Abstract
The limited regenerative capacity of neuronal cells requires tight orchestration of cell death and survival regulation in the context of longevity, age-associated diseases as well as during the development of the nervous system. Subordinate to genetic networks epigenetic mechanisms like DNA methylation and histone modifications are involved in the regulation of neuronal development, function and aging. DNA methylation by DNA methyltransferases (DNMTs), mostly correlated with gene silencing, is a dynamic and reversible process. In addition to their canonical actions performing cytosine methylation, DNMTs influence gene expression by interactions with histone modifying enzymes or complexes increasing the complexity of epigenetic transcriptional networks. DNMTs are expressed in neuronal progenitors, post-mitotic as well as adult neurons. In this review, we discuss the role and mode of actions of DNMTs including downstream networks in the regulation of neuronal survival in the developing and aging nervous system and its relevance for associated disorders.
Keywords: DNA methyltransferase 1, cortical interneurons, PAK6, neuronal aging, neuropsychiatric diseases, neurodevelopment, neuronal death
Introduction
Due to the limited regenerative capacity of neurons in the adult brain the precise regulation of their survival and death is essential for proper brain function and longevity (Götz et al., 2016). Moreover, survival and cell death regulation plays a pivotal role during brain development to ensure the correct establishment of functional circuits that are composed of particular neuronal subsets. Thereby, neurons are generated to a greater extent than finally needed, which makes the regulated cell death an elementary feature of brain development (Miller, 1995; Bartolini et al., 2013). Hence, the generation, fine tuning and maintenance of correct numbers and subtypes of neurons destined for diverse brain regions requires tight orchestration during the distinct stages of development.
In general, diverse signaling molecules activating intrinsic signaling cascades contribute to neuronal survival regulation (Kristiansen and Ham, 2014). To integrate and process pro- survival and pro-apoptotic cues cell type specifically, particular downstream proteins are shown to regulate each other's expression or functionality in an interconnected network deciding about survival or cell death inducing programs (Pfisterer and Khodosevich, 2017). Although alternative cell death pathways exist (Yuan et al., 2003), in developing as well as in matured brains the elimination of the majority of neurons occurs via the programmed cell death (Buss et al., 2006; Dekkers and Barde, 2013). In this regard, apoptotic processes are regulated by different protein families of specific biochemical signal transduction pathways (Dekkers et al., 2013).
Superordinate to genetic transcriptional networks epigenetic mechanisms like DNA methylation by DNA methyltransferases (DNMTs) as well as histone modifications contribute to the regulation of cell death through development, aging and disease (Fagiolini et al., 2009; Akbarian et al., 2013). In the developing but also in the adult brain the DNA methyltransferases DNMT1 and DNMT3A are expressed and perform promoter as well as gene body cytosine methylation often correlated with repression of transcription (Jang et al., 2017). Traditionally, DNMT1 was regarded as the maintenance methyltransferase copying methylation marks of hemimethylated DNA to the newly synthesized daughter strand during DNA replication, making the enzyme indispensable for dividing progenitor cells (Hermann et al., 2004; Hirasawa et al., 2008). This is supported by the finding that DNMT1 has a higher affinity to hemimethylated DNA (Bashtrykov et al., 2012) and that a total gene knockout of Dnmt1 in the central nervous system is lethal in mice (Fan et al., 2001). Although, Dnmt1 deletion in all dividing somatic cells is also lethal (Li et al., 1992; Fan et al., 2001; Jackson-Grusby et al., 2001; Trowbridge et al., 2009; Sen et al., 2010), mouse embryonic stem cells are viable, despite the resulting global loss of DNA methylation (Tsumura et al., 2006). Notably, human embryonic stem cells (ESCs) also displayed a global demethylation upon Dnmt1 deletion (Liao et al., 2015). However, in contrast to mice they undergo rapid cell death resulting in total lethality implicating distinct functions in embryonic stem cells of rodents and humans (Liao et al., 2015).
DNMTs Regulate Cellular Survival During Neuronal Development and Maturation
Besides its major role in retaining the methylation pattern in progenitor cells, different studies have already linked DNMT1 to post-mitotic developmental processes as well as to adult neuron functionality (Fagiolini et al., 2009; Akbarian et al., 2013; Lv et al., 2013). A surprisingly high Dnmt1 expression was detected in post-mitotic neurons of the central nervous system, suggesting functional implications in these cells (Chestnut et al., 2011; Kadriu et al., 2012). Indeed, decreased Dnmt1 gene function in embryonic excitatory neurons of the neocortex caused progressive cell death and a subsequent reduction in cortical thickness as revealed by conditional deletion of Dnmt1 in Emx1-expressing telencephalic precursors from embryonic day 13.5 on (Hutnick et al., 2009). In addition to neocortical excitatory cells, the survival and post-mitotic maturation of hippocampal neurons is also promoted by DNMT1 (Hutnick et al., 2009). For both, neocortical and hippocampal neurons, a prominent DNA hypomethylation was detected in Dnmt1 deficient cells during development and over life span coupled with a misexpression of genes involved in cell death and postnatal maturation, like layer-specification and ion channel functionality (Hutnick et al., 2009). In addition to its function in regulating cell survival and maturation of embryonic cortical neurons, DNMT1 further was reported to suppress precocious astroglial differentiation during neurogenesis in progenitors of the developing cerebral cortex (Fan et al., 2005). Thereby, DNMT1 was described to maintain the methylation states of astrocytic marker genes as well as genes related to crucial components of the gliogenic JAK-STAT pathway (Fan et al., 2005).
Besides regulating cell death associated gene expression in cortical and hippocampal projection neuron development, DNMT1 was also reported to promote the survival of photoreceptor cells and other types of neurons in the postnatal retina (Rhee et al., 2012) as well as of proliferative cells in the adult dentate gyrus (Noguchi et al., 2015). Interestingly, as soon as these newly generated hippocampal neurons in the adult brain accomplished their differentiation, DNMT1 expression was dispensable for further survival maintenance (Noguchi et al., 2015).
DNMT1 is A Key Regulator of the Survival of Inhibitory Cortical Interneurons
By shaping the responses of the excitatory glutamatergic principal neurons in complex interconnected neuronal circuits inhibitory gamma-aminobutyric acid (GABA)-expressing local interneurons contribute essentially to the neuronal information processing in the cerebral cortex, the seat of higher cognitive functions (Kepecs and Fishell, 2014). In contrast to the excitatory cortical neurons that arise from the cortical proliferative zones, inhibitory GABAergic interneurons originate in particular domains of the basal telencephalon, including the medial and caudal ganglionic eminence (MGE and CGE) as well as the pre-optic area (POA) (Gelman et al., 2011; Bandler et al., 2017). Post-mitotic immature cortical interneurons adopt a polarized migratory morphology with leading and trailing processes and perform long-range tangential migration through the basal telencephalon up to the cerebral cortex (Martini et al., 2009) directed by diverse guidance cues (Peyre et al., 2015).
Throughout the extended period of cortical interneuron development, including their progenitor state as well as their prolonged post-mitotic migration, cell survival has to be regulated and maintained. Misguided targeting or other defects in migration of immature cortical interneurons could induce cell death by activating intrinsic cascades (Uribe and Wix, 2012). Upon reaching their final position cortical interneurons lose their migratory morphology, form axons and dendrites as well as synaptic contacts (Wamsley and Fishell, 2017). At postnatal stages the fine-tuning of interneuron numbers occurs through intrinsically determined apoptotic events (Southwell et al., 2012), diminishing improperly or non-connected cells to ensure inhibitory network establishment and functionality.
In addition to its relevance during development, survival regulation of cortical interneurons is crucial during life time, due to their important function in cortical information processing (Kepecs and Fishell, 2014). Especially fast-firing interneuron subtypes show high energy consumption rates and are exposed to extensive oxidative stress (Laughlin et al., 1998; Sullivan and O’Donnell, 2012; Kann et al., 2014), which makes them vulnerable for damage and cell death (Kannan and Jain, 2000). As the generation of new neurons at adult stages is highly limited (Ming and Song, 2011), matured neurons employ multiple and often redundant strategies to prevent cell death and to promote survival (Kole et al., 2013).
In addition to excitatory cortical neurons, single cell transcriptomic profiling revealed that Dnmt1 is expressed in progenitors as well as in post-mitotic embryonic GABA-expressing cells of the cortical interneuron generating domains, the POA and MGE (Pensold et al., 2016). This points to a potential function in the post-mitotic development and maturation of these immature cortical GABAergic interneurons.
Rhee et al. (2012) highlighted in their studies on postnatal eye development an increased mortality of retinal interneurons associated to a diminished Dnmt1 expression. To approach whether Dnmt1 promotes the survival and maturation of post-mitotic cortical interneurons, Dnmt1 was conditionally deleted in Hmx3-Cre-expressing POA cells in the mouse model (Pensold et al., 2016). This post-mitotic deletion caused diminished numbers of POA-derived interneurons in the adult cerebral cortex. Phenotypic analysis of embryonic stages of Hmx3-Cre/tdTomato/Dnmt1 wild-type and knockout revealed that the reduced cell densities in the adult cortex were due to increased cell death of immature embryonic interneurons in basal parts of the telencephalon (Pensold et al., 2016). Dnmt1-deficient cells further displayed defective migration due to morphological abnormalities (Pensold et al., 2016). These were characterized by a loss of their polarized migratory shape and an adoption of a multipolar morphology indicative of precocious maturation. Thereby, Dnmt1-deficient cells showed prolonged side-processes, no prominent leading process and increased process branching.
To approach potential target genes of Dnmt1 involved in the regulation of interneuron survival, correlative methylome and transcriptome analysis by MeDIP and RNA sequencing of embryonic FAC-sorted Hmx3-Cre/tdTomato/Dnmt1 wild-type and knockout cells was performed (Pensold et al., 2016). Consistent with the observed morphological abnormalities and increased cell death events, many cell death- and cytoskeleton-associated genes were changed in expression upon Dnmt1 deletion. Among them Pak6 coding for a member of the p21-activated kinases (Kumar et al., 2017), was up-regulated in expression in Dnmt1-deficient cells (Pensold et al., 2016).
Potential Downstream Targets of DNMT1 Mediating the Regulation of Cortical Interneuron Survival
PAKs are known to be involved in cell survival regulation as well as cytoskeletal rearrangements (Kumar et al., 2017) and PAK6 was already shown to promote neurite complexity in excitatory cortical neurons (Civiero et al., 2015). Consistently, forced expression of PAK6 induced by a Pak6-green fluorescent protein (GFP) expression construct caused a multipolar morphology of embryonic POA-derived cells comparable to the Dnmt1-deficient migrating interneurons (Pensold et al., 2016). In contrast, siRNA-mediated Pak6 depletion reduced neurite complexity. As several PAKs were reported to influence cell survival (Kumar et al., 2017) and as increased cell death was observed in Dnmt1-deficient cells that display elevated Pak6 expression levels, it was tested whether PAK6 also affects the survival of embryonic POA cells (Pensold et al., 2016). To this end, the number of TUNEL-positive cells after Pak6 siRNA-mediated knockdown was determined. Consistent with the increased rate of cell death in Dnmt1-deficient mice, Pak6 depletion diminished the number of TUNEL-positive cells. Hence, Pak6 represents a potential downstream target of DNMT1-dependent transcriptional repression involved in cell death and cytoskeleton regulation.
The relevance of PAK6 for proper brain function was already suggested by in silico studies proposing PAK6 as a potential candidate for epileptic encephalopathy (Oliver et al., 2016) and gene deletion resulted in deficits in learning, memorizing and movement (Nekrasova et al., 2008; Furnari et al., 2013) underlying the physiological relevance of DNMT1-dependent regulation of Pak6 expression.
In regard to cell death regulation, some members of the PAK family including PAK6 were already shown to regulate the function of proteins of the BCL2 family like BAD (Schürmann et al., 2000; Tang et al., 2000; Zhang et al., 2010; Ye et al., 2011) and BCL6 (Barros et al., 2009) by phosphorylation. Both factors, BAD and BCL6, regulate pro-apoptotic and pro-survival effects in various cell types including neurons (Hatok and Racay, 2016). Interestingly, we detected a significantly altered expression of Bcl6 in Dnmt1-deficient POA-derived interneurons (unpublished data), indicating that Dnmt1 controls different factors of the survival-regulating network.
Furthermore, phosphorylation by PAKs was also shown to regulate the activity of mitogen-activated protein (MAP) kinases that in turn activate programmed cell death pathways (Déléris et al., 2011; De la Mota-Peynado et al., 2011; Qing et al., 2012). Some transcripts of MAP-kinases (MAPKs) we found up-regulated in Dnmt1-deficient mice (unpublished data). This includes Mapk4, encoding for the atypical MAP-kinase ERK3, which was already shown to be activated by PAK1-mediated phosphorylation and to activate downstream kinases (Déléris et al., 2011; De la Mota-Peynado et al., 2011). Furthermore, RNA-sequencing revealed an increased expression of Map3k5 in Dnmt1 knockout cells (unpublished data), coding for the MAPK-kinase-kinase apoptosis signal regulating kinase 1 (ASK1) (Pensold et al., 2016). In neurons ASK1 is associated with the regulation of JNK/p38/MAPK-mediated apoptotic processes (Tobiume et al., 2001; Nishitoh et al., 2002). However, whether PAK6 indeed activates specific MAPKs is not yet investigated, but it was already proposed by others (Lee et al., 2002; Déléris et al., 2011).
Taken together, the elevated Pak6 expression in Dnmt1-deficient immature cortical interneurons could lead to enhanced phosphorylation levels of BCL2 family members and MAPKs, which could be involved in mediating the increased cell mortality seen in Dnmt1 deficient cells. As mitogen activated protein kinases, as well as some BCL2 family members are phospho-regulated, further studies determining the phosphorylation levels of relevant BCL2 proteins and MAPKs as a function of altered PAK6 expression levels could shed light on these open questions. Alternatively, as it was also shown that PAK6 translocates into the nucleus and regulates gene expression through interaction with specific transcription factors (Lee et al., 2002), a potential direct transcriptional control of Bcl6 or genes encoding for specific MAPKs by PAK6 is conceivable and under current investigation.
In addition to Pak6, we also found increased Foxo1 expression as one of the cell death-associated transcripts in Dnmt1 deficient cells of the interneuron generating Hmx3-expressing cell population from the POA (unpublished data). Foxo1 encodes for a “pioneer” transcription factor that is also able to bind to condensed chromatin regions and regulates gene transcription (Hatta and Cirillo, 2007; Zaret and Mango, 2016). Members of the Forkhead Rabdomyosarcoma (FoxO) transcription factor family can be activated by MAPK-mediated phosphorylation (Asada et al., 2007), but also by serine/threonine protein kinases as it was already shown for PAK1 (Mazumdar and Kumar, 2003; de la Torre-Ubieta et al., 2010). They contribute to the regulation of a variety of genes, including genes encoding for different pro-apoptotic factors, via the dynamic modulation of chromatin states (Fu and Tindall, 2008). Recently it was also shown that FOXO1 together with the protein kinase MST1 triggers cell death in cerebellar granular neurons (Yuan et al., 2009). Thus, the repression of Foxo1-mediated by DNMT1 might be relevant for the survival of embryonic POA-derived interneurons, possibly together with the repression of Pak6 and therefore a PAK6 dependent phospho-activation. However, this requires further investigations to uncover potential interrelationships.
All together, these data emphasize the relevance of DNMT1 for regulating the expression of genes as part of a complex network involved in the regulation of cell death and survival in embryonic post-mitotic neurons, which is summarized in Figure 1.
Figure 1.
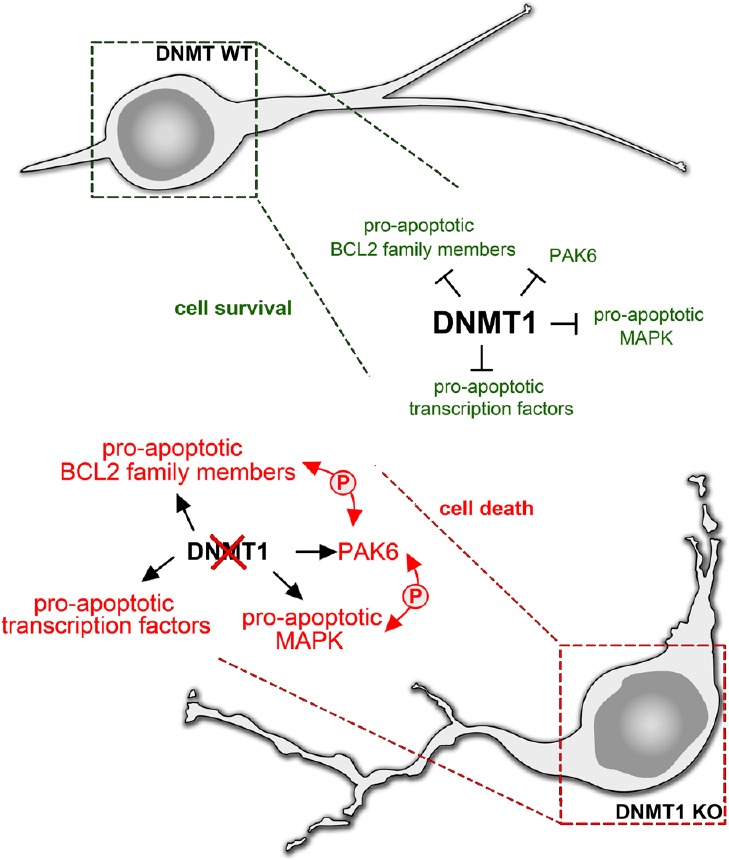
DNA methyltransferase 1 (DNMT1) promotes the survival of immature migrating inhibitory interneurons of the cerebral cortex.
DNMT1 seems to inhibit the expression of pro-apoptotic genes of the PAK family like Pak6 as well as genes encoding for MAP-kinases (MAPK) and BCL2 family members to ensure the proper survival and typical migratory phenotype of immature interneurons with leading and trailing process (DNMT1 wild type (WT)). Furthermore, Dnmt1 seems to repress transcription factors like FOXO1 with potential impact on cell death induction. Dnmt1 deletions abolish the repression of these pro-apoptotic genes resulting in increased expression in migrating interneurons during development (DNMT1 knockout (KO)). The subsequent activation of each other and downstream signaling pathways by phosphorylation events could facilitate morphological defects and results in increased mortality.
Potential Crosstalk of DNMTs with Histone Modifications in Cell Death Regulation
In addition to their canonical function performing DNA methylation, DNMTs were described to influence transcription through interactions with histone modifications (Du et al., 2015). To explore, whether DNMT1 regulates Pak6 expression through its DNA-methylating activity in post-mitotic interneurons, MeDIP-sequencing of FACS-enriched embryonic Dnmt1-deficient and wild-type cells was applied (Pensold et al., 2016). In contrast to changes in gene expression, no differences in the methylation level between wild-type and Dnmt1 knockout samples were detected, neither in Pak6 gene locus, nor up- or downstream (Pensold et al., 2016). Consistently, elevated Pak6 expression levels in a neuroblastoma cell culture model (N2a cells) were only observed after siRNA-mediated Dnmt1 depletion but not after inhibiting the DNA-methylating activity of DNMTs by RG108 (Pensold et al., 2016). This indicates that direct DNA methylation of Pak6 is not the way of action by which DNMT1 regulates its transcription. Moreover, for all transcription factors with predicted binding sites in the Pak6 gene locus, no changes in DNA methylation were identified in post-mitotic interneurons that correlated with transcriptional alterations (Pensold et al., 2016). This implies that DNMT1 modifies Pak6 gene expression in developing interneurons through non-canonical actions, potentially via crosstalk with histone-modifying enzymes.
Interactions between DNA methylation and histone modifications were already shown to be partially regulated by methylcytosin binding proteins, recruiting histone deacetylases to methylated DNA sequences (Jones et al., 1998; Nan et al., 1998). Acetylations at histones H3 and H4 are associated with higher gene accessibility compared to non-acetylated histones (Eberharter and Becker, 2002). Furthermore, there is also evidence that DNA methylation inhibits permissive and supports repressive histone methylation to ensure gene silencing (Hashimshony et al., 2003; Lande-Diner et al., 2007). Recent studies revealed that there are direct interactions between DNA-methylating and histone-modifying enzymes via specific binding domains, possibly regulating the recruitment of proteins to complexes and mediating the catalytic activity of their binding partners (Viré et al., 2006; Smallwood et al., 2007; Clements et al., 2012).
For instance, DNMT1 was shown to interact with EZH2 (Viré et al., 2006; Ning et al., 2015; Purkait et al., 2016), the core enzyme of the polycomp repressor complex 2. PCR2 mediates repressive trimethylations on lysine 27 at the N terminal amino acid tail of histone 3 (H3K27me3) (Margueron and Reinberg, 2011). It was reported that EZH2 is able to recruit DNMT1 to polycomp target genes (Viré et al., 2006; Ning et al., 2015), but does not alter the expression level of the DNA methyltransferase (Purkait et al., 2016). In contrast, manipulation of DNMT1 function or expression was found to be associated with changes in EZH2 expression levels as well as the occurrence of H3K27 trimethylation (So et al., 2011; Purkait et al., 2016). Inhibiting DNMT1 enzymatic function by 5-azacytidine or reducing Dnmt1 expression by target-specific siRNAs decrease the expression levels of PCR2 components (So et al., 2011). Additionally, an enhanced expression of miRNAs was detected, that target polycomp repressor complex proteins (So et al., 2011). Gain or loss of function experiments of PCR2 components like EZH2 as well as SUZ12 and EED already revealed an epigenetic control of Pak6 expression by H3K27 trimethylation in hepatoma cells (Liu et al., 2015). In addition, further studies suggested an epigenetic control of Pak6 transcript level by miRNAs (Cai et al., 2015), which were already shown to be regulated by DNA methylation activity (Han et al., 2007; Lujambio et al., 2008).
Taken together, in POA-derived interneurons DNMT1 could indirectly repress Pak6 expression by facilitating the assembly of H3K27 trimethylation in Pak6 promoter regions, either through modulating Ezh2 expression and other PCR2 components or by promoting PCR2 function. To what extent the Pak6 expression is indeed regulated via crosstalk of DNMT1 with EZH2 in neurons modulating repressive histone lysine methylation is subject of current studies. Beyond that, a possible contribution of specific DNMT1-dependent miRNAs in regulating Pak6 expression is also under investigation.
However, polycomb-induced repression by H3K27 trimethylation was shown to be easily reversible and PC-target genes are often marked bivalently by co-occurrence of H3K27me3 and H3K4me/me2/me3 to realize an optimal transition between repressive and active transcriptional states (Bernstein et al., 2006; Barski et al., 2007; Mikkelsen et al., 2007; Pan et al., 2007; Voigt et al., 2013). H3K4 mono-, di- and trimethylation are regulated by the MLL complex 1 and 2, consisting of the respective histone methyltransferases MLL1 and MLL2 (KMT2B) as well as other regulatory proteins like WDR5 and ASH2 that mediate complex stability, specificity and efficiency (Zhang et al., 2012; Carbonell et al., 2013; Jiang et al., 2013).
In support of this bivalent model of chromatin state, we detected increased transcript levels of Kmt2d (also known as MLL2) in Dnmt1-deficient cells (unpublished data). Kmt2d encodes for a histone methyltransferase which is the core enzyme of the MLL2 complex regulating mono- and dimethylation of H3K4, which are associated with activated gene transcription (Froimchuk et al., 2017). Furthermore, reduced expression of Cul4b in Dnmt1-deficient cells points to a possible contribution of an altered H3K4 methylation status in DNA methylation-independent transcriptional changes in developing Dnmt1-deficient interneurons (unpublished data). Cul4b encodes for a small scaffold protein in the E3 ubiquitin-protein ligase complex targeting WDR5, which is one part of the MLL complex that promotes permissive H3K4 trimethylation (Nakagawa and Xiong, 2011). Apart from that, reduced Cul4b expression was also shown to result in a decreased retention of the SIN3A/HDAC complex followed by increasing acetylation of histone 3 and 4 (Ji et al., 2014). Histone acetylation facilitates transcriptional expression of respective genes (Eberharter and Becker, 2002) and could contribute to an overall open chromatin state enabling abnormal Pak6 expression in migrating POA-deriving interneurons after Dnmt1 deletion. Hence, DNMT1-dependent repression of Pak6 gene transcription in immature cortical interneurons could be mediated by hampering the establishment of active histone marks like H3K4 methylation or histone acetylation. Together these studies emphasize the complexity of epigenetic transcriptional networks in regulating cell viability and highlight a pivotal role of DNMT1 in the regulation of survival genes in immature cortical interneurons. Further studies on DNMT1-dependent changes of histone H3 lysine 4 methylation as well as of acetylation at histone 3 and 4 could give us more insights into the methylation-independent activities of the DNA methyltransferase 1.
The Role of DNA Methylation Mediating Neuronal Survival in the Adult and Diseased Brain
In contrast to studies on immature developing neurons it was reported that in adult mice degeneration and apoptosis of motor neurons is caused by an aberrant high DNA methylation state through increased expression of DNMT1 and DNMT3a in a disease-relevant background (Chestnut et al., 2011). Although context-dependently different, alterations in DNA methylation seem to also play a fundamental role in the adult nervous system and in the etiology of neuropsychiatric disorders. While DNA demethylation of neuronal cell death-associated genes together with neuronal cell loss were related with major depression disorder (Duman, 2009; Xin et al., 2013; Chen et al., 2014), increased Dnmt1 expression and subsequently elevated DNA methylation levels were observed in cortical interneurons of patients diagnosed with schizophrenia (Costa et al., 2003; Veldic et al., 2004; Ruzicka et al., 2007). Thereby, increased methylation was reported for genes like Reln and Gad1 encoding for transcripts associated to GABAergic neurotransmission and interneuron function (Costa et al., 2003; Veldic et al., 2004; Ruzicka et al., 2007). The changed methylation patterns correlate with reduced expression of these genes pointing to impaired interneuron function (Costa et al., 2003; Veldic et al., 2004; Ruzicka et al., 2007). Disruption of GABAergic interneuron functionality has been associated with the pathophysiology of schizophrenia as well as of other psychological disorders including autism and epilepsy (Costa et al., 2003; Veldic et al., 2004; Levitt, 2005; Konradi et al., 2011a, b; Marín, 2012). In addition to the reported transcriptional changes caused by altered DNA methylation, a significant layer-specific loss of interneurons as well as of projection neurons was found in post-mortem studies of schizophrenia patients (Benes et al., 1991, 1998). These findings suggest that DNMT1 might indirectly influence interneuron survival possibly by impairing their functionality. Moreover, a death receptor pathway was recently shown to be implicated in the pathology of schizophrenia (Catts and Weickert, 2012). Whether the reported hypermethylation in schizophrenia patients (Veldic et al., 2004; Ruzicka et al., 2007; Catts and Weickert, 2012) also affects the transcriptional activity of gene promoters of respective death receptors, similar to how it was shown for cancer cells (van Noesel et al., 2002; Petak et al., 2003), needs to be investigated. The transcriptional regulation by DNA methylation in cortical interneurons in disease-relevant contexts reported so far mostly refers to genes relevant for brain development and physiology including neuronal activity (Costa et al., 2003; Veldic et al., 2004; Ruzicka et al., 2007). The modulation of signal transmission, synaptic plasticity and membrane excitability by DNMT1 was also reported in cortical excitatory neurons under normal conditions (Levenson et al., 2006; Feng et al., 2010; Meadows et al., 2016). That neuronal activity is closely linked to neuron survival has already been shown in various studies (Pfisterer and Khodosevich, 2017; Rozycka and Liguz-Lecznar, 2017). Hence, DNMT-dependent DNA methylation could regulate cell death in the healthy and diseased adult brain indirectly by affecting the expression of genes involved in synaptic neurotransmission.
Elevated Dnmt1 expression in cortical interneurons is also related to the pathogenesis of mental impairments and psychosis due to neural injury and drug abuse (Veldic et al., 2005; Guidotti et al., 2007; Lewis et al., 2012; Moore et al., 2013). Hence, the modulation of DNMT1 expression and function seems to be important for proper neuronal network performance and the functionality of the adult brain with potential impact on neuronal survival.
DNA Methylation in the Aging Brain
In addition to the developing and adult brain, dynamic changes in DNA methylation landscape were observed during aging including a global hypomethylation (Bollati et al., 2009; Shimoda et al., 2014; Lardenoije et al., 2015; Moore et al., 2016) with site-specific hypermethylation predominantly in promoter regions (Xu et al., 2007; Numata et al., 2012). Dynamic changes in methylation, not restricted to neurons, even enable the use of methylation marks to predict the age of the organism very exactly (Horvath, 2013; Moore et al., 2016).
These data point to a relevance of DNA methylation and demethylation for the age-associated alterations of the brain involving decreased functionality and neurodegeneration of particular neuronal subsets. In the cerebral cortex, we (unpublished data) and others found reduced densities of cortical interneuron numbers (Pugliese et al., 2004; Hua et al., 2008). Due to their important function for cortical information processing, this loss of interneurons likely contributes to the cognitive decline and somato-motoric defects observed in elderly. Many of the genes that are hypermethylated in the aged brain are implicated in neurodevelopmental functions (Siegmund et al., 2007; Rakyan et al., 2010). Hypermethylation mostly correlates with gene silencing (Mo et al., 2015). Consistently, aging, especially of the cerebral cortex, is rather associated with transcriptional repression than induction (Xu et al., 2007). Thereby, the targets of age-associated gene silencing are implicated in synaptic function and plasticity (Jiang et al., 2001; Lu et al., 2004), which is closely linked to neuronal survival (Bito and Takemoto-Kimura, 2003; Segal, 2010). As DNMT1 modulates synaptic plasticity often through regulation of the brain derived neurotrophic factor (Martinowich et al., 2003; Levenson et al., 2006; Feng et al., 2007), it may play indirectly a role in the long-term survival of neurons.
However, the underlying mechanisms of neuronal aging that can culminate in neuronal death or neurodegeneration seem manifold. They involve oxidative stress, disturbed calcium homeostasis, chromosomal instability, impaired DNA repair, and the accumulation of nuclear and mitochondrial DNA damage (Chouliaras et al., 2010) contributing either individually or combined to the age-associated cell death in the central nervous system. Although in cancer cells DNMT1 was already reported to function coordinately with the DNA damage repair machinery (Jin and Robertson, 2013), potential involvements in regulating neuronal aging-related cell death still remains elusive.
Another aspect is the age-associated decrease in enzymatic activity described for DNMT1 (Casillas et al., 2003), which is consistent with the global hypomethylation shown for the aging brain (Bollati et al., 2009; Shimoda et al., 2014; Lardenoije et al., 2015). Moreover, in patients diagnosed with Alzheimer´s disease, an age-related neurodegenerative disorder, 5-methylcytosine (5mC) immunoreactivity in neurons of post-mortem cortical tissue was found significantly reduced compared to age-matched controls (Mastroeni et al., 2010). As the levels of 5mC inversely correlate with the markers of late-stage neurofibrillary tangles in the same neurons, a significant global loss of 5mC was suggested to take place in brains of Alzheimer´s patients (Mastroeni et al., 2010). As active ways of DNA demethylation have been described for differentiated cells (Bhutani et al., 2011; Chen and Riggs, 2011), the age-associated inability to re-establish the methylation pattern upon DNA demethylation could be involved in the regulation of long-term neuronal survival and age-associated neurodegeneration. Similar to studies in the developing brain, potential interactions of DNMTs with histone modifications could further contribute to the regulation of neuronal function and survival upon aging.
Conclusion
Taken together, these studies underline a dynamic and complex network of DNMT-dependent regulation of neuron survival during development, in adults, during aging and in related diseases. Thereby, the functions and mode of actions seem to differ at the distinct stages of life, emphasizing the relevance of context-specific epigenetic regulation. Hence, the development of therapeutic strategies necessitates the consideration of cell and stage-specific approaches, which are based on the detailed and context-specific analysis of the epigenetic transcriptional networks.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Özgür Demir, Gaziosmanpasa University, Turkey; omar Abdel Hamed Ahmed-Farid, Gaziosmanpasa University, Turkey.
References
- 1.Akbarian S, Beeri MS, Haroutunian V. Epigenetic determinants of healthy and diseased brain aging and cognition. JAMA Neurol. 2013;70:711–718. doi: 10.1001/jamaneurol.2013.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 2007;19:519–527. doi: 10.1016/j.cellsig.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Bandler RC, Mayer C, Fishell G. Cortical interneuron specification: the juncture of genes, time and geometry. Curr Opin Neurobiol. 2017;42:17–24. doi: 10.1016/j.conb.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barros P, Jordan P, Matos P. Rac1 signaling modulates BCL-6-mediated repression of gene transcription. Mol Cell Biol. 2009;29:4156–4166. doi: 10.1128/MCB.01813-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Bartolini G, Ciceri G, Marín O. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 2013;79:849–864. doi: 10.1016/j.neuron.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Bashtrykov P, Jankevicius G, Smarandache A, Jurkowska RZ, Ragozin S, Jeltsch A. Specificity of Dnmt1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chem Biol. 2012;19:572–578. doi: 10.1016/j.chembiol.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 9.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146:866–872. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bito H, Takemoto-Kimura S. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. doi: 10.1016/s0143-4160(03)00140-4. [DOI] [PubMed] [Google Scholar]
- 13.Bollati V, Schwartz J, Wright R, Litonjua A, Tarantini L, Suh H, Sparrow D, Vokonas P, Baccarelli A. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130:234–239. doi: 10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- 15.Cai S, Chen R, Li X, Cai Y, Ye Z, Li S, Li J, Huang H, Peng S, Wang J, Tao Y, Huang H, Wen X, Mo J, Deng Z, Wang J, Zhang Y, Gao X, Wen X. Downregulation of microRNA-23a suppresses prostate cancer metastasis by targeting the PAK6-LIMK1 signaling pathway. Oncotarget. 2015;6:3904–3917. doi: 10.18632/oncotarget.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbonell A, Mazo A, Serras F, Corominas M. Ash2 acts as an ecdysone receptor coactivator by stabilizing the histone methyltransferase Trr. Mol Biol Cell. 2013;24:361–372. doi: 10.1091/mbc.E12-04-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casillas MA, Jr, Lopatina N, Andrews LG, Tollefsbol TO. Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol Cell Biochem. 2003;252:33–43. doi: 10.1023/a:1025548623524. [DOI] [PubMed] [Google Scholar]
- 18.Catts VS, Weickert CS. Gene expression analysis implicates a death receptor pathway in schizophrenia pathology. PLoS One. 2012;7:e35511. doi: 10.1371/journal.pone.0035511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Wang Y, Zhang J, Ma L, Gu J, Ho G. Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Dis Model Mech. 2014;7:723–730. doi: 10.1242/dmm.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ZX, Riggs AD. DNA methylation and demethylation in mammals. J Biol Chem. 2011;286:18347–18353. doi: 10.1074/jbc.R110.205286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci. 2011;31:16619–16636. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouliaras L, Rutten BP, Kenis G, Peerbooms O, Visser PJ, Verhey F, van Os J, Steinbusch HW, van den Hove DL. Epigenetic regulation in the pathophysiology of Alzheimer's disease. Prog Neurobiol. 2010;90:498–510. doi: 10.1016/j.pneurobio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Civiero L, Cirnaru MD, Beilina A, Rodella U, Russo I, Belluzzi E, Lobbestael E, Reyniers L, Hondhamuni G, Lewis PA, Van den Haute C, Baekelandt V, Bandopadhyay R, Bubacco L, Piccoli G, Cookson MR, Taymans JM, Greggio E. Leucine-rich repeat kinase 2 interacts with p21-activated kinase 6 to control neurite complexity in mammalian brain. J Neurochem. 2015;135:1242–1256. doi: 10.1111/jnc.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements EG, Mohammad HP, Leadem BR, Easwaran H, Cai Y, Van Neste L, Baylin SB. DNMT1 modulates gene expression without its catalytic activity partially through its interactions with histone-modifying enzymes. Nucleic Acids Res. 2012;40:4334–4346. doi: 10.1093/nar/gks031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa E, Grayson DR, Guidotti A. Epigenetic downregulation of GABAergic function in schizophrenia: potential for pharmacological intervention? Mol Interv. 2003;3:220–229. doi: 10.1124/mi.3.4.220. [DOI] [PubMed] [Google Scholar]
- 26.Déléris P, Trost M, Topisirovic I, Tanguay P-L, Borden KLB, Thibault P, Meloche S. Activation loop phosphorylation of ERK3/ERK4 by group I p21-activated kinases (PAKs) defines a novel PAK-ERK3/4-MAPK-activated protein kinase 5 signaling pathway. J Biol Chem. 2011;286:6470–6478. doi: 10.1074/jbc.M110.181529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De la Mota-Peynado A, Chernoff J, Beeser A. Identification of the atypical MAPK Erk3 as a novel substrate for p21-activated kinase (Pak) activity. J Biol Chem. 2011;286:13603–13611. doi: 10.1074/jbc.M110.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Torre-Ubieta L, Gaudilliere B, Yang Y, Ikeuchi Y, Yamada T, DiBacco S, Stegmuller J, Schuller U, Salih DA, Rowitch D, Brunet A, Bonni A. A FOXO-Pak1 transcriptional pathway controls neuronal polarity. Genes Dev. 2010;24:799–813. doi: 10.1101/gad.1880510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekkers MP, Barde YA. Developmental biology. Programmed cell death in neuronal development. Science. 2013;340:39–41. doi: 10.1126/science.1236152. [DOI] [PubMed] [Google Scholar]
- 30.Dekkers MP, Nikoletopoulou V, Barde YA. Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. J Cell Biol. 2013;203:385–393. doi: 10.1083/jcb.201306136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du J, Johnson LM, Jacobsen SE, Patel DJ. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol. 2015;16:519–532. doi: 10.1038/nrm4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin Neurosci. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H, ten Hoeve J, Shuai K, Sun YE. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- 36.Fan G, Beard C, Chen RZ, Csankovszki G, Sun Y, Siniaia M, Biniszkiewicz D, Bates B, Lee PP, Kuhn R, Trumpp A, Poon C, Wilson CB, Jaenisch R. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J Neurosci. 2001;21:788–797. doi: 10.1523/JNEUROSCI.21-03-00788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- 38.Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Froimchuk E, Jang Y, Ge K. Histone H3 lysine 4 methyltransferase KMT2D. Gene. 2017;627:337–342. doi: 10.1016/j.gene.2017.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furnari MA, Jobes ML, Nekrasova T, Minden A, Wagner GC. Functional deficits in PAK5, PAK6 and PAK5/PAK6 knockout mice. PLoS One. 2013;8:e61321. doi: 10.1371/journal.pone.0061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Götz M, Nakafuku M, Petrik D. Neurogenesis in the Developing and Adult Brain-Similarities and Key Differences. Cold Spring Harb Perspect Biol. 2016;8:a018853. doi: 10.1101/cshperspect.a018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marin O. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci. 2011;31:16570–16580. doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, Costa E. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport. 2007;18:57–60. doi: 10.1097/WNR.0b013e32800fefd7. [DOI] [PubMed] [Google Scholar]
- 45.Han L, Witmer PD, Casey E, Valle D, Sukumar S. DNA methylation regulates MicroRNA expression. Cancer Biol Ther. 2007;6:1284–1288. doi: 10.4161/cbt.6.8.4486. [DOI] [PubMed] [Google Scholar]
- 46.Hashimshony T, Zhang J, Keshet I, Bustin M, Cedar H. The role of DNA methylation in setting up chromatin structure during development. Nat Genet. 2003;34:187–192. doi: 10.1038/ng1158. [DOI] [PubMed] [Google Scholar]
- 47.Hatok J, Racay P. Bcl-2 family proteins: master regulators of cell survival. Biomol Concepts. 2016;7:259–270. doi: 10.1515/bmc-2016-0015. [DOI] [PubMed] [Google Scholar]
- 48.Hatta M, Cirillo LA. Chromatin opening and stable perturbation of core histone: DNA contacts by FoxO1. J Biol Chem. 2007;282:35583–35593. doi: 10.1074/jbc.M704735200. [DOI] [PubMed] [Google Scholar]
- 49.Hermann A, Goyal R, Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem. 2004;279:48350–48359. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 50.Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, Sasaki H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008;22:1607–1616. doi: 10.1101/gad.1667008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hua T, Kao C, Sun Q, Li X, Zhou Y. Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain Res Bull. 2008;75:119–125. doi: 10.1016/j.brainresbull.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, Silva AJ, Schweizer FE, Fan G. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum Mol Genet. 2009;18:2875–2888. doi: 10.1093/hmg/ddp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet. 2001;27:31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- 55.Jang HS, Shin WJ, Lee JE, Do JT. CpG and non-CpG methylation in epigenetic gene regulation and brain function. Genes. 2017;8:E148. doi: 10.3390/genes8060148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji Q, Hu H, Yang F, Yuan J, Yang Y, Jiang L, Qian Y, Jiang B, Zou Y, Wang Y, Shao C, Gong Y. CRL4B interacts with and coordinates the SIN3A-HDAC complex to repress CDKN1A and drive cell cycle progression. J Cell Sci. 2014;127:4679–4691. doi: 10.1242/jcs.154245. [DOI] [PubMed] [Google Scholar]
- 57.Jiang CH, Tsien JZ, Schultz PG, Hu Y. The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci U S A. 2001;98:1930–1934. doi: 10.1073/pnas.98.4.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang H, Lu X, Shimada M, Dou Y, Tang Z, Roeder RG. Regulation of transcription by the MLL2 complex and MLL complex-associated AKAP95. Nat Struct Mol Biol. 2013;20:1156–1163. doi: 10.1038/nsmb.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin B, Robertson KD. DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol. 2013;754:3–29. doi: 10.1007/978-1-4419-9967-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 61.Kadriu B, Guidotti A, Chen Y, Grayson DR. DNA methyltransferases1 (DNMT1) and 3a (DNMT3a) colocalize with GAD67-positive neurons in the GAD67-GFP mouse brain. J Comp Neurol. 2012;520:1951–1964. doi: 10.1002/cne.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34:1270–1282. doi: 10.1038/jcbfm.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 64.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kole AJ, Annis RP, Deshmukh M. Mature neurons: equipped for survival. Cell Death Dis. 2013;4:e689. doi: 10.1038/cddis.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2011a;68:340–350. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011b;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kristiansen M, Ham J. Programmed cell death during neuronal development: the sympathetic neuron model. Cell Death Differ. 2014;21:1025–1035. doi: 10.1038/cdd.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar R, Sanawar R, Li X, Li F. Structure, biochemistry, and biology of PAK kinases. Gene. 2017;605:20–31. doi: 10.1016/j.gene.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lande-Diner L, Zhang J, Ben-Porath I, Amariglio N, Keshet I, Hecht M, Azuara V, Fisher AG, Rechavi G, Cedar H. Role of DNA methylation in stable gene repression. J Biol Chem. 2007;282:12194–12200. doi: 10.1074/jbc.M607838200. [DOI] [PubMed] [Google Scholar]
- 71.Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, Coleman P, Lemere CA, Hof PR, van den Hove DL, Rutten BP. The epigenetics of aging and neurodegeneration. Prog Neurobiol. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laughlin SB, de Ruyter van Steveninck RR, Anderson JC. The metabolic cost of neural information. Nat Neurosci. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- 73.Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP. AR and ER interaction with a p21-activated kinase (PAK6) Mol Endocrinol. 2002;16:85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]
- 74.Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 75.Levitt P. Disruption of interneuron development. Epilepsia. 2005;46(Suppl 7):22–28. doi: 10.1111/j.1528-1167.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 76.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 78.Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, Akopian V, Gifford CA, Donaghey J, Galonska C, Pop R, Reyon D, Tsai SQ, Mallard W, Joung JK, Rinn JL, Gnirke A, Meissner A. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat Genet. 2015;47:469–478. doi: 10.1038/ng.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W, Liu Y, Liu H, Zhang W, Fu Q, Xu J, Gu J. Tumor suppressive function of p21-activated kinase 6 in hepatocellular carcinoma. J Biol Chem. 2015;290:28489–28501. doi: 10.1074/jbc.M115.658237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 81.Lujambio A, Calin GA, Villanueva A, Ropero S, Sanchez-cespedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso MS, Faller WJ, Gallagher WM, Eccles SA, Croce CM, Esteller M. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lv J, Xin Y, Zhou W, Qiu Z. The epigenetic switches for neural development and psychiatric disorders. J Genet Genomics. 2013;40:339–346. doi: 10.1016/j.jgg.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 83.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 84.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martini FJ, Valiente M, Lopez Bendito G, Szabo G, Moya F, Valdeolmillos M, Marin O. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development. 2009;136:41–50. doi: 10.1242/dev.025502. [DOI] [PubMed] [Google Scholar]
- 86.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 87.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic changes in Alzheimer's disease: decrements in DNA methylation. Neurobiol Aging. 2010;31:2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. doi: 10.1016/s0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- 89.Meadows JP, Guzman-Karlsson MC, Phillips S, Brown JA, Strange SK, Sweatt JD, Hablitz JJ. Dynamic DNA methylation regulates neuronal intrinsic membrane excitability. Sci Signal. 2016;9:ra83. doi: 10.1126/scisignal.aaf5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller MW. Relationship of the time of origin and death of neurons in rat somatosensory cortex: barrel versus septal cortex and projection versus local circuit neurons. J Comp Neurol. 1995;355:6–14. doi: 10.1002/cne.903550104. [DOI] [PubMed] [Google Scholar]
- 92.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mo A, Mukamel EA, Davis FP, Luo C, Henry GL, Picard S, Urich MA, Nery JR, Sejnowski TJ, Lister R, Eddy SR, Ecker JR, Nathans J. Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron. 2015;86:1369–1384. doi: 10.1016/j.neuron.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore AZ, Hernandez DG, Tanaka T, Pilling LC, Nalls MA, Bandinelli S, Singleton AB, Ferrucci L. Change in epigenome-wide DNA methylation over 9 years and subsequent mortality: results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2016;71:1029–1035. doi: 10.1093/gerona/glv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakagawa T, Xiong Y. Chromatin regulation by CRL4 E3 ubiquitin ligases: CUL4B targets WDR5 ubiquitylation in the nucleus. Cell Cycle. 2011;10:4197–4198. doi: 10.4161/cc.10.24.18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 98.Nekrasova T, Jobes ML, Ting JH, Wagner GC, Minden A. Targeted disruption of the Pak5 and Pak6 genes in mice leads to deficits in learning and locomotion. Dev Biol. 2008;322:95–108. doi: 10.1016/j.ydbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Ning X, Shi Z, Liu X, Zhang A, Han L, Jiang K, Kang C, Zhang Q. DNMT1 and EZH2 mediated methylation silences the microRNA-200b/a/429 gene and promotes tumor progression. Cancer Lett. 2015;359:198–205. doi: 10.1016/j.canlet.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noguchi H, Kimura A, Murao N, Matsuda T, Namihira M, Nakashima K. Expression of DNMT1 in neural stem/precursor cells is critical for survival of newly generated neurons in the adult hippocampus. Neurosci Res. 2015;95:1–11. doi: 10.1016/j.neures.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 102.Numata S, Ye T, Hyde TM, Guitart-Navarro X, Tao R, Wininger M, Colantuoni C, Weinberger DR, Kleinman JE, Lipska BK. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90:260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oliver KL, Lukic V, Freytag S, Scheffer IE, Berkovic SF, Bahlo M. In silico prioritization based on coexpression can aid epileptic encephalopathy gene discovery. Neurology Genetics. 2016;2:e51. doi: 10.1212/NXG.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Pensold D, Symmank J, Hahn A, Lingner T, Salinas-Riester G, Downie BR, Ludewig F, Rotzsch A, Haag N, Schubert NAK, Hübner CA, Pieler T, Zimmer G. The DNA methyltransferase 1 (DNMT1) controls the shape and dynamics of migrating POA-derived interneurons fated for the murine cerebral cortex. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw341. doi: 10.1093/cercor/bhw341. [DOI] [PubMed] [Google Scholar]
- 106.Petak I, Danam RP, Tillman DM, Vernes R, Howell SR, Berczi L, Kopper L, Brent TP, Houghton JA. Hypermethylation of the gene promoter and enhancer region can regulate Fas expression and sensitivity in colon carcinoma. Cell Death Differ. 2003;10:211–217. doi: 10.1038/sj.cdd.4401132. [DOI] [PubMed] [Google Scholar]
- 107.Peyre E, Silva CG, Nguyen L. Crosstalk between intracellular and extracellular signals regulating interneuron production, migration and integration into the cortex. Front Cell Neurosci. 2015;9:129. doi: 10.3389/fncel.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pfisterer U, Khodosevich K. Neuronal survival in the brain: neuron type-specific mechanisms. Cell Death Dis. 2017;8:e2643. doi: 10.1038/cddis.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pugliese M, Carrasco JL, Geloso MC, Mascort J, Michetti F, Mahy N. Gamma-aminobutyric acidergic interneuron vulnerability to aging in canine prefrontal cortex. J Neurosci Res. 2004;77:913–920. doi: 10.1002/jnr.20223. [DOI] [PubMed] [Google Scholar]
- 110.Purkait S, Sharma V, Kumar A, Pathak P, Mallick S, Jha P, Sharma MC, Suri V, Julka PK, Suri A, Sharma BS, Sarkar C. Expression of DNA methyltransferases 1 and 3B correlates with EZH2 and this 3-marker epigenetic signature predicts outcome in glioblastomas. Exp Mol Pathol. 2016;100:312–320. doi: 10.1016/j.yexmp.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Qing H, Gong W, Che Y, Wang X, Peng L, Liang Y, Wang W, Deng Q, Zhang H, Jiang B. PAK1-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Tumour Biol. 2012;33:985–994. doi: 10.1007/s13277-012-0327-1. [DOI] [PubMed] [Google Scholar]
- 112.Rakyan VK, Down TA, Maslau S, Andrew T, Yang TP, Beyan H, Whittaker P, McCann OT, Finer S, Valdes AM, Leslie RD, Deloukas P, Spector TD. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 2010;20:434–439. doi: 10.1101/gr.103101.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rhee KD, Yu J, Zhao CY, Fan G, Yang XJ. Dnmt1-dependent DNA methylation is essential for photoreceptor terminal differentiation and retinal neuron survival. Cell Death Dis. 2012;3:e427. doi: 10.1038/cddis.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rozycka A, Liguz-Lecznar M. The space where aging acts: focus on the GABAergic synapse. Aging cell. 2017;16:634–643. doi: 10.1111/acel.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- 116.Schürmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Segal M. Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci. 2010;31:2178–2184. doi: 10.1111/j.1460-9568.2010.07270.x. [DOI] [PubMed] [Google Scholar]
- 118.Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimoda N, Izawa T, Yoshizawa A, Yokoi H, Kikuchi Y, Hashimoto N. Decrease in cytosine methylation at CpG island shores and increase in DNA fragmentation during zebrafish aging. Age (Dordr) 2014;36:103–115. doi: 10.1007/s11357-013-9548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One. 2007;2:e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smallwood A, Estève P-O, Pradhan S, Carey M. Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev. 2007;21:1169–1178. doi: 10.1101/gad.1536807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.So AY, Jung JW, Lee S, Kim HS, Kang KS. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS One. 2011;6:e19503. doi: 10.1371/journal.pone.0019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, Baraban SC, Alvarez-Buylla A. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–113. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sullivan EM, O’Donnell P. Inhibitory interneurons, oxidative stress, and schizophrenia. Schizophr Bull. 2012;38:373–376. doi: 10.1093/schbul/sbs052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- 126.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trowbridge JJ, Snow JW, Kim J, Orkin SH. DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell. 2009;5:442–449. doi: 10.1016/j.stem.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, Matsuoka C, Shimotohno K, Ishikawa F, Li E, Ueda HR, Nakayama J, Okano M. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 2006;11:805–814. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 129.Uribe E, Wix R. Neuronal migration, apoptosis and bipolar disorder. Rev Psiquiatr Salud Ment. 2012;5:127–133. doi: 10.1016/j.rpsm.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 130.van Noesel MM, van Bezouw S, Salomons GS, Voute PA, Pieters R, Baylin SB, Herman JG, Versteeg R. Tumor-specific down-regulation of the tumor necrosis factor-related apoptosis-inducing ligand decoy receptors DcR1 and DcR2 is associated with dense promoter hypermethylation. Cancer Res. 2002;62:2157–2161. [PubMed] [Google Scholar]
- 131.Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 134.Voigt P, Tee WW, Reinberg D. A double take on bivalent promoters. Genes Dev. 2013;27:1318–1338. doi: 10.1101/gad.219626.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wamsley B, Fishell G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci. 2017;18:299–309. doi: 10.1038/nrn.2017.30. [DOI] [PubMed] [Google Scholar]
- 136.Xin Y, O’Donell A, Ge Y, Chanrion B, Dwork A, Arango V, Mann JJ, Haghighi F. DNA demethylation of neuronal cell death genes in depression. Epigenetics Chromatin. 2013;6:P113. [Google Scholar]
- 137.Xu X, Zhan M, Duan W, Prabhu V, Brenneman R, Wood W, Firman J, Li H, Zhang P, Ibe C, Zonderman AB, Longo DL, Poosala S, Becker KG, Mattson MP. Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol. 2007;8:R234. doi: 10.1186/gb-2007-8-11-r234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ye DZ, Jin S, Zhuo Y, Field J. p21-Activated kinase 1 (Pak1) phosphorylates BAD directly at serine 111 in vitro and indirectly through Raf-1 at serine 112. PLoS One. 2011;6:e27637. doi: 10.1371/journal.pone.0027637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 140.Yuan Z, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284:11285–11292. doi: 10.1074/jbc.M900461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zaret KS, Mango SE. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr Opin Genet Dev. 2016;37:76–81. doi: 10.1016/j.gde.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang M, Siedow M, Saia G, Chakravarti A. Inhibition of p21-activated kinase 6 (PAK6) increases radiosensitivity of prostate cancer cells. Prostate. 2010;70:807–816. doi: 10.1002/pros.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang P, Lee H, Brunzelle JS, Couture JF. The plasticity of WDR5 peptide-binding cleft enables the binding of the SET1 family of histone methyltransferases. Nucleic Acids Res. 2012;40:4237–4246. doi: 10.1093/nar/gkr1235. [DOI] [PMC free article] [PubMed] [Google Scholar]


