Abstract
Cellular homeostasis requires a tightly controlled balance between protein synthesis, folding and degradation. Especially long-lived, post-mitotic cells such as neurons depend on an efficient proteostasis system to maintain cellular health over decades. Thus, a functional decline of processes contributing to protein degradation such as autophagy and general lysosomal proteolytic capacity is connected to several age-associated neurodegenerative disorders, including Parkinson's, Alzheimer's and Huntington's diseases. These so called proteinopathies are characterized by the accumulation and misfolding of distinct proteins, subsequently driving cellular demise. We recently linked efficient lysosomal protein breakdown via the protease cathepsin D to the Ca2+/calmodulin-dependent phosphatase calcineurin. In a yeast model for Parkinson's disease, functional calcineurin was required for proper trafficking of cathepsin D to the lysosome and for recycling of its endosomal sorting receptor to allow further rounds of shuttling. Here, we discuss these findings in relation to present knowledge about the involvement of cathepsin D in proteinopathies in general and a possible connection between this protease, calcineurin signalling and endosomal sorting in particular. As dysregulation of Ca2+ homeostasis as well as lysosomal impairment is connected to a plethora of neurodegenerative disorders, this novel interplay might very well impact pathologies beyond Parkinson's disease.
Keywords: neurodegeneration, Parkinson's disease, α-synuclein, cathepsin D, calcineurin, retromer, yeast, lysosome, endosomal sorting
Introduction
With the continuously rising age of our society, the prevalence of age-related pathologies such as neurodegenerative diseases is increasing drastically. Although tremendous effort is made to address this health issue, the cellular and molecular events promoting neurodegeneration are still not completely understood. A mutual feature of so-called proteinopathies, a subset of neurodegenerative disorders including Parkinson's disease (PD), Alzheimer's disease (AD), Huntington's disease (HD) and amyotrophic lateral sclerosis (ALS), is the accumulation and/or aggregation of certain proteins that ultimately results in neuronal cell death. This common pathogenic hallmark already suggests a critical role for protein clearance in the pathophysiology of these disorders, and cumulating evidence points towards general lysosomal function and in particular lysosomal degradative capacity as crucial determinants of proteinopathies (Stoka et al., 2016).
Cathepsin D (CatD) is a lysosomal aspartyl protease with particularly high expression levels in the brain and is critically involved in the degradation of unfolded, damaged and unused proteins that are delivered into lysosomes via processes like autophagy or endocytosis (Vidoni et al., 2016). Thus, a dysregulation of its enzymatic activity affects the accumulation and aggregation of various proteins, and alterations in CatD levels and/or activity have been described for most proteinopathies. Furthermore, CatD also modulates the activity of diverse polypeptides, enzymes and growth factors, and should therefore not only be seen as protease with unspecific degradative functions but rather as an important regulator of cellular signalling and homeostasis (Benes et al., 2008; Wagner et al., 2015).
CatD and Calcineurin in PD
PD pathology is characterized by the accumulation of α-synuclein (αSyn) and its deposition into proteinaceous, intracellular inclusions called Lewy bodies in dopaminergic neurons of the substantia nigra pars compacta (Spillantini et al., 1997; Baba et al., 1998). In different cellular and organismal models for PD, CatD has been shown to be the major protease involved in lysosomal clearance of αSyn and in line, high levels of this protease reduced αSyn aggregation and toxicity (Stoka et al., 2016).
Recently, we could establish a nexus between the cytoprotective activity of CatD and the Ca2+/calmodulin-dependent phosphatase calcineurin (Aufschnaiter et al., 2017). Using a well-established yeast model for PD, we have shown that the oligomerization and aggregation of heterologously expressed human αSyn was accompanied by an acidification of the cytosol and a severe reduction of the proteolytic activity of Pep4, the yeast orthologue of CatD. These toxic consequences of αSyn ultimately resulted in cell death. The reduction of yeast CatD activity was at least in part due to insufficient endosomal sorting and trafficking of this protease into the vacuole (the yeast counterpart to lysosomes), as we observed mislocalization of both CatD and its sorting receptor Pep1/Vps10, the yeast analogue of the mannose-6-phosphat (M6P) receptor. Upon high-level expression of αSyn, Pep1 was retained at the vacuolar membrane instead of being recycled back to the trans-Golgi network for iterative rounds of shuttling, consequently leading to mislocalization of yeast CatD in prevacuolar compartments. High dosage of yeast CatD compensated the αSyn-driven loss of vacuolar proteolytic capacity, decreased αSyn oligomers and aggregates, prevented the acidification of the cytosol and ultimately inhibited cell death.
Interestingly, these cytoprotective effects of yeast CatD required functional calcineurin signalling. Although the existence of a CatD-calcineurin interplay remains to be validated in higher eukaryotic cells, an evolutionary conservation of a coordinated action of these proteins seems likely, since the function of each protein per se is highly conserved between yeast and humans. While disturbances in Ca2+ homeostasis and signalling have been connected to all major proteinopathies (Shah et al., 2017), the exact role of calcineurin in neurotoxicity is controversially discussed and both positive and negative effects of its phosphatase activity were reported in various models of neurodegenerative disorders (Kipanyula et al., 2016). In yeast and neuronal cell culture models of PD, insufficient as well as excessive calcineurin activity has been shown to be fatal, while moderate activity levels counteracted αSyn cytotoxicity (Caraveo et al., 2014). Our recent findings might also indirectly indicate that calcineurin signalling is protective in the presence of high CatD activity. Genetic inactivation of calcineurin via deletion of CNB1, the gene coding for the regulatory calcineurin subunit, caused a prominent mislocalization of both yeast CatD and its sorting receptor Pep1. Comparable to the effects of αSyn expression, this resulted in decreased vacuolar CatD activity (Aufschnaiter et al., 2017). Thus, calcineurin is essential for efficient endosomal trafficking and recycling, which might contribute to the cytoprotective effects of moderate calcineurin activity observed in cellular PD models.
The Retromer as Nexus between CatD and Calcineurin in PD?
Interestingly, mislocalization of M6P receptors such as Pep1 at the lysosomal/vacuolar membrane points towards defects in its retrieval and recycling, a process requiring the so-called retromer complex. This highly conserved multimeric protein complex, originally discovered in yeast, consists of two parts: the trimeric cargo-selective complex, composed of Vps26, Vps29 and Vps35, and a sorting nexin dimer (Seaman et al., 1997; Cullen and Korswagen, 2011). The retromer represents a master regulator of endosomal sorting and governs the recycling of distinct transmembrane proteins and receptors from endosomes back to either the trans-Golgi network or the plasma membrane. Thus, deregulated retromer activity perturbs protein trafficking and impairs lysosomal function, partly due to insufficient delivery of acid hydrolases (Arighi et al., 2004). The point mutation D620N in the retromer subunit Vps35, which is responsible for cargo recognition, has been linked to familial and sporadic late-onset forms of PD (Zimprich et al., 2011). The D620N mutation as well as Vps35 deficiency have been shown to cause sorting defects of M6P receptors and in consequence insufficient lysosomal delivery of its ligand CatD (Follett et al., 2014). Still, the mechanisms underlying Vps35-associated PD phenotypes seem to be rather complex, as not only Vps35 deficiency and mutation but also high levels of Vps35 and pathogenic variants have been linked to neuronal degeneration in various cellular and animal models (for recent reviews, see (McMillan et al., 2017; Williams et al., 2017)). For instance, loss of Vps35 function has been shown to enhance Syn aggregation and toxicity in PD models ranging from yeast and fly to rodents, while in other studies, high levels of Vps35 or of the pathogenic variant D620N triggered neurodegeneration. The D620N mutation does not interfere with retromer assembly or stability, and several cargo proteins are thought to be sorted correctly, indicating that probably rather discrete effects involving specific ligands or cellular destinations are involved (Williams et al., 2017). Thus, depending on the cellular model and scenario used, slight alteration or impediment of retromer activity might have different outcomes.
While we show that calcineurin contributes to correct endosomal sorting of the yeast M6P receptor Pep1 and thus sufficient CatD activity in the vacuole (Aufschnaiter et al., 2017), the molecular underpinnings of this cytoprotective effect remain to be investigated. As the lack of calcineurin causes a sorting defect frequently observed in mutants defective in endosomal recycling due to mutations in retromer subunits, it might well be that calcineurin is directly or indirectly involved in the fine-tuning of retromer activity and/or assembly. High levels of αSyn might interfere with this regulatory circuit, thus impairing efficient endosomal protein sorting and, in consequence, reducing lysosomal proteolytic capacity. Furthermore, the localization of proton transporters at the plasma membrane and the limiting vacuolar membrane might be affected by these changes in endosomal sorting and recycling, leading to the observed acidification of the cytosol. As an acidic environment has been shown to increase the aggregation propensity of αSyn, this drop in cytosolic pH might subsequently enhance αSyn aggregation and might thereby reinforce its detrimental effects. High-level expression of yeast CatD reinstalled proper vacuolar proteolytic capacity and counteracted αSyn toxicity in wild type cells, while mutants lacking functional calcineurin were unable to deliver and/or activate CatD properly. Thus, at least basal calcineurin activity is required for efficient M6P receptor-mediated sorting of yeast CatD to the vacuole (Aufschnaiter et al., 2017). As mentioned above, moderate activation of calcineurin has been shown to be neuroprotective, while deficiency as well as hyperactivation were neurotoxic in various models, including yeast, C. elegans, neuronal cell culture, mice and post-mortem samples of PD patients (Kipanyula et al., 2016). Whether these pleiotropic effects of calcineurin on neuronal (dys)function involve a regulation of retromer activity, alternative endosomal sorting mechanisms or cellular processes such as cytoskeleton dynamics necessary for proper transport of endosomes remains to be investigated. Further research will be needed to investigate a potential connection between calcineurin, CatD trafficking and the retromer complex in PD and other proteinopathies.
While our results indicate that CatD-mediated cytoprotection against αSyn toxicity does not require the autophagic machinery (Aufschnaiter et al., 2017), the autophagy-lysosome system has a crucial role in the pathophysiology of PD (Beilina and Cookson, 2016). Many PD-associated gene products, including αSyn, LRRK2, Vps35, glucocerobrosidase, PINK1, Parkin, Fbox7 and ATP13A2 have a physiological role in distinct subtypes of autophagy or differentially influence this degradative pathway. Furthermore, at least some of these proteins also affect CatD function.
Mutation or depletion of ATP13A2, a lysosomal ATPase connected to distinct forms of PD, has been shown to cause severe lysosomal alterations in dopaminergic neuronal cell lines and PD patient samples, ranging from diminished lysosomal acidification and impairment of autophagy to reduced proteolytic capacity resulting from abnormal CatD processing (Dehay et al., 2012). High levels of pathogenic variants of the leucine-rich repeat kinase 2 (LRRK2) drive the formation of enlarged and non-functional lysosomes with reduced proteolytic activity and decreased pH. Interestingly, these LRRK2-induced changes were associated with an upregulation of ATP13A2 (Henry et al., 2015). As mutations in LRRK2 are frequently accompanied by additional variations in PD-associated gene products with lysosomal function such as ATP13A2, one might speculate that increased lysosomal burden due to these additional genetic alterations might cause penetrance of LRRK2 mutations in PD (Lubbe et al., 2016). Comparable to the effects of high levels of αSyn, LRRK2 has been shown to influence the retromer complex, and overexpression of Vps35 protected against the toxic consequences of pathogenic LRRK2 variants (MacLeod et al., 2013).
CatD, Calcineurin and the Retromer: a Common Theme in Proteinopathies
CatD, the retromer complex and calcineurin have also been implicated in the pathophysiology of AD, though no connection between this Ca2+-activated phosphatase and endosomal sorting has been established so far. However, in cell culture and mouse models, calcineurin has been shown to be involved in vesicle endocytosis as well as in the regulation of autophagy via lysosomal Ca2+ signalling (Wu et al., 2014; Medina et al., 2015), and disturbances of these processes are tightly linked to AD pathogenesis. AD is associated with abnormal processing of the amyloid precursor protein, leading to amyloid beta deposition as well as the hyperphosphorylation and intracellular accumulation of the microtubule-associated protein tau. Evidence for a role of CatD in AD came from studies that identified CatD immunoreactivity in neuritic plaques, histological hallmarks of AD (Bernstein et al., 1989; Cataldo et al., 1990, 1995). CatD is suggested to provide neuroprotection by preventing excessive tau accumulation and in line, CatD deficiency causes lysosomal expansion and an enrichment of C-terminally truncated tau variants that promote neurotoxicity (Khurana et al., 2010). Furthermore, a dysfunction of the retromer complex has been reported to cause increased pathogenic processing of the amyloid precursor protein, microglial abnormalities and a reduction of CatD levels, which in turn reinforces toxicity mediated by C-terminally truncated tau (Small and Petsko, 2015). The hyperphosphorylation of tau, a common feature of AD and, to some extent, also of HD pathology, might be directly influenced by calcineurin. This phosphatase has been shown to directly bind to and dephosphorylate tau, thereby regulating microtubule system dynamics (Hoffman et al., 2017). In this line, a reduction of calcineurin activity was reported in post-mortem samples of AD patients. However, as described for PD above, controversial studies exist, describing an upregulation of calcineurin activity in AD patients (Reese and Taglialatela, 2011). These findings not only suggest an important role of CatD and the retromer complex in this pathology, but also indicate a complex role of calcineurin in AD. Since alterations in CatD levels were also observed for other proteinopathies including HD and ALS (Stoka et al., 2016), this protease plays a central role in different neurodegenerative diseases and thus, dysregulation of proteins and processes contributing to its proper trafficking, such as the retromer complex and potentially calcineurin, impact their pathophysiologies.
Outlook
Taken together, impairment of lysosomal function represents a key event in neurodegenerative diseases. Even though the malfunction of a diverse set of proteins triggers the pathogenesis of these disorders, many of them directly or indirectly reduce the proteolytic activity of CatD and/or impair lysosomal function (Figure 1). A sophisticated machinery that ensures proper targeting and activation of CatD, including the retromer complex, is required for efficient breakdown of proteins delivered to the lysosomes via autophagy and endosomes and thus represents a common target of different proteinopathies. While we demonstrate that calcineurin activity essentially contributes to this process in a yeast model for PD, it remains to be evaluated whether this regulatory axis is conserved in higher eukaryotes and if it impacts neurodegenerative diseases beyond PD.
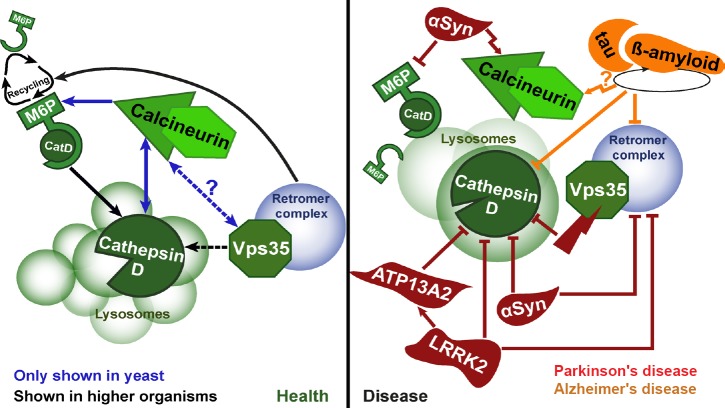
Figure 1.
Interplay of cathepsin D (CatD), calcineurin and the retromer in health and neurodegenerative diseases.
In healthy cells (left panel), a sophisticated and evolutionary conserved machinery governs the targeting and processing of CatD into lysosomes. CatD is an aspartyl protease important for the degradation of cargo delivered to lysosomes and thus contributes to cellular protein homeostasis. The retromer complex functions as a master regulator of endosomal sorting and facilitates correct trafficking and recycling of the mannose-6-phosphat (M6P) receptor, the major sorting receptor for CatD. We could show that at least basal levels of the Ca2+/calmodulin-dependent phosphatase calcineurin are required for efficient sorting of M6P and CatD in yeast, leading to speculations that calcineurin might contribute to the regulation of the retromer (Aufschnaiter et al., 2017). Blue arrows indicate pathways only identified in yeast so far; black arrows represent pathways established in yeast and higher eukaryotes; arrows with question marks reflect potential but unverified pathways. In neurodegenerative diseases (right panel), multiple proteins and pathogenic variants associated with these disorders (here illustrated for Parkinson's disease and Alzheimer's disease related gene products) have been shown to impair sorting processes of CatD via the M6P receptor and the retromer, ultimately resulting in reduced protease activity. This reduction in lysosomal degradative capacity is accompanied by morphological alterations, resulting in a reduced number of lysosomes and lysosomal enlargement. Dysregulation of calcineurin signalling is frequently observed in different neurodegenerative diseases and we reported an essential role of calcineurin in CatD-mediated cytoprotection against αSyn toxicity. In aggregate, a complex interplay of calcineurin, CatD and the retromer might essentially contribute to lysosomal function and thus neuronal health. Bar-headed lines represent pathways that are inhibited in the course of Parkinson's disease (red) or Alzheimer's disease (orange). Bar-headed lines with question marks reflect potential but unverified interferences.
Footnotes
Funding: This work was supported by the Austrian Science Fund FWF (No. P27183-B24), the Swedish Research Council Vetenskapsrådet (No. 2015-05468), Åke Wiberg Stiftelse (No. M16-0130), Carl Trygger Stiftlese (No. CTS16:85), and Goljes Stiftelse (No. LA2016-0123).
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Hans-Gert Bernstein, Otto von Guericke University Magdeburg, Germany.
References
- 1.Aufschnaiter A, Habernig L, Kohler V, Diessl J, Carmona-Gutierrez D, Eisenberg T, Keller W, Büttner S. The coordinated action of calcineurin and cathepsin D protects against α-synuclein toxicity. Front Mol Neurosci. 2017;10:207. doi: 10.3389/fnmol.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 4.Beilina A, Cookson MR. Genes associated with Parkinson's disease: regulation of autophagy and beyond. J Neurochem 139 Suppl. 2016;1:91–107. doi: 10.1111/jnc.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benes P, Vetvicka V, Fusek M. Cathepsin D--many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein HG, Bruszis S, Schmidt D, Wiederanders B, Dorn A. Immunodetection of cathepsin D in neuritic plaques found in brains of patients with dementia of Alzheimer type. J Hirnforsch. 1989;30:613–618. [PubMed] [Google Scholar]
- 7.Caraveo G, Auluck PK, Whitesell L, Chung CY, Baru V, Mosharov EV, Yan X, Ben-Johny M, Soste M, Picotti P, Kim H, Caldwell KA, Caldwell GA, Sulzer D, Yue DT, Lindquist S. Calcineurin determines toxic versus beneficial responses to alpha-synuclein. Proc Natl Acad Sci U S A. 2014;111:E3544–3552. doi: 10.1073/pnas.1413201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cataldo AM, Thayer CY, Bird ED, Wheelock TR, Nixon RA. Lysosomal proteinase antigens are prominently localized within senile plaques of Alzheimer's disease: evidence for a neuronal origin. Brain Res. 1990;513:181–192. doi: 10.1016/0006-8993(90)90456-l. [DOI] [PubMed] [Google Scholar]
- 9.Cataldo AM, Barnett JL, Berman SA, Li J, Quarless S, Bursztajn S, Lippa C, Nixon RA. Gene expression and cellular content of cathepsin D in Alzheimer's disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron. 1995;14:671–680. doi: 10.1016/0896-6273(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 10.Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2011;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, Vital A, Vila M, Klein C, Bezard E. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A. 2012;109:9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Follett J, Norwood SJ, Hamilton NA, Mohan M, Kovtun O, Tay S, Zhe Y, Wood SA, Mellick GD, Silburn PA, Collins BM, Bugarcic A, Teasdale RD. The Vps35 D620N mutation linked to Parkinson's disease disrupts the cargo sorting function of retromer. Traffic. 2014;15:230–244. doi: 10.1111/tra.12136. [DOI] [PubMed] [Google Scholar]
- 13.Henry AG, Aghamohammadzadeh S, Samaroo H, Chen Y, Mou K, Needle E, Hirst WD. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum Mol Genet. 2015;24:6013–6028. doi: 10.1093/hmg/ddv314. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman A, Taleski G, Sontag E. The protein serine/threonine phosphatases PP2A, PP1 and calcineurin: A triple threat in the regulation of the neuronal cytoskeleton. Mol Cell Neurosci. 2017;84:119–131. doi: 10.1016/j.mcn.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, Mulkearns E, Tyynela J, Scherzer CR, Feany MB. Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet. 2010;6:e1001026. doi: 10.1371/journal.pgen.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kipanyula MJ, Kimaro WH, Seke Etet PF. The emerging roles of the calcineurin-nuclear factor of activated T-lymphocytes pathway in nervous system functions and diseases. J Aging Res. 2016;2016:5081021. doi: 10.1155/2016/5081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubbe SJ, Escott-Price V, Gibbs JR, Nalls MA, Bras J, Price TR, Nicolas A, Jansen IE, Mok KY, Pittman AM, Tomkins JE, Lewis PA, Noyce AJ, Lesage S, Sharma M, Schiff ER, Levine AP, Brice A, Gasser T, Hardy J, et al. Additional rare variant analysis in Parkinson's disease cases with and without known pathogenic mutations: evidence for oligogenic inheritance. Hum Mol Genet. 2016;25:5483–5489. doi: 10.1093/hmg/ddw348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillan KJ, Korswagen HC, Cullen PJ. The emerging role of retromer in neuroprotection. Curr Opin Cell Biol. 2017;47:72–82. doi: 10.1016/j.ceb.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reese LC, Taglialatela G. A role for calcineurin in Alzheimer's disease. Curr Neuropharmacol. 2011;9:685–692. doi: 10.2174/157015911798376316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SZ, Hussain T, Zhao D, Yang L. A central role for calcineurin in protein misfolding neurodegenerative diseases. Cell Mol Life Sci. 2017;74:1061–1074. doi: 10.1007/s00018-016-2379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Small SA, Petsko GA. Retromer in Alzheimer disease. Parkinson disease and other neurological disorders. Nat Rev Neurosci. 2015;16:126–132. doi: 10.1038/nrn3896. [DOI] [PubMed] [Google Scholar]
- 25.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 26.Stoka V, Turk V, Turk B. Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Res Rev. 2016;32:22–37. doi: 10.1016/j.arr.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Vidoni C, Follo C, Savino M, Melone MA, Isidoro C. The role of cathepsin D in the pathogenesis of human neurodegenerative disorders. Med Res Rev. 2016;36:845–870. doi: 10.1002/med.21394. [DOI] [PubMed] [Google Scholar]
- 28.Wagner L, Wolf R, Zeitschel U, Rossner S, Petersén Š, Leavitt BR, Kästner F, Rothermundt M, Gärtner UT, Gündel D, Schlenzig D, Frerker N, Schade J, Manhart S, Rahfeld JU, Demuth HU, von Hörsten S. Proteolytic degradation of neuropeptide Y (NPY) from head to toe: Identification of novel NPY-cleaving peptidases and potential drug interactions in CNS and Periphery. J Neurochem. 2015;135:1019–1037. doi: 10.1111/jnc.13378. [DOI] [PubMed] [Google Scholar]
- 29.Williams ET, Chen X, Moore DJ. VPS35, the retromer complex and Parkinson's disease. J Parkinsons Dis. 2017;7:219–233. doi: 10.3233/JPD-161020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu XS, Zhang Z, Zhao WD, Wang D, Luo F, Wu LG. Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 2014;7:982–988. doi: 10.1016/j.celrep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]