There is mounting evidence that targeting mitochondrial dysfunction following neurotrauma could be key in developing effective therapeutic strategies since mitochondria are known to play a major role in cellular bioenergetics, function, and survival following traumatic spinal cord injury (SCI) (Rabchevsky et al., 2011). Our research group is one of the pioneers in targeting mitochondrial dysfunction to foster functional neuroprotection, having documented that pharmacological maintenance of mitochondrial function acutely results in long-term neuroprotection and improved functional recover. We have recently reported that treatment with the pleiotropic drug, pioglitazone, maintains acute mitochondrial integrity correlated with chronic tissue sparing and functional recovery after contusion SCI, but that this was not correlated with altered neuroinflammation (Patel et al., 2017). We herein propose that the mechanism(s) by which pioglitazone confers neuroprotection may not be entirely dependent upon its activation of peroxisome proliferator activated receptor (PPAR), a member of nuclear receptor superfamily that can heterodimerize in a ligand-dependent and -independent manner to regulate gene expression of multiple molecular processes. A class of drugs used to treat type 2 diabetes, called thiazolidinediones, can modulate PPAR-γ therapeutic effects. Also called glitazones, these drugs include pioglitazone, rosiglitazone, troglitazone and ciglitazone, which are reported to provide their therapeutic effects through varying interactions with PPAR-γ (Park et al., 2007).
While it has been hypothesized that PPAR-γ modulation of inflammation is the basis for reported therapeutic efficacy after neurotrauma, it is our contention that the noted anti-inflammatory effects of pioglitazone following neurotrauma are indirect and based, in part, on tissue preservation due to mitochondrial homeostasis and, consequently, greater functional neuroprotection. Similar to our recent report after SCI (Patel et al., 2017), we previously found that following contusion traumatic brain injury (TBI), pioglitazone attenuated mitochondria dysfunction and improved both tissue sparing and behavioral outcome, but without altering neuroinflammation (Sauerbeck et al., 2011). Accordingly, others used PPAR antagonists to show that the neuroprotective effects of pioglitazone treatment after TBI were independent of PPAR-γ activation (Thal et al., 2011). To further support our alternative hypothesis, studies have shown that pioglitazone binds to mitoNEET, a novel mitochondrial membrane protein, and that pioglitazone binding to mitoNEET is able to inhibit its [2Fe-2S] cluster transfer upon binding (see Tamir et al., 2015 and references within). However, the role of such a mechanism in providing therapy after central nervous system (CNS) injury is unknown, although it may be related to prevention of its dimerization.
MitoNEET is a protein localized in the brain, liver and skeletal muscles of rodents, a finding long after the discovery that pioglitazone has a binding affinity for the mitochondrial membrane that is mediated through a new m-17 kDa protein, later termed mitoNEET (Tamir et al., 2015). At the time of its discovery, mitoNEET was proposed to be a pivotal protein for mitochondrial metabolism that had the potential of being modulated by pioglitazone. Since its initial discovery, the exact role of mitoNEET in the cell remains uncertain. However, a handful of groups have studied mitoNEET's protein dynamics and suggested one possible role is to be a shuttle protein for the mitochondria (Tamir et al., 2015). Additionally, in mitoNEET knockout mice the mitochondria have decreased oxidative capacity, which suggests that it may be pivotal in controlling the rate of mitochondrial respiration, notably as a redox-sensitive protein that can be reduced by biological thiols such as glutathione (GSH), reversing the effect of mitoNEET oxidation (Tamir et al., 2015). This may account for our demonstration that novel GSH precursors prevent mitochondrial dysfunction and afford neuroprotection following traumatic SCI and TBI (Pandya et al., 2014; Patel et al., 2014), and we have more recently reported on the identification of small molecules that bind to mitoNEET that might be targeted pharmacologically after CNS trauma (Geldenhuys et al., 2016; Yonutas et al., 2016). These ligands are built on the glitazone backbone by truncating the PPAR binding moiety (Tamir et al., 2015), allowing these novel compounds to target mitoNEET directly without any subsequent direct PPAR activation.
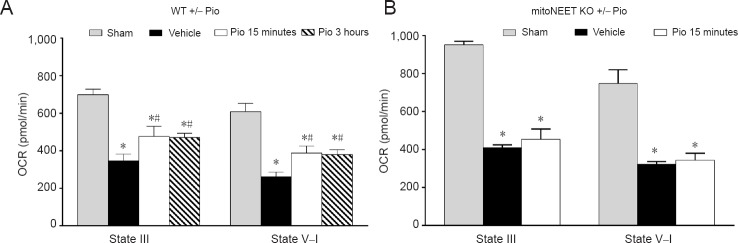
While we have documented that pioglitazone administered at 15 minutes or 3 hours after SCI significantly maintains mitochondrial respiration 24 hours post-injury (Figure 1A), our recent findings indicate that pioglitazone is neuroprotective following SCI by maintaining mitochondrial homeostasis via direct interactions with mitoNEET. Specifically, pioglitazone administration to mitoNEET knockout (KO) (–/–) mice does not maintain mitochondrial function following SCI (Figure 1B). Therefore, unlike beneficial effects seen in wild-type (WT) mice, pioglitazone treatment was ineffective at improving mitochondrial respiration in mitoNEET KO mice.
Figure 1.
Pioglitazone (Pio) is ineffective at maintaining mitochondrial state III respiration (OCR) in mitoNEET knockout (KO) mice.
(A) At 24 hours after traumatic spinal cord injury (SCI) (vehicle) in wild-type (WT) mice, there was a significant reduction in OCR (oxidative phosphorylation), as well as NADH-linked electron transport system (ETS) (state V–I) capacity. Administration of Pio (10 mg/kg) at 15 minutes or 3 hours post-injury followed by a booster at 24 hours significantly maintained mitochondrial respiration at 25 hours post-injury (Patel et al., 2017). (B) Compared to sham, SCI also resulted in reduced mitochondrial respiration in mitoNEET KO mice. However, unlike WT, similar treatment with 10 mg/kg Pio in injured mitoNEET KO mice did not restore mitochondrial respiration at 25 hours post-SCI. Bars represent the mean ± SEM, n = 7–10/group (A), 3/group (B). *P < 0.05, vs. sham group; #P < 0.05, vs. vehicle group.
Collectively, our data provide a foundation for the novel hypothesis that pioglitazone benefits following SCI are dependent upon its interactions with mitoNEET. Accordingly, ongoing experiments are directly testing this hypothesis to establish, mechanistically, the basis for pioglitazone neuroprotection with the ultimate goal of using novel specific mitoNEET ligands as novel therapeutics for both SCI and TBI, as well as a wide range of neurological disease states.
This work was funded by NIH R21NS096670 (AGR), Craig H. Neilsen Foundation 476719 (AGR), Kentucky Spinal Cord and Head Injury Research Trust #15-14A (PGS), Veterans Affairs Merit Review Award #I01BX003405 (PGS), University of Kentucky Spinal Cord and Brain Injury Center Chair Endowments (AGR & PGS), NIH/NINDS 2P30NS051220.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Joaquin Martí-clúa, Universitat Autònoma de Barcelona, Spain.
Comments to authors: After carefully reading this manuscript, my view is that this work has been very well carried out. It is suitable for publication in the Neural Regeneration Research. The continuation of this work may provide a novel strategy of benefit to patients with spinal cord injury.
Reviewer 2: Paul Lu, University of California San Diego, USA.
Comments to authors: This is a prospect following a recent publication titled “Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery” in Exp Neurol by Dr. Alexander Rabchevsky's group. This study shows different mechanism of pioglitazone treatment after SCI: maintenance of acute mitochondrial bioenergetics. A recent study demonstrates that pioglitazone can bind to a mitochondrial membrane protein called mitoNEET, which may involve maintenance of mitochondrial respiration. This prospect is well-written and discusses the future direction to exploit this potential treatment for spinal cord injury.
References
- 1.Geldenhuys WJ, Yonutas HM, Morris DL, Sullivan PG, Darvesh AS, Leeper TC. Identification of small molecules that bind to the mitochondrial protein mitoNEET. Bioorg Med Chem Lett. 2016;26:5350–5353. doi: 10.1016/j.bmcl.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, Rabchevsky AG, Sullivan PG. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp Neurol. 2014;257:106–113. doi: 10.1016/j.expneurol.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320:1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- 4.Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG. Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery. Exp Neurol. 2017;293:74–82. doi: 10.1016/j.expneurol.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SP, Sullivan PG, Pandya JD, Goldstein GA, VanRooyen JL, Yonutas HM, Eldahan KC, Morehouse J, Magnuson DS, Rabchevsky AG. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp Neurol. 2014;257:95–105. doi: 10.1016/j.expneurol.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabchevsky AG, Patel SP, Springer JE. Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol Ther. 2011;132:15–29. [Google Scholar]
- 7.Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol. 2011;227:128–135. doi: 10.1016/j.expneurol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamir S, Paddock ML, Darash-Yahana-Baram M, Holt SH, Sohn YS, Agranat L, Michaeli D, Stofleth JT, Lipper CH, Morcos F, Cabantchik IZ, Onuchic JN, Jennings PA, Mittler R, Nechushtai R. Structure-function analysis of NEET proteins uncovers their role as key regulators of iron and ROS homeostasis in health and disease. Biochim Biophys Acta. 2015;1853:1294–1315. doi: 10.1016/j.bbamcr.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Thal SC, Heinemann M, Luh C, Pieter D, Werner C, Engelhard K. Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-gamma-independent mechanisms. J Neurotrauma. 2011;28:983–993. doi: 10.1089/neu.2010.1685. [DOI] [PubMed] [Google Scholar]
- 10.Yonutas HM, Vekaria HJ, Sullivan PG. Mitochondrial specific therapeutic targets following brain injury. Brain Res. 2016;1640:77–93. doi: 10.1016/j.brainres.2016.02.007. [DOI] [PubMed] [Google Scholar]