Abstract
Ischemic brain injury triggers an inflammatory response. This response is necessary to clear damaged brain tissue but can also exacerbate brain injury. Microglia are the innate immune cells of the brain that execute this critical function. In healthy brain, microglia perform a housekeeping function, pruning unused synapses between neurons. However, microglia become activated to an inflammatory phenotype upon brain injury. Interferon regulatory factors modulate microglial activation and their production of inflammatory cytokines. This review briefly discusses recent findings pertaining to these regulatory mechanisms in the context of stroke recovery.
Keywords: interferon regulatory factors, interferon beta, microglia, interferon regulatory factor 2 binding protein 2, stroke, inflammation, synaptic pruning, anxiety
M1/M2 Polarization
The ischemic brain injury of stroke triggers an acute and sustained inflammatory response of the brain-resident macrophages called microglia. Upon stroke injury, debris of dying neurons and astrocytes is recognized as damage-associated molecular patterns (DAMP) that activate Toll-like receptors (TLR) and the TLR signalling cascade. Microglia activation after stroke proceeds in an orderly transition from a proinflammatory M1 phenotype to an anti-inflammatory and restorative M2 phenotype. Bacterial lipopolysaccharides (LPS) induce M1 markers, including the pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL) 1β, IL12, as well as monocyte chemoattractant protein-1 (MCP1), macrophage inflammatory protein 1a (MIP-1a), cyclooxygenase 2 (Cox2) and inducible nitric oxide synthetase (iNOS). IL4 induces M2 markers including the anti-inflammatory cytokine IL10, arginase 1 (Arg1), mannose receptor C type 1 (Mrc1; also as CD206), Ym1 and found in inflammatory zone 1 (Fizz1) (Murray et al., 2014; Chen et al., 2015). Experimental stroke studies showed that increased pro-inflammatory M1 polarization is associated with larger infarction and worse stroke outcome, whereas anti-inflammatory M2 polarization resolves inflammation, limits stroke injury progression and promotes tissue regeneration and recovery from stroke injury (Cruz et al., 2017).
Interferon Regulatory Factor (IRF) in Microglia Polarization
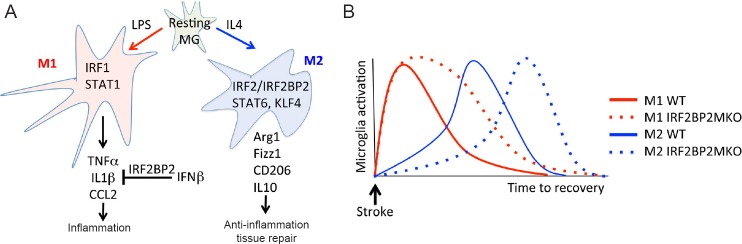
Growing evidence points to the IRF family of transcription factors as playing a key role to control the microglial inflammatory phenotype, and also a central role in defending against viral infection through the activation of the interferon response. IRF1 is a transcriptional activator that is activated by ischemic brain injury and promotes M1 polarization. IRF1-null mice have a reduced infarction after ischemic brain injury (Iadecola et al., 1999). On the other hand, IRF2 binds to the same sequences as IRF1 but works as a transcriptional repressor through its interaction with the corepressor IRF2 binding protein 2 (IRF2BP2) (Childs and Goodbourn, 2003; Teng et al., 2010, 2011). Although the effects of IRF2 in the context of stroke remain to be seen, overexpression of IRF2 was shown to limit ischemia/reperfusion injury in the liver (Klune et al., 2012). Moreover, a recent study by Cruz et al. (2017) showed that microglial-specific ablation of IRF2BP2 enhanced M1 inflammatory gene expression, impaired M2 phenotypic transition of microglia and worsened ischemic brain injury after stroke (Figure 1A). Thus, this study indicates that IRF2 acts to counterbalance the inflammatory response caused by IRF1.
Figure 1.
IRF2BP2 is required for microglia M2 polarization and anti-inflammatory effect of IFNβ.
(A) Resting MG are activated to the M1 phenotype by LPS and to the M2 phenotype by IL4. IFNβ suppresses IL1β expression and this effect requires IRF2BP2. (B) IRF2BP2-deficient MG have prolonged M1 and delayed M2 activation after stroke and this is associated with larger infarction and worsened functional deficits. IRF2BP2: Interferon regulatory factor 2 binding protein 2; IFNβ: interferon beta; MG: microglia; LPS: lipopolysaccharides; IL4: interleukin 4; IL1β: interleukin 1β; IRF1: interferon regulatory factor 1; IRF2: interferon regulatory factor 2; STAT1: signal transducer and activator of transcription 1; TNF-α: tumor necrosis factor-α; IL1: interleukin-1; CCL2: chemokine (C-C motif) ligand 2; IFN1β: interferon 1 beta; STAT6: signal transducer and activator of transcription 6; KLF4: Kruppel-like factor 4; Arg1: arginase 1; Fizz1: found in inflammatory zone 1; CD206: mannose receptor C type 1 (Mrc1); IL10: interleukin 10; WT: wild type; IRF2BP2MKO: IRF2BP2 microglia/macrophage knockout.
Interferon Beta (IFNβ) Protects from Stroke Injury
IFNβ expression and signalling are activated by bacterial and viral infection to limit the spread of infection. IFNβ signaling is not only important to ensure pathogen clearance but also to prevent excess inflammation (IL1β activation) and tissue destruction, a process that was reported to be dependent on IRF5 (Castiglia et al., 2016). IFNβ is also activated by ischemic brain injury (Marsh et al., 2009) and both endogenous and exogenous IFNβ reduce inflammation and infarction (Marsh et al., 2009; Inácio et al., 2015; Kuo et al., 2016). A recent report by Cruz et al. (2017) showed that activation of IFNβ signaling in the context of stroke also suppresses IL1β and limits brain inflammation and tissue damage. Importantly, and unexpectedly, this process requires IRF2BP2 in microglia. Since IRF2BP2 only interacts with and co-represses IRF2 (and not IRF1 or IRF5), this also implicates IRF2 in the protective effect of IFNβ signaling after stroke. Whether the IRF2 and IRF5 pathways work in parallel or converge to control IL1β remains to be determined.
IRF2BP2 Expression in Microglia/Macrophages
The effect of IFNβ on IRF2BP2 expression in microglia is not known. In macrophages, expression of IRF2BP2 is dynamically regulated; inflammation suppresses IRF2BP2 expression whereas anti-inflammatory stimulation by IL4 elevates IRF2BP2 expression (Chen et al., 2015). As in microglia, ablation of IRF2BP2 in macrophages promotes the inflammatory M1 phenotype and interferes with IL4-induced M2 polarization and worsens atherosclerosis in susceptible mice (Chen et al., 2015).
The inflammation caused by ischemic brain injury would suppress IRF2BP2 expression and this may compromise the full beneficial effect of IFNβ to limit stroke injury. Recent studies identified several ischemia-induced microRNAs, including miR-107 (Yang et al., 2014; Bhatia et al., 2016) and miR-155 (Arruda et al., 2015) that target and suppress IRF2BP2 expression. Antagonism of miR-155 promotes stroke recovery (Caballero-Garrido et al., 2015), an effect likely mediated by increased IRF2BP2 protein levels. Future studies will be required to test whether the therapeutic effect of IFNβ for stroke recovery can be improved by antagonism of miR-107 and miR-155 in order to maintain IRF2BP2 expression.
Synaptic Pruning in Stroke Recovery
Focal ischemic lesions produce a well-documented reorganization of the sensorimotor cortex (Harrison et al., 2013), a process that is dependent on tissue repair and synaptic pruning by microglia (Paolicelli et al., 2011). Whether M2 microglia are better at synaptic pruning than M1 microglia, or target different types of synapses for pruning, are important questions that remain to be elucidated. Nonetheless, the study by Cruz et al. (2017) showed more severe functional deficits using the adhesive removal test, suggesting that in the absence of IRF2BP2, the reorganization process in the sensorimotor cortex after stroke is impaired likely due to more severe inflammation. In this paper, IRF2BP2-deficient microglia showed no difference in their phagocytic ability to engulf micro-particles after LPS stimulation compared to wild type (WT) microglia. Whether synaptic pruning in vivo is defective in IRF2BP2-deficient microglia remains to be seen. However, since there were fewer IRF2BP2-deficient M2 microglia in the peri-infarct area compared to littermate controls, and M2 microglia are associated with tissue repair, this could account for the delayed regression of the ischemic lesion. Figure 1B illustrates how IRF2BP2-deficient microglia likely have a delayed transition to the M2 phenotype that accounts for a prolonged inflammatory lesion and impaired recovery.
Inflammatory cytokines can also directly affect synaptic structure and neuronal network connectivity. TNFβ modulates synaptic transmission and synaptic scaling (Stellwagen and Malenka, 2006) and also increases the turnover of dendritic spines and axonal boutons, contributing to early synaptic abnormality in somatosensory cortex in mouse models of experimental autoimmune encephalomyelitis (Yang et al., 2013). Pathological levels of IL1β have been shown to impede synaptic long-term potentiation (Ross et al., 2003). The inflammatory cytokine IL1β is elevated not only at the area surrounding the infarction but also at the contralateral cortex 48 hours after ischemic stroke (Davies et al., 1999).
Astrocytes as Mediators of Microglial Inflammation
Although not addressed in the Cruz et al. (2017) study, astrocytes are another key player in the response to stroke injury. Like microglia, astrocytes undergo an A1 and A2 polarization in response to brain injury. A recent study by Liddelow et al. (2017) showed that activated microglia activate an inflammatory A1 phenotype of adjacent astrocytes that precipitate neuronal death following injury. Since IRF2BP2 is expressed in astrocytes and its expression is elevated in activated astrocytes (Liu et al., 2006), the function of IRF2BP2 in astroctyes and whether it affects their A1 polarization are important questions for future studies and may be relevant to stroke therapy.
Inflammation and Anxiety
Another consequence of inflammation after stroke is the appearance of affective mood disorders (anxiety and depression) that can occur in the absence of an obvious sensory-motor deficit, long after the initial ischemic insult. Histological (Nilupul Perera et al., 2006) and positron emission tomography (PET) imaging (Gerhard et al., 2005; Gulyás et al., 2012) studies detect inflammatory brain microglia/macrophages in the area of the ischemic lesion that can persist for several months. Ischemic lesions to the left prefrontal cortex, a brain region important for mood control, can produce anxiety and depression with minimal deficits in sensory and motor function in humans and mice (Terroni et al., 2011; Vahid-Ansari et al., 2016). Inflammatory microglia are tied to anxiety-like behaviours in mice (Li et al., 2014; McGuiness et al., 2016) and mice with IRF2BP2-deficient microglia are resistant to the anxiolytic effect of enhanced postnatal care (Hari et al., 2017). It will be interesting to see whether mice with IRF2BP2-deficient microglia display more severe post-stroke affective mood disorders. Together, these reports confirm a deleterious effect of brain inflammation and that its rapid resolution after stroke is desirable for functional recovery.
Footnotes
Funding: This study was supported by a grant from the Heart and Stroke Foundation of Canada (HHC, AFRS), a grant from the Natural Science & Engineering Research Council of Canada (HHC, AFRS), a Mid-Career Investigator Award from the Heart and Stroke Foundation of Ontario, Canada (HHC).
Conflicts of interest: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Arruda LC, Lorenzi JC, Sousa AP, Zanette DL, Palma PV, Panepucci RA, Brum DS, Barreira AA, Covas DT, Simoes BP, Silva WA, Jr, Oliveira MC, Malmegrim KC. Autologous hematopoietic SCT normalizes miR-16, -155 and -142-3p expression in multiple sclerosis patients. Bone Marrow Transplant. 2015;50:380–389. doi: 10.1038/bmt.2014.277. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia H, Pattnaik BR, Datta M. Inhibition of mitochondrial beta-oxidation by miR-107 promotes hepatic lipid accumulation and impairs glucose tolerance in vivo. Int J Obes (Lond) 2016;40:861–869. doi: 10.1038/ijo.2015.225. [DOI] [PubMed] [Google Scholar]
- 3.Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35:12446–12464. doi: 10.1523/JNEUROSCI.1641-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castiglia V, Piersigilli A, Ebner F, Janos M, Goldmann O, Dambock U, Kroger A, Weiss S, Knapp S, Jamieson AM, Kirschning C, Kalinke U, Strobl B, Muller M, Stoiber D, Lienenklaus S, Kovarik P. Type I interferon signaling prevents IL-1beta-driven lethal systemic hyperinflammation during invasive bacterial infection of soft tissue. Cell Host Microbe. 2016;19:375–387. doi: 10.1016/j.chom.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen HH, Keyhanian K, Zhou X, Vilmundarson RO, Almontashiri NA, Cruz SA, Pandey NR, Lerma Yap N, Ho T, Stewart CA, Huang H, Hari A, Geoffrion M, McPherson R, Rayner KJ, Stewart AF. IRF2BP2 reduces macrophage inflammation and susceptibility to atherosclerosis. Circ Res. 2015;117:671–683. doi: 10.1161/CIRCRESAHA.114.305777. [DOI] [PubMed] [Google Scholar]
- 6.Childs KS, Goodbourn S. Identification of novel co-repressor molecules for interferon regulatory factor-2. Nucleic Acids Res. 2003;31:3016–3026. doi: 10.1093/nar/gkg431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz SA, Hari A, Qin Z, Couture P, Huang H, Lagace DC, Stewart AFR, Chen HH. Loss of IRF2BP2 in microglia increases inflammation and functional deficits after focal ischemic brain injury. Front Cell Neurosci. 2017;11:201. doi: 10.3389/fncel.2017.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1999;19:87–98. doi: 10.1097/00004647-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage. 2005;24:591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Gulyás B, Tóth M, Schain M, Airaksinen A, Vas A, Kostulas K, Lindstrom P, Hillert J, Halldin C. Evolution of microglial activation in ischaemic core and peri-infarct regions after stroke: a PET study with the TSPO molecular imaging biomarker [((11))C]vinpocetine. J Neurol Sci. 2012;320:110–117. doi: 10.1016/j.jns.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Hari A, Cruz SA, Qin Z, Couture P, Vilmundarson RO, Huang H, Stewart AFR, Chen HH. IRF2BP2-deficient microglia block the anxiolytic effect of enhanced postnatal care. Sci Rep. 2017;7:9836. doi: 10.1038/s41598-017-10349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison TC, Silasi G, Boyd JD, Murphy TH. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke. 2013;44:2300–2306. doi: 10.1161/STROKEAHA.113.001272. [DOI] [PubMed] [Google Scholar]
- 13.Iadecola C, Salkowski CA, Zhang F, Aber T, Nagayama M, Vogel SN, Ross ME. The transcription factor interferon regulatory factor 1 is expressed after cerebral ischemia and contributes to ischemic brain injury. J Exp Med. 1999;189:719–727. doi: 10.1084/jem.189.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inácio AR, Liu Y, Clausen BH, Svensson M, Kucharz K, Yang Y, Stankovich T, Khorooshi R, Lambertsen KL, Issazadeh-Navikas S, Deierborg T. Endogenous IFN-beta signaling exerts anti-inflammatory actions in experimentally induced focal cerebral ischemia. J Neuroinflammation. 2015;12:211. doi: 10.1186/s12974-015-0427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klune JR, Dhupar R, Kimura S, Ueki S, Cardinal J, Nakao A, Nace G, Evankovich J, Murase N, Tsung A, Geller DA. Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G666–673. doi: 10.1152/ajpgi.00050.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo PC, Scofield BA, Yu IC, Chang FL, Ganea D, Yen JH. Interferon-beta modulates inflammatory response in cerebral ischemia. J Am Heart Assoc. 2016;5:e002610. doi: 10.1161/JAHA.115.002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Ma L, Kulesskaya N, Voikar V, Tian L. Microglia are polarized to M1 type in high-anxiety inbred mice in response to lipopolysaccharide challenge. Brain Behav Immun. 2014;38:237–248. doi: 10.1016/j.bbi.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J Neurosci. 2006;26:7532–7540. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuiness B, Gibney SM, Beumer W, Versnel MA, Sillaber I, Harkin A, Drexhage HA. Exaggerated increases in microglia proliferation, brain inflammatory response and sickness behaviour upon lipopolysaccharide stimulation in non-obese diabetic mice. Neuroimmunomodulation. 2016;23:137–150. doi: 10.1159/000446370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA. Inflammation following stroke. J Clin Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 24.Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 26.Teng AC, Al-Montashiri NA, Cheng BL, Lou P, Ozmizrak P, Chen HH, Stewart AF. Identification of a phosphorylation-dependent nuclear localization motif in interferon regulatory factor 2 binding protein 2. PLoS One. 2011;6:e24100. doi: 10.1371/journal.pone.0024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng AC, Kuraitis D, Deeke SA, Ahmadi A, Dugan SG, Cheng BL, Crowson MG, Burgon PG, Suuronen EJ, Chen HH, Stewart AF. IRF2BP2 is a skeletal and cardiac muscle-enriched ischemia-inducible activator of VEGFA expression. FASEB J. 2010;24:4825–4834. doi: 10.1096/fj.10-167049. [DOI] [PubMed] [Google Scholar]
- 28.Terroni L, Amaro E, Iosifescu DV, Tinone G, Sato JR, Leite CC, Sobreiro MF, Lucia MC, Scaff M, Fraguas R. Stroke lesion in cortical neural circuits and post-stroke incidence of major depressive episode: a 4-month prospective study. World J Biol Psychiatry. 2011;12:539–548. doi: 10.3109/15622975.2011.562242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vahid-Ansari F, Lagace DC, Albert PR. Persistent post-stroke depression in mice following unilateral medial prefrontal cortical stroke. Transl Psychiatry. 2016;6:e863. doi: 10.1038/tp.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang G, Parkhurst CN, Hayes S, Gan WB. Peripheral elevation of TNF-alpha leads to early synaptic abnormalities in the mouse somatosensory cortex in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2013;110:10306–10311. doi: 10.1073/pnas.1222895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang ZB, Zhang Z, Li TB, Lou Z, Li SY, Yang H, Yang J, Luo XJ, Peng J. Up-regulation of brain-enriched miR-107 promotes excitatory neurotoxicity through down-regulation of glutamate transporter-1 expression following ischaemic stroke. Clin Sci (Lond) 2014;127:679–689. doi: 10.1042/CS20140084. [DOI] [PubMed] [Google Scholar]