads/unos/MELD_PELD_Calculator_Documentation.pdf, accessed 2016-05-30).
Table 2.
Effects of alcoholic cirrhosis (case versus control) and recent drinking (reported abstinence for previous 60 days) on laboratory test results. For bilirubin, creatinine, AST, ALT, and GGT the significance of differences in means and of the interaction term was assessed on log-transformed data to reduce the effects of skewed distributions. To allow for multiple testing, p values less than 0.0038 (0.05/13) may be considered significant.
| Controls
|
Cases
|
p values
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Case–control | Abstinence | Interaction | ||
| Hemoglobin (g/L) | Abstinent | 143.6 | 15.7 | 16 | 117.4 | 23.6 | 618 | 1.16 × 10−19 | 0.944 | 0.849 |
| Non-Abstinent | 142.9 | 15.7 | 557 | 117.7 | 25.6 | 362 | ||||
| White cell count (cells/L × 10−9) | Abstinent | 7.907 | 2.549 | 17 | 6.267 | 2.819 | 616 | 0.063 | 0.036 | 0.029 |
| Non-Abstinent | 7.877 | 2.575 | 556 | 8.006 | 4.413 | 359 | ||||
| Platelet count (cells/L × 10−9) | Abstinent | 251.9 | 64.3 | 17 | 135.8 | 71.6 | 613 | 3.02 × 10−27 | 0.742 | 0.480 |
| Non-Abstinent | 248.3 | 81.3 | 555 | 146.0 | 82.8 | 361 | ||||
| INR (ratio) | Abstinent | 1.008 | 0.243 | 17 | 1.402 | 0.455 | 595 | 3.14 × 10−17 | 0.817 | 0.495 |
| Non-Abstinent | 0.986 | 0.154 | 497 | 1.447 | 0.508 | 326 | ||||
| Albumin (g/L) | Abstinent | 41.5 | 4.4 | 17 | 35.4 | 6.9 | 596 | 4.01 × 10−18 | 0.864 | 0.134 |
| Non-Abstinent | 43.0 | 5.6 | 545 | 34.2 | 7.7 | 333 | ||||
| Bilirubin (μmol/L) | Abstinent | 10.6 | 8.1 | 17 | 50.8 | 81.6 | 621 | 5.20 × 10−31 | 0.176 | 0.032 |
| Non-Abstinent | 9.3 | 7.3 | 553 | 88.7 | 130.1 | 363 | ||||
| Creatinine (μmol/L) | Abstinent | 75.1 | 13.6 | 17 | 94.5 | 67.1 | 622 | 0.235 | 0.0068 | 0.122 |
| Non-Abstinent | 71.9 | 17.8 | 558 | 75.3 | 39.2 | 360 | ||||
| ALT (units/L) | Abstinent | 56.4 | 110.7 | 17 | 34.5 | 48.7 | 620 | 0.920 | 0.075 | 0.030 |
| Non-Abstinent | 38.0 | 34.4 | 554 | 45.0 | 38.8 | 363 | ||||
| AST (units/L) | Abstinent | 48.2 | 66.2 | 17 | 50.1 | 48.9 | 606 | 1.24 × 10−9 | 3.25 × 10−4 | 8.71 × 10−4 |
| Non-Abstinent | 41.1 | 33.9 | 552 | 83.4 | 59.5 | 356 | ||||
| GGT (units/L) | Abstinent | 285.5 | 924.2 | 17 | 126.4 | 171.4 | 581 | 1.35 × 10−9 | 7.39 × 10−7 | 3.54 × 10−4 |
| Non-Abstinent | 113.6 | 156.8 | 553 | 424.0 | 627.2 | 348 | ||||
| AST/ALT ratio | Abstinent | 1.136 | 0.386 | 17 | 1.743 | 1.029 | 606 | 8.18 × 10−13 | 0.051 | 0.124 |
| Non-Abstinent | 1.184 | 0.435 | 547 | 2.113 | 0.952 | 355 | ||||
| AST/platelet ratio | Abstinent | 0.190 | 0.225 | 17 | 0.524 | 0.595 | 596 | 5.24 × 10−25 | 0.017 | 0.061 |
| Non-Abstinent | 0.201 | 0.247 | 547 | 0.841 | 1.017 | 353 | ||||
| MELD score | Abstinent | 7.24 | 1.78 | 17 | 13.57 | 5.95 | 591 | 7.42 × 10−25 | 0.566 | 0.387 |
| Non-Abstinent | 7.05 | 1.36 | 490 | 14.52 | 6.94 | 324 | ||||
For comparison of means between groups, test results showing positively skewed distributions (bilirubin, creatinine, ALT, AST, and GGT) were log10-transformed. For ROC curve analysis, test results where the case mean was lower than the control mean (hemoglobin; white cell count; platelet count; albumin) had the assumption of higher results indicating abnormality reversed so that areas under the ROC curve (AUC) were greater than 0.5. Statistical analyses were performed using SPSS (IBM Corporation, Armonk, New York).
Results
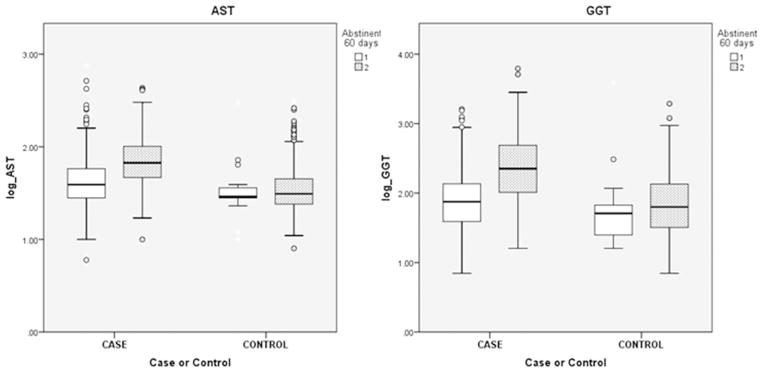
The test means for abstinent and non-abstinent cases and controls are summarized in Table 2, with results for men and women shown separately in Supplementary Table 1. p values for both the effects of presence of cirrhosis and of abstinence on the means, and for case/control by abstinent/non-abstinent interaction, are also shown. Most of the tests showed differences between the case and control groups, but only AST and GGT showed significant effects of abstinence. These two tests also showed significant case/control by abstinent/non-abstinent interaction terms. Plots for AST and GGT by case–control status and by abstinence, to illustrate the main effects and interaction, are shown in Fig. 1; reported abstinence was associated with lower AST and GGT in cases but not in controls (but very few control patients had abstained from alcohol).
Fig. 1.
Boxplots of AST and GGT results by Case–Control and Abstinent/Non-Abstinent status. Boxes show 25th, 50th, and 75th centiles, whiskers indicate 95% range. For the legend ‘Abstinent 60 days’ 1 = Yes (abstinent) and 2 = No (drinking). For each test, values differ significantly by both case/control and abstinent drinking status but there is also case/control by abstinent/drinking interaction (see Table 2). Abstinent/drinking status has significant effects in cases but not in controls.
The ability of the laboratory tests to distinguish cases from controls is summarized in Table 3. ROC curves (which plot test sensitivity, true positive rate, against [1–specificity], false positive rate) are shown for the most discriminating tests (hemoglobin, platelet count, INR, bilirubin, and albumin) and the MELD score in Supplementary Fig. 1. Because there is always a trade-off between better sensitivity and better specificity, determined by the chosen cut-off value separating ‘normal’ from ‘abnormal’ results, comparisons of sensitivity between tests or between groups of patients should be based on the same specificity for each. For our comparisons, we have chosen 90% specificity (10% false positive rate) unless otherwise noted, and report the cut-off values and sensitivities associated with that specificity.
Table 3.
Results of ROC curve analysis; for alcoholic cirrhosis (Cases versus Controls), and for abstinence among patients with alcoholic cirrhosis. To allow for multiple testing, p values less than 0.0038 (0.05/13) may be considered significantly different from chance (i.e., from AUC = 0.500).
| Cases versus Controls
|
Abstinent Cases versus Drinking Cases
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Cases | N Controls | AUC | Std. Error | p value | N Drinking | N Abstinent | AUC | Std. Error | p value | |
| Hemoglobin | 982 | 573 | 0.802a | 0.011 | 7.63 × 10−88 | 362 | 618 | 0.501 | 0.019 | 0.960 |
| White cell count | 977 | 574 | 0.644a | 0.014 | 2.05 × 10−21 | 359 | 616 | 0.616 | 0.019 | 1.43 × 10−9 |
| Platelet count | 976 | 573 | 0.852a | 0.010 | 7.23 × 10−119 | 361 | 613 | 0.528 | 0.019 | 0.143 |
| INR | 923 | 515 | 0.914 | 0.008 | 2.80 × 10−150 | 326 | 595 | 0.522 | 0.020 | 0.273 |
| Bilirubin | 986 | 571 | 0.875 | 0.009 | 2.50 × 10−134 | 363 | 621 | 0.599 | 0.019 | 2.40 × 10−7 |
| Albumin | 931 | 563 | 0.821a | 0.011 | 1.78 × 10−96 | 333 | 596 | 0.543 | 0.020 | 0.031 |
| AST | 964 | 570 | 0.685 | 0.014 | 8.35 × 10−34 | 356 | 606 | 0.737 | 0.017 | 8.85 × 10−35 |
| ALT | 985 | 572 | 0.483 | 0.015 | 0.275 | 363 | 620 | 0.649 | 0.018 | 6.19 × 10−15 |
| GGT | 931 | 571 | 0.643 | 0.014 | 1.35 × 10−20 | 348 | 581 | 0.771 | 0.016 | 2.03 × 10−43 |
| Creatinine | 984 | 576 | 0.573 | 0.014 | 1.52 × 10−06 | 360 | 622 | 0.643 | 0.018 | 6.26 × 10−14 |
| AST/ALT ratio | 963 | 565 | 0.774 | 0.012 | 2.00 × 10−71 | 355 | 606 | 0.627 | 0.019 | 4.61 × 10−11 |
| AST/platelet ratio | 951 | 565 | 0.815 | 0.011 | 5.73 × 10−94 | 353 | 596 | 0.641 | 0.018 | 3.60 × 10−13 |
| MELD score | 917 | 508 | 0.914 | 0.008 | 7.89 × 10−148 | 324 | 591 | 0.527 | 0.020 | 0.173 |
Positive status (Case) is associated with lower test result.
Most (77%) of the patients with alcoholic cirrhosis had current or prior ascites. In order to test how much this affected the test results and their diagnostic performance, we conducted further analyses on case sub-groups defined by presence or history of ascites, comparing those with and without ascites. For most tests, ascites was significantly associated with more-abnormal results (Supplementary Table 2), and exclusion of cases with reported ascites decreased the case–control AUCs (Table 4). The notable exceptions were AST and GGT, where ascites was associated with lower (less abnormal) mean values and with higher AUCs.
Table 4.
Comparison of selected ROC curve results for all Cases, and for Cases with or without current or past ascites.
| All
|
With ascites
|
No ascites
|
|
|---|---|---|---|
| AUC ± SE | AUC ± SE | AUC ± SE | |
| Case versus Control comparison | |||
| INR | 0.914 ± 0.008 | 0.924 ± 0.008 | 0.884 ± 0.014 |
| MELD score | 0.913 ± 0.008 | 0.928 ± 0.008 | 0.865 ± 0.016 |
| Bilirubin | 0.875 ± 0.009 | 0.881 ± 0.009 | 0.853 ± 0.015 |
| Platelet count | 0.852 ± 0.010 | 0.855 ± 0.010 | 0.842 ± 0.017 |
| Albumin | 0.821 ± 0.011 | 0.838 ± 0.011 | 0.762 ± 0.021 |
| Hemoglobin | 0.802 ± 0.011 | 0.831 ± 0.011 | 0.703 ± 0.022 |
| AST | 0.685 ± 0.014 | 0.669 ± 0.015 | 0.738 ± 0.020 |
| GGT | 0.643 ± 0.014 | 0.606 ± 0.016 | 0.762 ± 0.019 |
| Cases only, Abstinent versus Drinking comparison | |||
| AST | 0.737 ± 0.017 | 0.717 ± 0.021 | 0.784 ± 0.031 |
| GGT | 0.771 ± 0.016 | 0.753 ± 0.020 | 0.762 ± 0.032 |
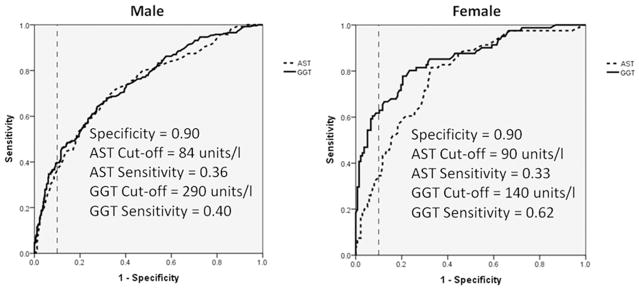
Because only 17 of the controls reported abstinence for 60 days preceding recruitment, analysis of the ability of tests to distinguish abstinence from continued drinking was confined to the cases (Table 3). The only tests showing AUC above 0.70 were AST and GGT, and results for these are shown in more detail in Table 5 and Fig. 2. When data from men and women were analyzed together, the AUC for AST was 0.737, and was 0.771 for GGT. This analysis was then repeated for male and female cases separately (also shown in Table 5). For AST, the AUC, test sensitivities, and cut-off values were similar in men and women, but for GGT the AUC was greater in women than in men and the appropriate cut-off values (determined by the desired specificity) were substantially higher in men.
Table 5.
Details of ROC curve analysis for AST and GGT in distinguishing between Cases with reported abstinence for 60 days and Cases reported as non-abstinent.
| AST
|
GGT
|
|||||
|---|---|---|---|---|---|---|
| Combined | Female | Male | Combined | Female | Male | |
| AUC (95% CI) | 0.737 (0.705–0.770) | 0.774 (0.713–0.835) | 0.726 (0.688–0.764) | 0.771 (0.739–0.802) | 0.851 (0.798–0.904) | 0.744 (0.706–0.781) |
| Standard error | 0.017 | 0.031 | 0.020 | 0.016 | 0.027 | 0.019 |
| p value | 8.85 × 10−35 | 2.30 × 10−12 | 2.31 × 10−24 | 2.03 × 10−43 | 2.28 × 10−18 | 1.92 × 10−27 |
| 70% Specificity: Sensitivity | 0.67 | 0.70 | 0.66 | 0.69 | 0.82 | 0.66 |
| Cut-off (units/L) | 53 | 53 | 53 | 122 | 85 | 133 |
| 80% Specificity: Sensitivity | 0.54 | 0.59 | 0.53 | 0.60 | 0.74 | 0.54 |
| Cut-off (units/L) | 63 | 64 | 63 | 168 | 108 | 200 |
| 85% Specificity: Sensitivity | 0.46 | 0.50 | 0.45 | 0.51 | 0.68 | 0.49 |
| Cut-off (units/L) | 72 | 73 | 72 | 215 | 126 | 232 |
| 90% Specificity: Sensitivity | 0.37 | 0.34 | 0.36 | 0.46 | 0.63 | 0.40 |
| Cut-off (units/L) | 85 | 87 | 84 | 265 | 138 | 288 |
| 95% Specificity: Sensitivity | 0.23 | 0.22 | 0.24 | 0.35 | 0.54 | 0.28 |
| Cut-off (units/L) | 105 | 108 | 103 | 363 | 220 | 422 |
Fig. 2.
Comparison of ROC curves for AST and GGT in men and women. Classification of Cases as Abstainer for the previous 60 days versus Non-Abstainer.
Discussion
We have compared the performance of routine tests, and the composite MELD score, for distinguishing between patients with alcoholic cirrhosis (cases) and patients with similar lifetime exposure to alcohol but no liver disease (controls). The best of these tests show good discrimination, consistent with the comparison of selected groups and with clinical experience. We have also compared results from abstinent and non-abstinent patients with alcoholic cirrhosis. The tests that perform best for making the distinction between abstinent and non-abstinent cases are GGT and AST, and they perform well in patients with advanced liver disease as long as appropriately high cut-off limits are used.
It is generally accepted that conventional liver function tests have poor sensitivity in detecting cirrhosis, particularly in the early stages. Although our cases have (or have had) clinical symptoms, and we accept that we are comparing selected extremes of the spectrum of potential patients, we find that INR, bilirubin, platelet count, and albumin – in that order – give good discrimination between cases and controls (Table 3). The best single test, INR, had an AUC of 0.914 and test sensitivity of 78% at a specificity of 90%. Even in less advanced disease, i.e., after restricting the analyses to patients without ascites, INR and bilirubin continued to show good separation between the case and control groups.
The calculated AST/ALT ratio showed better discrimination than either of its components in the case/control comparison (see Table 3). The AST/platelet ratio showed no advantages, being significantly worse than platelet count for case/control discrimination or AST for drinking/abstinence (again, see Table 3); this is consistent with a previous evaluation (Lieber, Weiss, Morgan, & Paronetto, 2006). The MELD score, being based on INR, bilirubin, and creatinine, gives results equivalent to (but no better than) the INR measurement alone for the case versus control comparison (although MELD may still be superior to any single test for other purposes, such as prognosis, which we did not evaluate).
To be an improvement on what is already available, any new test or test combination would need to achieve either an AUC above 0.91 in patients equivalent to ours, or an equal sensitivity and specificity in patients with less advanced disease. Indicators of fibrosis might be valuable in patients with less advanced disease, and a number have been investigated. Results were summarized in Mueller et al., 2014, with some markers having high reported AUCs or promising sensitivity and specificity values in comparatively small studies. A direct comparison of three fibrosis markers, tissue inhibitor of metalloproteinase 1, aminoterminal propeptide of type III collagen, and hyaluronic acid, showed highly significant differences in mean values between alcoholic patients with mild and advanced fibrosis, but the AUCs were in the range 0.67–0.69 and sensitivity was approximately 33% at 90% specificity.
Another important clinical question is whether people with known alcoholic cirrhosis are abstaining from alcohol. Taking the cases only, ROC curve analysis was performed to assess the ability of the laboratory tests to classify people as abstinent or non-abstinent (Tables 3 and 5, Fig. 2). The tests that were best at distinguishing cases from controls (INR, bilirubin, platelet count, albumin) performed poorly in distinguishing abstinent and non-abstinent cases; they are detecting cirrhosis rather than drinking. It is unexpected that test results are not closer to normal in the abstinent than in the drinking cases, although the period of abstinence specified (60 days or more) might be too short to have made a difference.
On the other hand, AST and GGT, which did not perform well for the case–control comparison, did surprisingly well in the abstinent-drinking comparison. These are tests which primarily measure liver cell damage and/or enzyme induction and which have not previously been considered useful in the presence of liver disease. In fact, the test performance (Table 5) for GGT is very similar to that derived from meta-analysis of data from studies on people without liver disease (Conigrave, Davies, Haber, & Whitfield, 2003) (which estimated GGT sensitivity of 44% and AST sensitivity of 27%, each at 90% specificity). However, the GGT value giving this specificity and sensitivity in our cases (about 250 units/L) is much higher than it would be in alcoholics without known liver disease.
Another point to notice is that AST and GGT performed slightly better, both in the case–control and abstinent-drinking comparisons, in patients with cirrhosis but no ascites (Table 4). This is in contrast to the other tests, and is probably due to decreased liver cell mass in the patients with more advanced disease who have or have had ascites. As these enzymes originate from the liver, very low functioning liver cell mass will lead to less enzyme release into the circulation.
For the evaluation of abstinence in individuals with cirrhosis, we found relevant differences in test performance between men and women. The performance of GGT was better in women than in men (Table 5, Fig. 2) and the appropriate cut-off values for various levels of specificity were higher in men. For example, a cut-off value of approximately 290 units/L would give 40% sensitivity and 90% specificity in men but a cut-off value of 140 units/L would give 63% sensitivity and 90% specificity in women. (The cut-off value for equivalent specificity in the absence of liver disease would be approximately 40–50 units/L.) On the other hand, AST (which performs about as well as GGT as an alcohol marker in the alcoholic cirrhosis context) showed similar test performance and cut-off limits in men and women (Table 5), with a cut-off of approximately 85 units/L (still substantially above the appropriate value for people without liver disease), giving sensitivity of about 35% and 90% specificity.
As mentioned above, there is a trade-off between diagnostic sensitivity and specificity. So far, we have compared test performance at 90% specificity. If prevalence of the condition is low, it is appropriate to use a high cut-off value to attain high specificity because of the need to minimize false positives. However, there are clinical situations where high sensitivity is needed and poor specificity can be tolerated, and detection of continued drinking in patients with alcoholic cirrhosis may be one of these. If specificity of only 70% can be accepted, then the sensitivity of GGT for detection of continued drinking in the presence of cirrhosis increases to about 65% in men (at 133 units/L) and 80% in women (at 85 units/L). However, even though GGT and AST have some ability to distinguish currently drinking patients from currently abstinent patients with alcoholic cirrhosis, it would be inappropriate to place too much reliance on them. As with patients who do not have liver disease, high GGT should be considered suggestive of excessive or continuing alcohol use and a finding which warrants further investigation.
We acknowledge some limitations due to our study design, particularly the existence of spectrum bias because of comparison of extremes rather than unselected patients. Participants were recruited for a case–control genetic association study, so it was important to select cases with strong evidence of cirrhosis. This limitation should be less of a problem in the comparison of abstinent and non-abstinent cirrhotic patients, if we assume that alcoholics are either abstinent or drinking heavily and cannot maintain controlled drinking. Despite assessment for the absence of past or current symptoms, a few control subjects may have had some liver damage from alcohol, though this was probably insignificant given our stringent eligibility criteria. If liver damage were present in some controls, this would tend to decrease the difference between cases and controls, and therefore impair test performance.
Another limitation is that test evaluations depend on having a reliable diagnosis. Liver biopsy is often the most commonly used test for cirrhosis, but it is invasive, not always justifiable, and may be subject to sampling error. Clinical symptoms in the presence of high long-term alcohol intake, and exclusion of alternative causes of cirrhosis, formed the basis for diagnosis in our cases, and controls were recruited with similar alcohol intake and absence of symptoms or history of liver disease.
Finally, we used self-report to assess alcohol intake and abstinence, again with no widely accepted standard. This has been the method of alcohol use assessment in many studies on alcohol consumption, both those that have focused on epidemiological associations between alcohol and health or disease, and those that have evaluated alcohol biomarkers. In general, self-report is a valid approach to assessment, particularly in a setting in which there are no negative consequences for a participant who reports ongoing alcohol use, but it may be subject to bias (Del Boca & Darkes, 2003). Accuracy of self-reported alcohol use may vary according to sex, country, case/control status, or other unmeasured factors. However, it is reasonable to assume that patients with cirrhosis who report continued drinking are giving correct information, while the group who report abstinence contains some who are drinking. If so, any bias will be conservative in that test performance will be underestimated.
Conclusions
We have documented and compared tests related to liver function in alcoholic cirrhosis, and have shown the best performance for INR and bilirubin. AST and GGT are increased by liver disease but they may still give useful information on recent alcohol intake in patients with alcoholic cirrhosis if appropriately higher and sex-specific cut-off values are used.
Supplementary Material
Acknowledgments
Recruitment, data, and sample collection were funded by grant AA018389 from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism. The contributions of all participants, and the work of personnel involved in specimen and data collection are gratefully acknowledged.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.alcohol.2017.07.006.
References
- Anttila P, Järvi K, Latvala J, Blake JE, Niemelä O. Diagnostic characteristics of different carbohydrate-deficient transferrin methods in the detection of problem drinking: Effects of liver disease and alcohol consumption. Alcohol and Alcoholism. 2003;38:415–420. doi: 10.1093/alcalc/agg102. [DOI] [PubMed] [Google Scholar]
- Bell H, Tallaksen C, Sjåheim T, Weberg R, Raknerud N, Orjasaeter H, et al. Serum carbohydrate-deficient transferrin as a marker of alcohol consumption in patients with chronic liver diseases. Alcoholism: Clinical and Experimental Research. 1993;17:246–252. doi: 10.1111/j.1530-0277.1993.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Chrostek L, Cylwik B, Gruszewska E, Panasiuk A, Szmitkowski M. N-Latex CDT results in liver diseases. Alcohol and Alcoholism. 2012;47:428–432. doi: 10.1093/alcalc/ags053. https://doi.org/10.1093/alcalc/ags053. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98(Suppl 2):31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- DiMartini A, Day N, Lane T, Beisler AT, Dew MA, Anton R. Carbohydrate deficient transferrin in abstaining patients with end-stage liver disease. Alcoholism: Clinical and Experimental Research. 2001;25:1729–1733. [PMC free article] [PubMed] [Google Scholar]
- Fagan KJ, Irvine KM, McWhinney BC, Fletcher LM, Horsfall LU, Johnson LA, et al. BMI but not stage or etiology of nonalcoholic liver disease affects the diagnostic utility of carbohydrate-deficient transferrin. Alcoholism: Clinical and Experimental Research. 2013;37:1771–1778. doi: 10.1111/acer.12143. https://doi.org/10.1111/acer.12143. [DOI] [PubMed] [Google Scholar]
- Gonzalo P, Pecquet M, Bon C, Gonzalo S, Radenne S, Augustin-Normand C, et al. Clinical performance of the carbohydrate-deficient transferrin (CDT) assay by the Sebia Capillarys2 system in case of cirrhosis. Interest of the Bio-Rad %CDT by HPLC test and Siemens N-Latex CDT kit as putative confirmatory methods. Clinica Chimica Acta. 2012;413:712–718. doi: 10.1016/j.cca.2011.12.022. https://doi.org/10.1016/j.cca.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Johnston DE. Special considerations in interpreting liver function tests. American Family Physician. 1999;59:2223–2230. [PubMed] [Google Scholar]
- Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. https://doi.org/10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- Li W, Amet T, Xing Y, Yang D, Liangpunsakul S, Puri P, et al. Alcohol abstinence ameliorates the dysregulated immune profiles in patients with alcoholic hepatitis: A prospective observational study. Hepatology. 2017;66:575–590. doi: 10.1002/hep.29242. https://doi.org/10.1002/hep.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate amino-transferase to platelet ratio index in patients with alcoholic liver fibrosis. The American Journal of Gastroenterology. 2006;101:1500–1508. doi: 10.1111/j.1572-0241.2006.00610.x. https://doi.org/10.1111/j.1572-0241.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Gut. 2017;66:705–715. doi: 10.1136/gutjnl-2016-311861. https://doi.org/10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World Journal of Gastroenterology. 2014;20:14626–14641. doi: 10.3748/wjg.v20.i40.14626. https://doi.org/10.3748/wjg.v20.i40.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä O. Biomarker-based approaches for assessing alcohol use disorders. International Journal of Environmental Research and Public Health. 2016;13:166. doi: 10.3390/ijerph13020166. https://doi.org/10.3390/ijerph13020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä O, Sorvajärvi K, Blake JE, Israel Y. Carbohydrate-deficient transferrin as a marker of alcohol abuse: Relationship to alcohol consumption, severity of liver disease, and fibrogenesis. Alcoholism: Clinical and Experimental Research. 1995;19:1203–1208. doi: 10.1111/j.1530-0277.1995.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Scouller K, Conigrave KM, Macaskill P, Irwig L, Whitfield JB. Should we use carbohydrate-deficient transferrin instead of gamma-glutamyltransferase for detecting problem drinkers? A systematic review and metaanalysis. Clinical Chemistry. 2000;46:1894–1902. [PubMed] [Google Scholar]
- Staufer K, Yegles M. Biomarkers for detection of alcohol consumption in liver transplantation. World Journal of Gastroenterology. 2016;22:3725–3734. doi: 10.3748/wjg.v22.i14.3725. https://doi.org/10.3748/wjg.v22.i14.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SF, Day CP. Alcoholic liver disease. In: Bittar E, editor. The liver in biology and disease. 1. Vol. 15. Amsterdam: Elsevier Science; 2004. pp. 317–359. [Google Scholar]
- Stewart SH, Reuben A, Anton RF. Relationship of abnormal chromatographic pattern for carbohydrate-deficient transferrin with severe liver disease. Alcohol and Alcoholism. 2017;52:24–28. doi: 10.1093/alcalc/agw069. https://doi.org/10.1093/alcalc/agw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Rahman K, Haber PS, Day CP, Masson S, Daly AK, et al. Brief report: Genetics of alcoholic cirrhosis-GenomALC multinational study. Alcoholism: Clinical and Experimental Research. 2015;39:836–842. doi: 10.1111/acer.12693. https://doi.org/10.1111/acer.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Thon N, Yegles M, Schrück A, Preuss UW, Weinmann W. Ethanol metabolites: Their role in the assessment of alcohol intake. Alcoholism: Clinical and Experimental Researcher. 2015;39:2060–2072. doi: 10.1111/acer.12851. https://doi.org/10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.