Abstract
The B-cell lymphoma-extra large (Bcl-xL) is a mitochondrial anti-apoptotic protein that plays a role in neuroprotection. However, during excitotoxic stimulation, Bcl-xL undergoes caspase-dependent cleavage and produces a fragmented form, ΔN-Bcl-xL. Accumulation of ΔN-Bcl-xL is associated with mitochondrial dysfunction and neuronal death. Therefore, strategies to inhibit the activity or formation of ΔN-Bcl-xL protect the brain against excitotoxic injuries. Our team found that the pharmacological inhibitor ABT-737 exerts dose dependent effects in primary neurons. When primary hippocampal neurons were treated with 1 μM ABT-737, glutamate-mediated mitochondrial damage and neuronal death were exacerbated, whereas 10 nM ABT-737, a 100-fold lower concentration, protected mitochondrial function and enhanced neuronal viability against glutamate toxicity. In addition, we suggested acute vs. prolonged formation of ΔN-Bcl-xL may have different effects on mitochondrial or neuronal functions. Unlike acute production of ΔN-Bcl-xL by glutamate, overexpression of ΔN-Bcl-xL did not cause drastic changes in neuronal viability. We predicted that neurons undergo adaptation and may activate altered metabolism to compensate for ΔN-Bcl-xL-mediated mitochondrial dysfunction. Although the detailed mechanism of ABT-mediated neurotoxicity neuroprotection is still unclear, our study shows that the mitochondrial membrane protein ΔN-Bcl-xL is a central target for interventions.
Keywords: B-cell lymphoma-extra large, ΔN-Bcl-xL, mitochondria, ABT-737
Introduction
The B-cell lymphoma-extra large (Bcl-xL) is a mitochondrial protein and a member of the Bcl2 family. It plays anti-apoptotic functions by preventing oligomerization of pro-apoptotic Bax and Bak (Cheng et al., 1996; Sattler et al., 1997; Ivanovska et al., 2004; Soane et al., 2008), reducing cytochrome c release (Kharbanda et al., 1997; Kim et al., 1997; Carthy et al., 2003) and regulating caspase activity (Zaidi et al., 2001; Bruey et al., 2007). In addition to its significance in cell survival, Bcl-xL is reported to play critical roles in neurophysiology by controlling intracellular energy metabolism, mitochondrial and other intracellular membrane dynamics, and neuronal growth. Bcl-xL enhances mitochondrial adenosine triphosphate (ATP) production by decreasing unnecessary proton leakage across the mitochondrial inner membrane via direct interaction with F1F0 ATP synthase (Alavian et al., 2011; Chen et al., 2011). It also promotes synaptic vesicle endocytosis and regulates homeostasis of synaptic vesicle pools; these functions are required for normal neurotransmission (Li et al., 2013). Bcl-xL promotes neurite outgrowth and branching (Park et al., 2015); thus it protects from the loss of axons and dendrites during neurotoxic insults and it supports proper synapse formation (Li et al., 2008).
Neuroprotection via ΔN-Bcl-xL Regulation
Despite its neuroprotective roles, Bcl-xL also participates in the promotion of neuronal death. Full length Bcl-xL protein contains caspase dependent cleavage sites within its N-terminus (Clem et al., 1998). Accumulation of a fragmented form of Bcl-xL, ΔN-Bcl-xL, is associated with mitochondrial injury, or neuronal injury in both in vitro and in vivo models of cerebral ischemia (Jonas et al., 2004, 2005; Ofengeim et al., 2012; Park et al., 2017). Application of recombinant ΔN-Bcl-xL protein within the presynaptic terminal induces abnormally large mitochondrial membrane conductance (Jonas et al., 2004). Recombinant ΔN-Bcl-xL protein also forms mitochondrial pores and enhances the release of apoptogenic factors including cytochrome c (Basañez et al., 2001). In addition to the effects of recombinant ΔN-Bcl-xL protein, we have found that glutamate-mediated excitotoxicity and ischemic stroke enhance the formation of endogenous ΔN-Bcl-xL in hippocampal neurons; this event leads eventually to neuronal death (Ofengeim et al., 2012; Park et al., 2017). Therefore, strategies that prevent ΔN-Bcl-xL appearance, activity, or accumulation in the neurons have been found to be neuroprotective. ΔN-Bcl-xL may be an important therapeutic target in the treatment of brain injury caused by cerebral ischemia or neurodegenerative diseases.
The most recent study from our laboratory reported that application of the pharmacological inhibitor, ABT-737, prevents ΔN-Bcl-xL-mediated neurotoxicity (Park et al., 2017). Our in silico model shows that cleavage of the Bcl-xL N-terminus does not alter ABT-737 binding sites, thus ΔN-Bcl-xL can form a complex with ABT-737. Although ABT-737 interacts with both full length and ΔN-Bcl-xL, the binding affinity to each protein may be different. BH3 containing proteins Bak or Bad are capable of binding to the BH1, BH2 and BH3 pocket of the 3-dimensional structure of Bcl-xL (Sattler et al., 1997). ABT-737 is a BH3 mimetic; it may interact with Bcl-xL similarly to Bak or Bad. Since full-length Bcl-xL contains a BH4 domain that is removed by caspase cleavage, creation of ΔN-Bcl-xL from full length Bcl-xL may expose the binding sites, thus ΔN-Bcl-xL may have a higher efficiency to form the ABT-737-ΔN-Bcl-xL complex compared to full length Bcl-xL. In neurons treated with a low concentration of ABT-737, ΔN-Bcl-xL may be preferentially targeted compared to full length Bcl-xL, while a high concentration of ABT-737 blocks both anti-apoptotic full length and pro-apoptotic cleaved ΔN-Bcl-xL. Although we have not investigated the mechanism of ΔN-Bcl-xL-ABT-737 binding in detail, we nevertheless predict that ΔN-Bcl-xL undergoes conformational changes after losing the N-terminus or upon binding to mitochondrial membranes. The new conformation of ΔN-Bcl-xL may be more accessible to ABT-737, presumably by exposing the hydrophobic groove that contains ABT-737 binding sites (Lee et al., 2007).
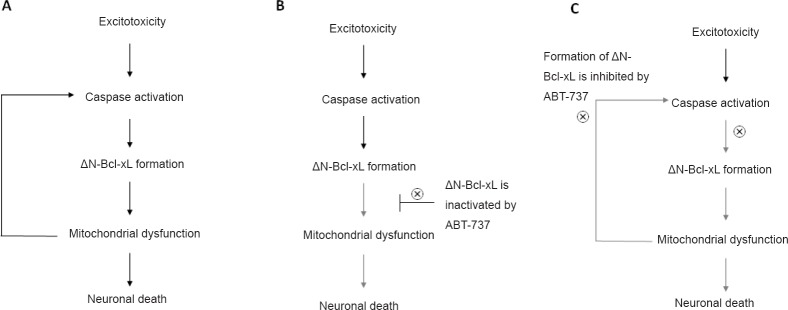
Our data show that ABT-737 exerts dose dependent effects in primary hippocampal neurons. At a high concentration, ABT-737 exacerbates glutamate-induced mitochondrial dysfunction and neuronal death. However, at a low concentration, it conserves mitochondrial function and protects neurons against excitotoxic insult by blocking pro-apoptotic functions of ΔN-Bcl-xL. We further report that ΔN-Bcl-xL is located at both the outer and inner membranes of mitochondria, and that it directly alters mitochondrial inner membrane potential. Our data suggest that application of ABT-737 prevents ΔN-Bcl-xL-mediated mitochondrial dysfunction and neuronal death by multiple pathways (Figure 1). An immediate mechanism of ABT-737's action is to bind directly to ΔN-Bcl-xL, inactivating it and protecting mitochondrial membranes from injury. In addition to sequestering already formed ΔN-Bcl-xL, prevention of the appearance of ΔN-Bcl-xL by ABT-737 also attenuates traditional apoptotic cascades mediated by, for example, the mitochondrial permeability transition pore, cytochrome c release and caspase activation; these actions of ABT-737 eventually block more ΔN-Bcl-xL production by caspases by inhibiting a positive feedback loop. Together, ABT-737 prevents the function of ΔN-Bcl-xL both by inhibiting its activity and blocking its formation (Figure 1).
Figure 1.
ABT-737 inhibits activity and formation of ΔN-Bcl-xL.
(A) Excitotoxic stimulation caused by cerebral ischemia triggers caspase-dependent cleavage of Bcl-xL and forms ΔN-Bcl-xL. Accumulation of ΔN-Bcl-xL at the inner membrane leads to mitochondrial dysfunction associated with mPTP opening and cytocrome c release, then eventually causes neuronal death. (B) Excitotoxic stimulation produces ΔN-Bcl-xL protein, but ABT-737 binds with the active site of ΔN-Bcl-xL, therefore inactivates neurotoxic functions of ΔN-Bcl-xL at the mitochondria (the immediate mechanism of action of ABT-737). (C) Inactivation of ΔN-Bcl-xL by ABT-737 blocks mPTP opening and cytocrome c release which eventually inhibits caspase activation (delayed function of ABT-737). Therefore, application of ABT-737 also blocks formation of ΔN-Bcl-xL. Bcl-xL: B-cell lymphoma-extra large; mPTP: mitochondrial permeablity transition pore.
In addition to our current publication (Park et al., 2017), various approaches to protect the brain by preventing ΔN-Bcl-xL action have been reported previously. Ofengeim et al. (2012) reported neuroprotective properties of ABT-737 in an in vivo system. The authors found that stereotaxic injection of ABT-737 before or after 4 vessel occlusion protected CA1 hippocampal neurons from ischemia-induced cell death. This study also showed that ΔN-Bcl-xL is capable of triggering cell death under conditions of Bax or Bak depletion indicating that ΔN-Bcl-xL-mediated cell death mechanisms may occur independently of traditional Bax or Bak mediated cell death pathways. Application of ABT-737 attenuated ischemia-induced large-channel opening in mitochondrial membranes and reduced the number of degenerating neurons in the hippocampus. Miyawaki et al. (2008) reported that in ischemic preconditioning, where a short exposure of ischemia prior to the main ischemic event protects rodent brains, the conversion of procaspase 3 to form the active caspase 3 in CA1 pyramidal neurons was attenuated. They further found that prevention of formation of ΔN-Bcl-xL was correlated with decreased activation of caspase 3, suggesting that preconditioning protects neurons from apoptotic death through inhibition of the onset of the positive feedback loop of caspase activation and full length Bcl-xL cleavage. In investigating Bcl-xL binding partners that potentially interfere with Bcl-xL cleavage, Arena et al. (2013) reported that PTEN-induced putative kinase 1 (PINK1) binds to Bcl-xL. Interaction between PINK1 and Bcl-xL causes Bcl-xL phosphorylation which impairs cleavage of Bcl-xL's N terminus thereby exerting protection against apoptotic stimulation.
Our current publication also demonstrates the effect of prolonged ΔN-Bcl-xL exposure in the primary hippocampal neurons through a system of exogenous ΔN-Bcl-xL overexpression in addition to glutamate-mediated acute endogenous ΔN-Bcl-xL formation (Park et al., 2017). Unlike during acute ΔN-Bcl-xL formation, over expression of ΔN-Bcl-xL alone did not demonstrate dramatic neurotoxicity. Neurons overexpressing ΔN-Bcl-xL did not die even over 2 weeks of overexpression in our study. During neuronal development, neurons may undergo adaptation to compensate for ΔN-Bcl-xL-mediated mitochondrial dysfunction to retain their survival. Despite having no significant change in viability, however, ΔN-Bcl-xL overexpressing neurons demonstrated a reduced mitochondrial potential. It is still unclear if ΔN-Bcl-xL-mediated mitochondrial potential loss is exclusively due to alteration of mitochondrial inner membrane leak or due to cytochrome c release and the inability to generate a mitochondrial membrane potential. Nevertheless, we expect that ΔN-Bcl-xL-induced inner membrane depolarization may cause the onset of mitochondrial fission and mitophagy, the latter of which is the degradation or self-digestion of mitochondria. In another proposed function for Bcl-xL in mitophagy, Maiuri et al. (2007) and Pedro et al. (2015) reported that the interaction between Bcl-xL and the pro-autophagic protein Beclin-1 suppresses autophagy, but that the BH3 protein Bad and other BH3-containing proteins induce autophagy by causing a disassociation of Beclin-1 from Bcl-xL. The BH3 mimetic ABT-737 also inhibits Beclin-1/Bcl-xL interaction and enhances autophagy (Malik et al., 2011). Since ΔN-Bcl-xL is a BH3 containing fragment, ΔN-Bcl-xL could also play the part of the BH3-only proteins or mimetics in causing a disassociation of Beclin-1 from its inhibitory binding partners. Prolonged exposure to ΔN-Bcl-xL (12–14 days of transfection) could induce formation of autophagosomes containing mitochondria thus initiating mitochondrial degradation. It is known that inhibition of apoptosis may shift the balance to other forms of cell death/survival, including autophagy/mitophagy (Lalaoui et al., 2015; Radogna et al., 2015; White, 2015), therefore we suggest that ΔN-Bcl-xL overexpression in the absence of death-inducing stimuli may tip the balance toward autophagy as a survival mechanism. Gustafsson's group also reported involvement of the BH3 protein, Bnip3 in mitochondrial autophagy. Bnip3 induces translocation of Parkin, a ubiquitin ligase that is downstream of PINK1. Parkin-expressing mitochondria then enter autophagosomes (Lee et al., 2011; Rikka et al., 2011). Mitophagy due to Parkin accumulation has been reported in glutamate-induced excitotoxicity (Van Laar et al., 2015). Various studies reported that defects in mitophagy are associated with other neurological disorders (Youle and Narendra, 2011; Scott et al., 2017), and regulation of mitophagy homeostasis controls survival or recovery of neurons (Amato et al., 2017; Zhan et al., 2017; Zhang et al., 2017).
Conclusion
In summary, our results support that production of ΔN-Bcl-xL by cleavage of full length Bcl-xL controls mitochondrial inner membrane dynamics (e.g., mitochondrial permeability transition and mitochondrial membrane potential). In addition, ΔN-Bcl-xL interaction with the inner mitochondrial membrane may induce mitochondrial remodeling in surviving neurons presumably via mitophagy during neuronal development or during prolonged low level excitotoxic exposure. Together, arresting ΔN-Bcl-xL activity, production or accumulation protects the brain from neurotoxic insults but may prevent the activation of autophagic pathways, therefore, we suggest that ΔN-Bcl-xL is a novel but complex therapeutic target to treat brain injuries that are relevant to stroke, Alzheimer's and Parkinson's diseases.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Ryan Hirschi, University of Utah, USA.
Comments to authors: This paper describes a novel yet complex therapeutic target to treat brain injuries relevant to common neurological disorders. Overall, the article is well written and provides a good overview of previously published work. References are abundant and appropriate. The topic is interesting and the findings are novel.
References
- 1.Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, Lazrove E, Nabili P, Flaherty B, Graham M, Chen Y, Messerli SM, Mariggio MA, Rahner C, McNay E, Shore GC, Smith PJ, Hardwick JM, Jonas EA. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amato R, Catalani E, Dal Monte M, Cammalleri M, Di Renzo I, Perrotta C, Cervia D, Casini G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol Res. 2017 doi: 10.1016/j.phrs.2017.09.022. doi: 10.1016/j.phrs.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Arena G, Gelmetti V, Torosantucci L, Vignone D, Lamorte G, De Rosa P, Cilia E, Jonas EA, Valente EM. PINK1 protects against cell death induced by mitochondrial depolarization, by phosphorylating Bcl-xL and impairing its pro-apoptotic cleavage. Cell Death Differ. 2013;20:920–930. doi: 10.1038/cdd.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basañez G, Zhang J, Chau BN, Maksaev GI, Frolov VA, Brandt TA, Burch J, Hardwick JM, Zimmerberg J. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J Biol Chem. 2001;276:31083–31091. doi: 10.1074/jbc.M103879200. [DOI] [PubMed] [Google Scholar]
- 5.Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, Matsuzawa S, Terskikh AV, Faustin B, Reed JC. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Carthy CM, Yanagawa B, Luo H, Granville DJ, Yang D, Cheung P, Cheung C, Esfandiarei M, Rudin CM, Thompson CB, Hunt DW, McManus BM. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology. 2003;313:147–157. doi: 10.1016/s0042-6822(03)00242-3. [DOI] [PubMed] [Google Scholar]
- 7.Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, Dayhoff-Brannigan M, Zou S, Pineda FJ, O’Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 9.Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U S A. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanovska I, Galonek HL, Hildeman DA, Hardwick JM. Regulation of cell death in the lymphoid system by Bcl-2 family proteins. Acta Haematol. 2004;111:42–55. doi: 10.1159/000074485. [DOI] [PubMed] [Google Scholar]
- 11.Jonas EA, Hickman JA, Hardwick JM, Kaczmarek LK. Exposure to hypoxia rapidly induces mitochondrial channel activity within a living synapse. J Biol Chem. 2005;280:4491–4497. doi: 10.1074/jbc.M410661200. [DOI] [PubMed] [Google Scholar]
- 12.Jonas EA, Hickman JA, Chachar M, Polster BM, Brandt TA, Fannjiang Y, Ivanovska I, Basanez G, Kinnally KW, Zimmerberg J, Hardwick JM, Kaczmarek LK. Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc Natl Acad Sci U S A. 2004;101:13590–13595. doi: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S, Weichselbaum R, Nalin C, Kufe D. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CN, Wang X, Huang Y, Ibrado AM, Liu L, Fang G, Bhalla K. Overexpression of Bcl-X(L) inhibits Ara-C-induced mitochondrial loss of cytochrome c and other perturbations that activate the molecular cascade of apoptosis. Cancer Res. 1997;57:3115–3120. [PubMed] [Google Scholar]
- 15.Lalaoui N, Lindqvist LM, Sandow JJ, Ekert PG. The molecular relationships between apoptosis, autophagy and necroptosis. Semin Cell Dev Biol. 2015;39:63–69. doi: 10.1016/j.semcdb.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Lee EF, Czabotar PE, Smith BJ, Deshayes K, Zobel K, Colman PM, Fairlie WD. Crystal structure of ABT-737 complexed with Bcl-xL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007;14:1711–1713. doi: 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1924–1931. doi: 10.1152/ajpheart.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Alavian KN, Lazrove E, Mehta N, Jones A, Zhang P, Licznerski P, Graham M, Uo T, Guo J, Rahner C, Duman RS, Morrison RS, Jonas EA. A Bcl-xL-Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat Cell Biol. 2013;15:773–785. doi: 10.1038/ncb2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, McNay EC, Yu T, Schwarzenbacher R, Bossy B, Bossy-Wetzel E, Bennett MV, Pypaert M, Hickman JA, Smith PJ, Hardwick JM, Jonas EA. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik SA, Orhon I, Morselli E, Criollo A, Shen S, Mariño G, BenYounes A, Bénit P, Rustin P, Maiuri MC, Kroemer G. BH3 mimetics activate multiple pro-autophagic pathways. Oncogene. 2011;30:3918–3929. doi: 10.1038/onc.2011.104. [DOI] [PubMed] [Google Scholar]
- 22.Miyawaki T, Mashiko T, Ofengeim D, Flannery RJ, Noh KM, Fujisawa S, Bonanni L, Bennett MV, Zukin RS, Jonas EA. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ofengeim D, Chen YB, Miyawaki T, Li H, Sacchetti S, Flannery RJ, Alavian KN, Pontarelli F, Roelofs BA, Hickman JA, Hardwick JM, Zukin RS, Jonas EA. N-terminally cleaved Bcl-xL mediates ischemia-induced neuronal death. Nat Neurosci. 2012;15:574–580. doi: 10.1038/nn.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HA, Licznerski P, Alavian KN, Shanabrough M, Jonas EA. Bcl-xL is necessary for neurite outgrowth in hippocampal neurons. Antioxid Redox Signal. 2015;22:93–108. doi: 10.1089/ars.2013.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HA, Licznerski P, Mnatsakanyan N, Niu Y, Sacchetti S, Wu J, Polster BM, Alavian KN, Jonas EA. Inhibition of Bcl-xL prevents pro-death actions of DeltaN-Bcl-xL at the mitochondrial inner membrane during glutamate excitotoxicity. Cell Death Differ. 2017;24:1963–1974. doi: 10.1038/cdd.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedro JM, Wei Y, Sica V, Maiuri MC, Zou Z, Kroemer G, Levine B. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy. 2015;11:452–459. doi: 10.1080/15548627.2015.1017191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radogna F, Dicato M, Diederich M. Cancer-type-specific crosstalk between autophagy, necroptosis and apoptosis as a pharmacological target. Biochem Pharmacol. 2015;94:1–11. doi: 10.1016/j.bcp.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Rikka S, Quinsay MN, Thomas RL, Kubli DA, Zhang X, Murphy AN, Gustafsson ŠB. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ. 2011;18:721–731. doi: 10.1038/cdd.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 30.Scott L, Dawson VL, Dawson TM. Trumping neurodegeneration: Targeting common pathways regulated by autosomal recessive Parkinson's disease genes. Exp Neurol. 2017 doi: 10.1016/j.expneurol.2017.04.008. doi: 10.1016/j.expneurol.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soane L, Siegel ZT, Schuh RA, Fiskum G. Postnatal developmental regulation of Bcl-2 family proteins in brain mitochondria. J Neurosci Res. 2008;86:1267–1276. doi: 10.1002/jnr.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Laar VS, Roy N, Liu A, Rajprohat S, Arnold B, Dukes AA, Holbein CD, Berman SB. Glutamate excitotoxicity in neurons triggers mitochondrial and endoplasmic reticulum accumulation of Parkin, and, in the presence of N-acetyl cysteine, mitophagy. Neurobiol Dis. 2015;74:180–193. doi: 10.1016/j.nbd.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaidi AU, D’Sa-Eipper C, Brenner J, Kuida K, Zheng TS, Flavell RA, Rakic P, Roth KA. Bcl-X(L)-caspase-9 interactions in the developing nervous system: evidence for multiple death pathways. J Neurosci. 2001;21:169–175. doi: 10.1523/JNEUROSCI.21-01-00169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan L, Chen S, Li K, Liang D, Zhu X, Liu L, Lu Z, Sun W, Xu E. Autophagosome maturation mediated by Rab7 contributes to neuroprotection of hypoxic preconditioning against global cerebral ischemia in rats. Cell Death Dis. 2017;8:e2949. doi: 10.1038/cddis.2017.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Tao W, Yuan Z, Liu Y. Mst-1 deficiency promotes post-traumatic spinal motor neuron survival via enhancement of autophagy flux. J Neurochem. 2017;143:244–256. doi: 10.1111/jnc.14154. [DOI] [PubMed] [Google Scholar]