Abstract
The complex pathophysiology of spinal cord injury may explain the current lack of an effective therapeutic approach for the regeneration of damaged neuronal cells and the recovery of motor functions. Many efforts have been performed to design and develop suitable scaffolds for spinal cord regeneration, keeping in mind that the reconstruction of a pro-regenerative environment is the key challenge for an effective neurogenesis. The aim of this review is to outline the main features of an ideal scaffold, based on biomaterials, produced by the electrospinning technique and intended for the spinal cord regeneration. An overview of the polymers more investigated in the production of neural fibrous scaffolds is also provided.
Keywords: spinal cord injury, biomaterials, electrospun fibers, conductive scaffolds, morphological properties, biodegradability
Introduction
Spinal cord injury (SCI) is a highly debilitating pathology that results in devastating and, in most cases, irreversible sensory, motor and autonomic disabilities.
Huge emotional, social and financial costs of SCI solicit the development of more effective therapeutic strategies, considering that, nowadays, the treatment of SCI is largely palliative, modulating secondary complications and promoting functional recovery by rehabilitation (Oyinbo, 2011; Ramer et al., 2014).
SCI is, generally, caused by a mechanical trauma that exerts compression on the spinal cord, triggering, in a few minutes, a cascade of cellular, biochemical and vascular events. Such secondary injury responses induce an enlargement of the area of damage, contributing to the formation of a glial scar, which acts as both physical and chemical barrier to any further attempts to promote axonal regeneration. Moreover, secondary injury events, particularly oxidative stress and glutamate excitotoxicity, promote an extensive oligodendrocyte apoptosis and, thus, a widespread demyelination of axons, which are no longer able to guarantee a rapid salutatory conduction (Almad et al., 2011; Oyinbo, 2011; Plemel et al., 2014; Silva et al., 2014).
Therefore, the spinal cord regeneration represents a current challenge for the researchers in the biomedical and pharmaceutical field. Following SCI, the harsh microenvironment produced by the recruitment of pro-inflammatory and pro-apoptotic signalling molecules and the disruption of the native spinal cord architecture limits endogenous mechanisms able to promote neural repair and functional recovery. Moreover, at a chronic stage of the pathology, the high complexity of the post-injury milieu hinders the possibility for any therapeutic interventions to be really effective in the treatment of SCI (Kabu et al., 2015; Ahuja and Fehlings, 2016).
Current neuroregenerative therapies propose the use of biomaterials to repair the broken neuronal circuitry of the injured spinal cord. Implantable biomaterials can be mainly used to regenerate a damaged area of the spinal cord, bridging the formed gap and acting as support for axonal re-growth. These scaffolds represent also promising substrates for the transplantation of somatic and/or neural stem cells in SCI microenvironment and excellent systems for the delivery of neuroprotective drugs and bioactive molecules (Tian et al., 2015; Faccendini et al., 2017; Ziemba and Gilbert, 2017). Scaffolds based on biomaterials have been demonstrated a successful alternative to natural nerve guides, such as autografts, allografts or xenografts, reducing the risk of an inflammatory reaction and avoiding the potential loss of sensation and function at donor sites. Another advantage is that they could be developed with tailored physical, chemical and mechanical properties based on the application to which they are designed (Cao et al., 2009).
A successful approach in the design of neural biomaterials is to mimic the native extracellular matrix (ECM) of the spinal cord, providing an artificial pro-regenerative environment at the injury site to facilitate neural repair. According to biomimetic principles, an ideal neural scaffold should combine appropriate physicochemical, biochemical, mechanical, topographical and electrical cues, which could enhance and guide axon migration and extension. Based on ECM composition, mechanical framework and biochemical interactions, fibrous polymeric scaffolds are recognized as the most promising implanted biomaterials for spinal cord regeneration. In particular, the large surface area-to-volume ratio of a fibrous membrane maximizes the contact with the surrounding cells. Moreover, the architecture of the fibrous scaffolds can act as a guide for the axonal extension (Raspa et al., 2016; Sensharma et al., 2017).
In the last decades, electrospinning has emerged as a compelling technique for the production of biomimetic fibrous scaffolds with controlled architectural properties, such as morphology, geometry and topography. Many reviews have been recently published on the electrospinning process, proposing the use of electrospun fibers in several biomedical applications, such as in spinal cord injury regeneration (Guo et al., 2014; Johnson et al., 2016; Schaub et al., 2016; Faccendini et al., 2017). The basic principle of this method is to apply a high-voltage supply to overcome the surface tension of a polymeric solution, which flows through a syringe needle tip, in order to induce a jet formation and, thus, the deposition of dried fibers on grounded collector. Processing (applied voltage, flow rate and spinneret-collector distance), solution (polymer concentration, viscosity, surface tension and conductivity) and environmental (temperature and humidity) parameters can affect electrospun fiber diameters and orientation, which in turn influence cell migration, proliferation and differentiation (Rogina, 2014; Haider et al., 2015).
The aim of this review is to outline the main features of an ideal scaffold, based on biomaterials, produced by the electrospinning technique and intended for the spinal cord regeneration. An overview of the polymers more investigated in the production of neural fibrous scaffolds is also provided. Moreover, the development of electrically conductive scaffolds that represent a novel strategy for neural application is discussed.
Biomaterials
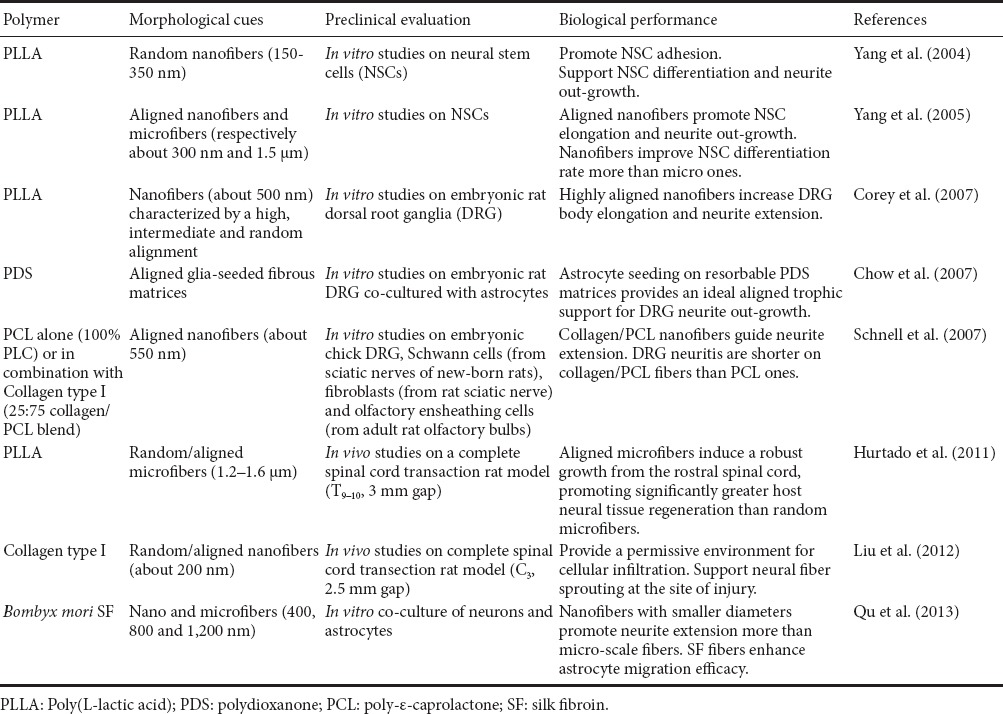
A wide range of natural and synthetic polymers and/or their blends could be electrospun (Schiffman and Schauer, 2008); fiber chemical composition, geometry and surface modifications can influence their biocompatibility, degradation kinetics and mechanical properties, as well as their biological performance (Kennedy et al., 2017). In the context of SCI treatment, the most promising polymers used for the development of electrospun fibers were summarized in Table 1; a brief comment of the characteristics of the resulting fibers was also provided.
Table 1.
Polymers and/or their blends used for the production of electrospun fibers for the treatment of spinal cord injury
Therefore, desirable implantable electrospun fibers can be developed for spinal cord regeneration following a list of fundamental criteria, which are related to each other (Figure 1).
Figure 1.
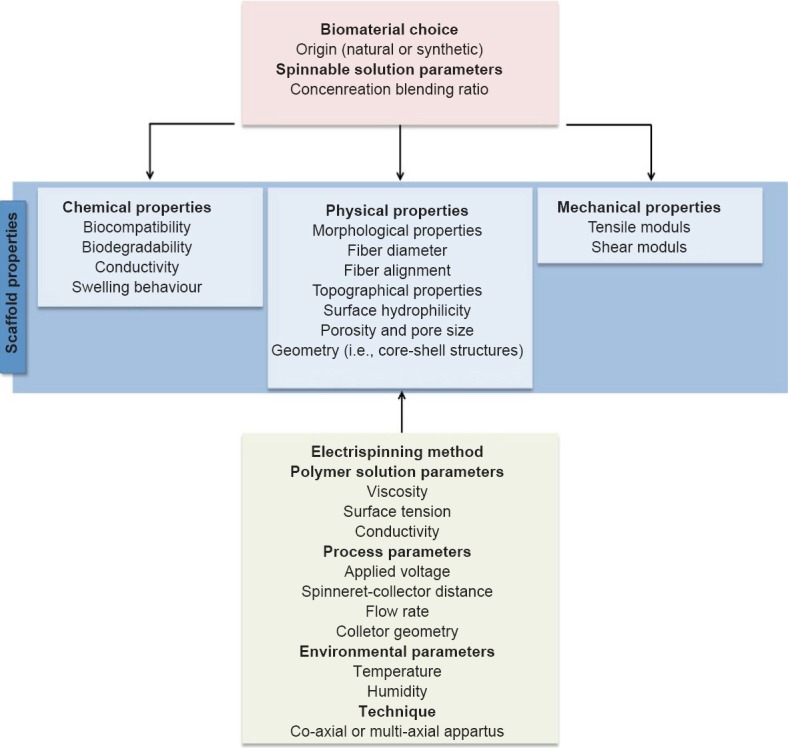
Schematic representation of design criteria of electrospun fibrous scaffolds for the treatment of spinal cord injury.
Biocompatibility and biodegradability
Electrospun fibers must be biocompatible specifically with the spinal cord environment. The success and the long-term effectiveness of implanted scaffolds, particularly at the injury site, are strictly correlated to their biocompatibility and, thus, to their chemical composition. Both natural and synthetic polymers are identified as suitable biomaterials for the design and production of fibrous scaffolds via electrospinning. Natural polymers, such as collagen, gelatin, laminin and chitosan, are generally preferred due to their similarity with the soft tissue components in terms of biomolecular recognition sites, physical and mechanical properties. On the other hand, synthetic polymers, such as polyesters and their co-polymers, appear attractive materials for neural tissue engineering purposes due to the possibility of tailoring their degradation kinetic and mechanical properties. Lots of studies, both in vitro and in vivo, have confirmed the ability of the electrospun fibers to act as permissive substrates for axons to penetrate the injured area, without eliciting any immune or inflammatory reaction that could compromise tissue regeneration (Cao et al., 2009).
In the context of neural tissue regeneration, the biological performance of scaffolds depends also on its degradation rate. Electrospun fibers, intended for spinal cord repair, may be designed in order to show a controlled biodegradability, which is strictly related to the nature, concentration and blending ratio of their components. The use of biodegradable polymers eliminates the need for a surgical removal of the implanted scaffold: re-growing axons migrate and extend within the framework of electrospun fibers, which slowly degrades in harmony with the regeneration of new tissues. Such scaffolds act as temporary substrates to fill the formed gap in the injured spinal cord: the peculiar morphology and porosity of implanted electrospun fibers enhance neighbouring cell infiltration and ECM deposition within their structure, which gradually lose its integrity leading to degradation products that can be resorbed by the surrounding tissues. Degradation kinetics of fibrous scaffolds may be accelerated by their large surface area-to-volume ratio that maximizes the contact with the biological fluids in the spinal cord environment. Electrospun fiber geometry (multi-axial fibers) and surface modifications could also affect their degradation profile (Sensharma et al., 2017).
Morphological features
Electrospun fibers have been demonstrated as effective therapeutic platforms in neural tissue regeneration due to their unique morphological features: fiber orientation and diameter, recognized as the parameters that mainly affect axon behaviour, can be tailored by tuning electrospinning parameters and polymeric solution properties. Aligned fibrous microstructures are designed to guide alignment and elongation of regenerating cells and to induce neural differentiation of stem cells at the site of spinal cord injury (Schaub et al., 2016).
Several studies have evidenced that highly aligned electrospun fibers enhance neurite outgrowth more than random-oriented ones, confirming that alignment patterns, closely mimicking the neural ECM, are pivotal in directing neural cell phenotype towards spinal cord regeneration (Schaub et al., 2016). The desirable fiber alignment can be obtained by using a rotating cylinder as collector, setting up an adequate rotating speed (Rogina, 2014).
Lots of evidence reveals that alterations in neural cell phenotype can be observed after fiber diameter modifications. Different in vitro studies have investigated the effect of fiber diameters, within nano- to micro-scale, on cell morphology as well as on cytoskeletal arrangement and focal adhesion localization: a more robust neurite outgrowth was induced on aligned electrospun fibers with diameters in the range of few microns (1–2 μm), if compared to nanofibrous scaffolds (Johnson et al., 2016; Kennedy et al., 2017). Dorsal root ganglia cultures have been routinely used to investigate the effects of fiber morphology on neurite extension. From a review of literature, it has been evidenced that i) random-oriented fibers, with diameters larger than 750 nm, induce neurite outgrowth in all directions, ii) aligned fibers, with diameters larger than 750 nm, strongly guide neurite extension, iii) aligned fibers, with diameters smaller than 750 nm, direct neurite elongation to a lesser extend (Schaub et al., 2016).
Moreover, micro and nanofibrous scaffolds exhibit a large surface area-to-volume ratio, which makes them effective delivery systems for neuroprotective drugs. These morphological properties ensure that any biochemicals (such as drugs and/or growth factors), incorporated into fiber matrix, can be efficiently release at the injury site and optimize the contact between the fibers and the damaged cells, favouring chemical uptake (Cao et al., 2009).
As above-mentioned, electrospun fiber morphology depends on the properties of the polymer solution, such as rheological properties, surface tension and conductivity. In our opinion, a design of experiments (DoE) approach could be a useful tool to investigate, on a statistical basis, the role of each polymeric solution parameter on the production of homogeneous fibers having optimal morphological properties. Such an approach could lead to draw up guidelines to be used for fiber production.
To date, no studies on the influence of viscoelasticity of polymer solution on electrospun fiber morphological cues are reported in literature. This evaluation is legitimate particularly when polymers (such as poly(ethilen oxide)) with well-known viscoelastic properties were electrospun alone or in combination with other polymers.
Porosity and pore size
In the context of neural tissue engineering, scaffold performance is extensively influenced by porosity and pore size, which, in turn, affect cell migration and proliferation, vascularization, mechanical stability, biochemical diffusion, nutrient flow and waste product removal. An interconnected architectural template is required to create a pro-regenerative environment at the spinal cord injury site, in which neurites can outgrowth. Porosity, in addition to size and alignment, contributes to define electrospun fiber density that is crucial for cell infiltration. Aligned nanofibers are generally characterized by pores in the order of 1 μm, which are prohibitively small for any regeneration process. In this case, fiber porosity can affect cell phenotype: when void size is too small, cells adhere on the rough surface of the scaffold with amoeboid movements, squeezing their cytoskeleton (Madigan et al., 2009; Kennedy et al., 2017; Sensharma et al., 2017).
Swelling properties
After implantation, electrospun fibers are exposed to the moist environment of spinal cord injury: cerebral spinal fluid freely flows within the network of fibrous scaffolds that swell producing a kind of hydrogel materials. An efficient neural scaffold must be designed in order to preserve its structural features, particularly an appropriate fiber alignment, even after hydration, to guarantee a temporary guide for regenerative axon migration and extension. Electrospun fiber swelling degree and rate depend on their chemical composition and, in turn, affect scaffold degradation. Polymers, with the tendency to form hydrogels, represent attractive materials for spinal cord applications. After swelling, fibrous scaffolds, based on these polymers, result soft enough to adequately fill the formed gap and not compress surrounding neural tissues. The swelling properties could also influence scaffold permeability to oxygen and nutrients, of which cerebral spinal fluid is poor. This is a crucial element to evaluate, particularly in designing tissue engineered scaffolds, which have to support cell metabolism and, thus, viability, prior and after implantation (Madigan et al., 2009; Kabu et al., 2015).
Mechanical properties
For an effective neuroregenerative intervention, scaffolds should be designed in order to exhibit similar mechanical properties to the native tissue ones. Chemical composition, morphology, geometry and porosity can influence the mechanical behaviour of a tissue engineered scaffold and, thus, its stability and effectiveness. Electrospun fibers display unique mechanical parameters, such as tensile and shear moduli, which seem to increase with fiber diameter decreasing. It has been hypothesized that this phenomenon is correlated to the higher crystallinity of nano-scale fibers with respect to micro-scale ones due to a higher macromolecular chain alignment reached by nanofibers during electrospinning.
A desirable scaffold for spinal cord application must resemble the anisotropic mechanical properties of neural tissue: cells sense the mechanical cues of the electrospun fibers through cell surface mechanoreceptors and grow along the direction of fiber alignment. Fibrous scaffolds must be strong enough to support and drive cell attachment and migration. The incorporation of crosslinking polymers, in different concentrations and blending ratios, can improve electrospun fiber strength and stiffness, but also their surface roughness and hydrophilicity and, thus, cell behaviour. As alternative, multi-axial electrospinning methods provide an efficient method for producing fibers with improved mechanical properties, without altering surface chemistry (Kennedy et al., 2017).
Electrical cues
Recently, a growing interest in bioelectricity leads to the development of electrically conductive scaffolds for neural applications. It is well known that neurons are highly responsive to electrical stimuli: the axon is responsible for the transmission of the electrical signal, which affects ion influx across neuronal membrane, inducing a membrane potential alteration and, thus, interference in the intracellular signal transduction pathways. Several studies, both in vitro and in vivo, have demonstrated that the application of an electrical stimulation could affect the rate and the orientation of neurite outgrowth as well as stem cell neuronal differentiation. In this context, the use of electrically conducting polymers, particularly polypirrole (Ppy) and polyaniline, appears particularly attractive: these materials simultaneously provide the physicochemical properties of organic polymers and the electrical cues of metals. Conducting polymer-based scaffolds, including electrospun fibers, can be act as architectural template through which the electrical stimulation could be applied to cells. The only drawback of these materials is the difficulty associated to their processing, particularly through an electrospinning apparatus. Different research groups have investigated the possibility of blending electrically conducting polymers with spinnable ones (such as poly(ethilenoxide) or polystyrene), even if compromising their conductive and morphological properties (Ghasemi-Mobarakeh et al., 2011; Balint et al., 2014). Tian et al. (2016) designed and produced an electrically conductive nanofibrous scaffold by electrospinning a blend of poly(lactic acid) (PLA) and Ppy. The obtained electrospun nanofibers were well characterized in terms of conductivity, surface and topography properties and, then, demonstrated to be able to support the adhesion, proliferation and differentiation of PC12 cells. Moreover, in presence of pulsed electrical stimulation, PLA-Ppy scaffold induced a more pronounced cell differentiation as evidenced by longer neurite extension (Tian et al., 2016). Another method to exploit the peculiar properties of conductive polymers is to use them for the coating of electrospun fibers (Balint et al., 2014). According to this strategy, Aznar-Cervantes et al. (2012) produced electrospun silk fibroin fibers coated with Ppy, which were demonstrated to be a suitable support for the adhesion and proliferation of both human mesenchymal stem cells and fibroblasts.
Conclusions
Several research groups have investigated the feasibility of electrospun fibers as scaffolds for neural tissue applications, outlining the main features that they must exhibit to guarantee an effective intervention. In the context of spinal cord regeneration, electrospun fibrous scaffolds, based on biomaterials, could be considered promising therapeutic platforms, if designed according to well-defined criteria. Firstly, they must be biocompatible and characterized by a degradation rate in equilibrium with neurite migration and extension. Secondly, they must be soft enough to not compress surrounding growing tissues, but sufficiently strong to support cell activities. Finally, they must display suitable topographical and conductive properties to enhance neuronal proliferation.
Many efforts have been performed to design and develop suitable scaffolds for spinal cord regeneration, keeping in mind that the reconstruction of a pro-regenerative environment is the key challenge for an effective neurogenesis.
Acknowledgments
BV conceived and wrote the review. SR helped in manuscript writing and reviewed the final version of the manuscript. FF reviewed the final version of the manuscript. GS and MCB helped in bibliographic research.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Ahuja CS, Fehlings M. Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies inspinal cord injury. Stem Cells Transl Med. 2016;5:914–924. doi: 10.5966/sctm.2015-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almad A, Sahinkaya FR, McTigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8:262–273. doi: 10.1007/s13311-011-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aznar-Cervantes S, Roca MI, Martinez JG, Meseguer-Olmo L, Cenis JL, Moraleda JM, Otero TF. Fabrication of conductive electrospun silk fibroin scaffolds by coating with polypyrrole for biomedical applications. Bioelectrochemistry. 2012;85:36–43. doi: 10.1016/j.bioelechem.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10:2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Cao H, Liu T, Chew SY. The application of nanofibrous scaffolds in neural tissue engineering. Adv Drug Deliv Rev. 2009;61:1055–1064. doi: 10.1016/j.addr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Chow WN, Simpson DG, Bigbee JW, Colello RJ. Evaluating neuronal and glial growth on electrospun polarized matrices: bridging the gap in percussive spinal cord injuries. Neuron Glia Biol. 2007;3:119–126. doi: 10.1017/S1740925X07000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL, Martin DC. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A. 2007;83:636–645. doi: 10.1002/jbm.a.31285. [DOI] [PubMed] [Google Scholar]
- 8.Faccendini A, Vigani B, Rossi S, Sandri G, Bonferoni MC, Caramella CM, Ferrari F. Nanofiber scaffolds as drug delivery systems to bridge spinal cord injury. Pharmaceuticals (Basel) 2017;10:E63. doi: 10.3390/ph10030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Eng Regen Med. 2011;5:e17–35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 10.Guo JS, Qian CH, Ling EA, Zeng YS. Nanofiber scaffolds for treatment of spinal cord injury. Curr Med Chem. 2014;21:4282–4289. doi: 10.2174/0929867321666140815124648. [DOI] [PubMed] [Google Scholar]
- 11.Haider A, Haider S, Kang IK. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem. 2015 doi: 10.1016/j.arabjc.2015.11.015. [Google Scholar]
- 12.Hurtado A, Cregg JM, Wang HB, Wendell DF, Oudega M, Gilbert RJ, McDonald JW. Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials. 2011;32:6068–6079. doi: 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson CD, D’Amato AR, Gilbert RJ. Electrospun fibers for drug delivery after spinal cord injury and the effects of drugincorporation on fiber properties. Cells Tissues Organs. 2016;202:116–135. doi: 10.1159/000446621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabu S, Gao Y, Kwon BK, Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J Control Release. 2015;219:141–154. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy KM, Bhaw-Luximon A, Jhurry D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance. Acta Biomater. 2017;50:41–55. doi: 10.1016/j.actbio.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Houle JD, Xu J, Chan BP, Chew SY. Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng Part A. 2012;18:1057–1066. doi: 10.1089/ten.tea.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madigan NN, McMahon S, O’Brien T, Yaszemski MJ, Windebank AJ. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir Physiol Neurobiol. 2009;169:183–199. doi: 10.1016/j.resp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 19.Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Qu J, Wang D, Wang H, Dong Y, Zhang F, Zuo B, Zhang H. Electrospun silk fibroin nanofibers in different diameters support neurite outgrowth and promote astrocyte migration. J Biomed Mater Res A. 2013;101:2667–2678. doi: 10.1002/jbm.a.34551. [DOI] [PubMed] [Google Scholar]
- 21.Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 2014;13:1241–1256. doi: 10.1016/S1474-4422(14)70144-9. [DOI] [PubMed] [Google Scholar]
- 22.Raspa A, Pugliese R, Maleki M, Gelain F. Recent therapeutic approaches for spinal cord injury. Biotechnol Bioeng. 2016;113:253–259. doi: 10.1002/bit.25689. [DOI] [PubMed] [Google Scholar]
- 23.Rogina A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl Surf Sci. 2014;296:221–230. [Google Scholar]
- 24.Schaub NJ, Johnson CD, Cooper B, Gilbert RJ. Electrospun fibers for spinal cord injury research and regeneration. J Neurotrauma. 2016;33:1405–1415. doi: 10.1089/neu.2015.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiffman JD, Schauer CL. A review: electrospinning of biopolymer nanofibers and their applications. Polym Rev. 2008;48:317–352. [Google Scholar]
- 26.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Sensharma P, Madhumathi G, Jayant RD, Jaiswal AK. Biomaterials and cells for neural tissue engineering: Current choices. Mater Sci Eng C Mater Biol Appl. 2017;77:1302–1315. doi: 10.1016/j.msec.2017.03.264. [DOI] [PubMed] [Google Scholar]
- 28.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: scaffolds, cells and biomolecules. Regen Biomater. 2015;2:31–45. doi: 10.1093/rb/rbu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian L, Prabhakaran MP, Hu J, Chen M, Besenbacher F, Ramakrishna S. Synergistic effect of topography, surface chemistry and conductivity of the electrospun nanofibrous scaffold on cellular response of PC12 cells. Colloids Surf B Biointerfaces. 2016;145:420–429. doi: 10.1016/j.colsurfb.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Xu CY, Kotaki M, Wang S, Ramakrishna S. Characterization of neural stem cells on electrospun poly(L-lactic acid) nanofibrous scaffold. J Biomater Sci Polym Ed. 2004;15:1483–1497. doi: 10.1163/1568562042459733. [DOI] [PubMed] [Google Scholar]
- 33.Ziemba AM, Gilbert RJ. Biomaterials for local, controlled drug delivery to the injured spinal cord. Front Pharmacol. 2017;8:245. doi: 10.3389/fphar.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]


