Keywords: nerve regeneration, virtual reality, Wolf motor function test, functional magnetic resonance imaging, stroke, Leap Motion, rehabilitation, upper limb, neural reorganization, neural regeneration
Abstract
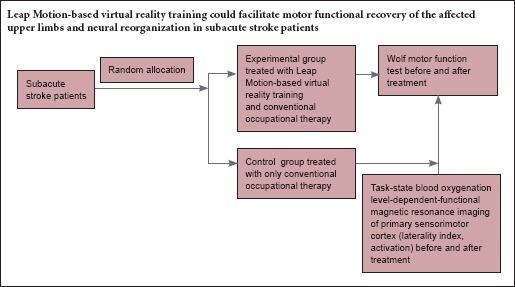
Virtual reality is nowadays used to facilitate motor recovery in stroke patients. Most virtual reality studies have involved chronic stroke patients; however, brain plasticity remains good in acute and subacute patients. Most virtual reality systems are only applicable to the proximal upper limbs (arms) because of the limitations of their capture systems. Nevertheless, the functional recovery of an affected hand is most difficult in the case of hemiparesis rehabilitation after a stroke. The recently developed Leap Motion controller can track the fine movements of both hands and fingers. Therefore, the present study explored the effects of a Leap Motion-based virtual reality system on subacute stroke. Twenty-six subacute stroke patients were assigned to an experimental group that received virtual reality training along with conventional occupational rehabilitation, and a control group that only received conventional rehabilitation. The Wolf motor function test (WMFT) was used to assess the motor function of the affected upper limb; functional magnetic resonance imaging was used to measure the cortical activation. After four weeks of treatment, the motor functions of the affected upper limbs were significantly improved in all the patients, with the improvement in the experimental group being significantly better than in the control group. The action performance time in the WMFT significantly decreased in the experimental group. Furthermore, the activation intensity and the laterality index of the contralateral primary sensorimotor cortex increased in both the experimental and control groups. These results confirmed that Leap Motion-based virtual reality training was a promising and feasible supplementary rehabilitation intervention, could facilitate the recovery of motor functions in subacute stroke patients. The study has been registered in the Chinese Clinical Trial Registry (registration number: ChiCTR-OCH-12002238).
Introduction
Chronic conditions such as stroke are becoming more prevalent as the world's population ages (Christensen et al., 2009). Although the number of fatalities caused by stroke has fallen in most countries, stroke is still a leading cause of acquired adult hemiparesis (Langhorne et al., 2009; Liu and Duan, 2017). Up to 85% of patients who survive a stroke experience hemiparesis, resulting in impaired movement of an arm and hand (Nakayama et al., 1994). Among them, a large proportion (46% to 95%) remain symptomatic six months after experiencing an ischemic stroke (Kong et al., 2011). The loss of upper limb function adversely affects the quality of life and impedes the normal use of other body parts. The motor function recovery of the upper limbs is more difficult than that of the lower extremities (Kwakkel et al., 1996; Nichols-Larsen et al., 2005; Día and Gutiérrez, 2013). Functional motor recovery in the affected upper extremities in patients with hemiparesis is the primary goal of physical therapists (Page et al., 2001). Evidence suggests that repetitive, task-oriented training of the paretic upper extremity is beneficial (Barreca et al., 2003; Wolf et al., 2006). Rehabilitation intervention is a critical part of the recovery and studies have reported that intensive repeated practice is likely necessary to modify the neural organization and favor the recovery of the functional upper limb motor skills of stroke survivors (Brunnstrom, 1966; Kopp et al., 1999; Taub et al., 1999; Wolf et al., 2006; Nudo, 2011). Meta-analyses of clinical trials have indicated that longer sessions of practice promote better outcomes in the case of impairments, thus improving the daily activities of people after a stroke (Nudo, 2011; Veerbeek et al., 2014; Sehatzadeh, 2015; French et al., 2016). However, the execution of these conventional rehabilitation techniques is tedious, resource-intensive, and often requires the transportation of patients to specialized facilities (Jutai and Teasell, 2003; Teasell et al., 2009).
Virtual reality training is becoming a promising technology that can promote motor recovery by providing high-intensity, repetitive, and task-orientated training with computer programs simulating three-dimensional situations in which patients play by moving their body parts (Saposnik et al., 2010, 2011; Kim et al., 2011; Laver et al., 2015; Tsoupikova et al., 2015). The gaming industry has developed a variety of virtual reality systems for both home and clinical applications (Saposnik et al., 2010; Bao et al., 2013; Orihuela-Espina et al., 2013; Gatica-Rojas and Méndez-Rebolledo, 2014). The most difficult task related to hemiparesis rehabilitation after a stroke is the functional recovery of the affected hand (Carey et al., 2002). To facilitate the functional recovery of a paretic hand along with that of the proximal upper extremity, an ideal virtual reality system should be able to track hand position and motion, which is not a feature of most existing virtual reality systems (Jang et al., 2005; Merians et al., 2009). The leap motion controller developed by Leap Motion (https://www.leapmotion.com) provides a means of capturing and tracking the fine movements of the hand and fingers, while controlling a virtual environment requiring hand-arm coordination as part of the practicing of virtual tasks (Iosa et al., 2015; Smeragliuolo et al., 2016).
Most virtual reality studies have often only involved patients who have experienced chronic stroke (Piron et al., 2003; Yavuzer et al., 2008; Saposnik et al., 2010; da Silva Cameirao et al., 2011). For patients in the chronic stage, who had missed the window of opportunity present at the acute and subacute stages (in which the brain plasticity peaks), rehabilitation-therapy-induced neuroplasticity can only be effective within a relatively narrow range (Chen et al., 2002). No motor function recovery of the hands, six months after the onset of a stroke, indicates a poor prognosis for hand function (Duncan et al., 1992).
We hypothesized that Leap Motion-based virtual reality training would facilitate motor functional recovery of the affected upper limb, as well as neural reorganization in subacute stroke patients. Functional magnetic resonance imaging (fMRI), also called blood oxygenation level-dependent fMRI (BOLD-fMRI), is widely used as a non-invasive, convenient, and economical method to examine cerebral function (Ogawa et al., 1990; Iosa et al., 2015; Yu et al., 2016). In the present study, we evaluated the brain function reorganization by fMRI, as well as the motor function recovery of the affected upper limb in patients with subacute stroke using Leap Motion-based virtual reality training.
Subjects and Methods
Subjects
This randomized controlled pilot study was performed at the China Rehabilitation Research Center at the Beijing Boai Hospital, China, from November 2014 to November 2015. Twenty-six inpatients (all of whom had suffered a subacute stroke) of the China Rehabilitation Research Center provided written consent according to the Declaration of Helsinki. All patients were assessed as being right-handed, as defined by the Edinburgh scale. Ethical approval was given by the Ethics Committee of the China Rehabilitation Research Center (approval number: 2012-045-1), and the study was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR-OCH-12002238). In the context of the study, “stroke” was diagnosed according to the diagnostic criteria of the Fourth National Academic Conference on cerebrovascular disease, held in 1995 (Wang, 1996). At the beginning of the study, each patient was subjected to a diagnostic procedure including a general physical examination, neurological examination, computed tomography (CT) or MRI scans, and a review of his/her medical history.
Inclusion criteria
Patients presenting with all of the following criteria were considered for study inclusion: (1) They were being treated for their first-ever supratentorial stroke in the area of the middle cerebral artery, as confirmed by MRI or a CT scan. (2) They exhibited mild to moderate motor impairment in the upper extremity, resulting directly from the stroke (hemorrhage or ischemia). (3) They were able to independently move the affected arm and wrist with their residual voluntary movement capacity (that is, a nadir function level of the hand beyond Brunnstrom IV (Brunnstrom, 1966)). (4) Since the onset of the stroke, between 4–24 weeks had elapsed (1–6 months, subacute phase of stroke (Teasell et al., 2014; Hebert et al., 2016); They were aged 18–75. (5) They were able to sit steadily without assistance. (6) They were able to sign the informed consent. (7) They had normal or corrected to normal visual acuity.
Exclusion criteria
Patients with one or more of the following conditions were excluded from this study: Severe psychiatric problems, cognitive impairment or aphasia, neglect/inattention, evidence of significant medical disease (e.g., cardiovascular disease, diabetes or hepatic cancer, renal, cardiac or pulmonary disorders), the presence of uncontrolled hypertension, hearing disabilities, visual field impairment, depression, and contraindications to MRI.
Assignment
Using random allocation software (NCSS-PASS 11.0, NCSS, Kaysville, UT, USA), 26 eligible subjects were assigned to either the control group (conventional rehabilitation group, n = 13; two females; mean age, 53.4 ± 7.6 years; mean elapsed time since stroke onset, 7.9 ± 2.1 weeks) or the experimental group (Leap Motion-based virtual reality training added to conventional rehabilitation, n = 13; two females; mean age 55.3 ± 8.4 years; mean elapsed time since stroke onset, 7.2 ± 2.0 weeks). The sample size was determined based on statistical calculations (NCSS-PASS 11.0, NCSS) to achieve a 90% power at a significance level of 0.05, and the parameters used to perform the power calculation were estimated from the results of a pre-experiment.
Leap Motion-based virtual reality intervention
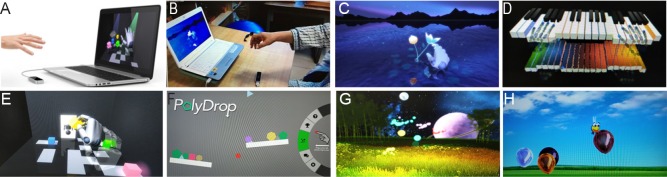
As depicted in Figure 1A, B, the Leap Motion-based virtual reality system consisted of a personal computer and the leap motion controller (LMC®; Leap Motion, Inc., San Francisco, CA, USA). The two parts were connected through a USB cable. The controller can track, with sub-millimeter accuracy, the movement of multiple hands and fingers. The leap motion controller is a low-cost, markerless motion-capture device that tracks the fine movements of fingers and hands using neither data gloves nor markers. It is also capable of controlling a virtual environment (Iosa et al., 2015; Smeragliuolo et al., 2016).
Figure 1.
Leap Motion-based virtual reality system and training games.
(A, B) Leap Motion-based virtual reality system; (C) petal-picking game; (D) piano-playing game; (E) robot-assembling game; (F) object-catching with balance board game; (G) firefly game; (H) bee-batting game.
The captured images of a hand were converted into digital format and projected on to the screen of the personal computer. The patient was able to view the movement of a representation (avatar) of his/her own hand and carry out the tasks involved in games played in the virtual world by controlling the avatar through hand-arm coordination in the real world. As such, the patient was able to immerse him/herself in the virtual environment. Thus, the Leap Motion-based virtual reality system is superior to competitive virtual reality systems in that it provides greater freedom of mobility without the need for extra devices such as data gloves, wires, or head-mounted displays.
Figure 1C–H shows six interfaced virtual exercise protocols programmed in the Leap Motion-based virtual reality system. These Leap Motion-based virtual reality protocols were designed to focus on the development of the pinching, grasping, and individuating motor skills of fingers; the improvement of the dexterity and coordination of the digits; the improvement of the ability to flex and extend the hand, the pronation and supination of the forearm; the increase in the joint range of motion of the hand, elbow, shoulder and wrist; the improvement of the movement speed, muscle strength, and motor control.
Petal-picking game: Designed to develop the pinching motor skills of the fingers by picking lotus petals in a virtual environment. This also improved the dexterity and coordination of the digits (Figure 1C).
Piano-playing: This game was designed to develop the individuating motor skills of the fingers while improving the dexterity and coordination of the digits (Figure 1D).
Robot-assembly game: This game was mainly used to develop the pinching motor skills of the fingers and to improve the pronation and supination capabilities of the forearm (Figure 1E).
Object-catching with balance board: This game focused on the flexion and extension of the hand, as well as the pronation and supination of the forearm (Figure 1F).
Firefly game: This game focused on the flexion and extension of the hand and the development of the grasping motor skills of the hand (Figure 1G).
Bee-batting game: This game focused on increasing the range of motion of the joints of the hand, elbow, shoulder, and wrist, the improvement of the speed of movement, muscle strength, and motor control (Figure 1H).
Experimental procedure
Training started after baseline evaluation and fMRI examination. Figure 2 shows the experimental protocol. In the experimental group, patients were given Leap Motion-based virtual reality training for 45 minutes, once a day, 5 times a week for 4 weeks, as well as conventional occupational therapy for 45 minutes, once a day, 5 times a week for 4 weeks. In the control group, the patients only received conventional occupational therapy training twice a day, each for 45 minutes, 5 times a week for 4 weeks. Both the experimental and control groups participated in conventional physiotherapy, which included stretches, strength, balance, gait, and functional training for 45 minutes, once a day, 5 times a week for 4 weeks. Conventional occupational therapy concentrates on both hand and arm movements that are similar to Leap Motion-based virtual reality training. During training, the virtual reality exercise was carried out five times for each game, with the therapists guiding each patient to exercise the related joints to their maximum achievable range of motion, while avoiding compensatory strategies (Martin et al., 2011). Conventional occupational exercise was also performed by each patient at the same frequency, and each patient was also guided to avoid compensatory strategies and to exercise related joints to their maximum achievable range of motion.
Figure 2.
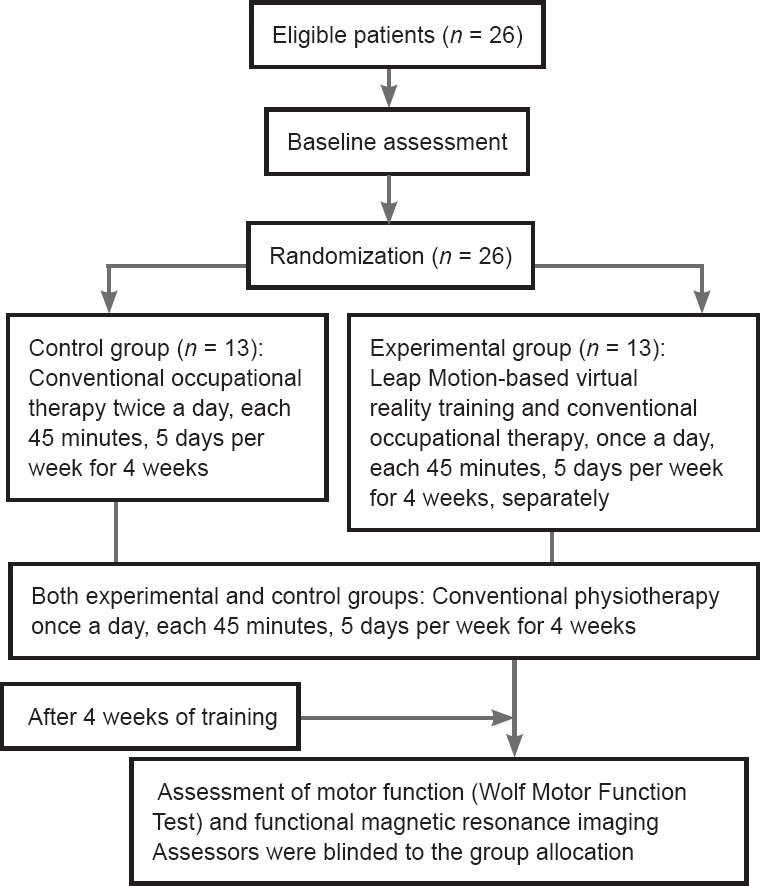
Flow chart of experimental protocol.
Outcome measures
Primary outcome measure
The primary outcome measure was the motor functions as assessed using the Wolf motor function test (WMFT) (Saposnik et al., 2010). Measurements were recorded at the baseline and post intervention by two certified therapists. The assessors were not aware of the group allocations. WMFT was used because (1) the test is standard throughout constraint-induced movement therapy research and other studies about upper limb rehabilitation techniques (Taub et al., 1999; Tarkka et al., 2008; Bao et al., 2013; Tong et al., 2015). The inclusion criteria of our present investigation were a modification of those for constraint-induced movement therapy (Taub et al., 1993). (2) This test measures the movement ability of the upper extremity. It is composed of a series of tasks with timed single or multiple joint motions of progressive complexity that measures movement capability and speed with minimal training and with the need for few tools (Wolf et al., 2001). In the present study, a patient's paretic hand and arm coordination in the real world was utilized to carry out the tasks required of the games played in a virtual environment. WMFT (Bao et al., 2013): Tasks 1–6 of the WMFT are timed joint-segment movements, while tasks 7–15 are timed integrative functional movements. Higher function scores indicate a superior upper-limb function. A reduction in the performance time corresponded to an improvement in the functioning of the upper extremity.
Patients in both groups were also questioned about their experience with the Leap Motion-based virtual reality training and conventional therapy, specifically with the aim of identifying any side-effects or preference for virtual reality over conventional therapy (Yin et al., 2014).
Secondary outcome measure
The secondary outcome measure was the fMRI results. Measurements were recorded at the baseline and post intervention. The task-state BOLD-fMRI experiment was divided into six 1-minute task cycles with a 24-second preparatory phase. Each task cycle consisted of a 30-second motion and a 30-second rest. During the motion, a patient performed an auditory-directed 1-Hz movement whereby he/she used the thumb of the affected hand to touch the opposite palm (Baeck et al., 2012). During the fMRI scanning, the room lights were dimmed, and the patients were instructed to close their eyes. The patient's body parts were also secured by straps to prevent translational movement and the scanning was done with the patients in the supine position.
A 1.5-T General Electric Signa scanner (Signa; General Electric Medical Systems, Milwaukee, WI, USA) was used to measure the BOLD contrast. The fMRI acquisition parameters were: echo time = 40 ms, repetition time = 3,000 ms, field of view = 24 mm × 24 mm, slice thickness = 5 mm, image matrix = 64 × 64. The mid-sagittal scout image was used, and a total of 24 5-mm contiguous axial slices were laid along the anterior-posterior commissure plane, thus covering the entire brain.
The MATLAB7.1 platform (Mathworks, Inc., Natick, MA, USA) SPM8 package (Welcome Department of Cognitive Neurology, Queen's College, London, England) was used to analyze the fMRI data. The preprocessing steps included realignment, co-registration, spatial normalization, and smoothing. The statistics threshold was at P < 0.05 (corrected). A false discovery rate of 0.05 was used to perform correction for multiple comparisons. The activated cluster level was more than 10 voxels. For all the patients, the bilaterally predefined regions of interest, including the primary sensorimotor cortex (SMC) containing the primary sensory cortex and the primary motor cortex, the supplementary motor area (SMA), and the cerebellum, were studied to identify the occurrence of neuroplastic changes (Cramer et al., 1997; Carey et al., 2002; Lefebvre et al., 2012; Szameitat et al., 2012; Bao et al., 2013).
The BOLD signal carries a large within-subject variability; therefore, to determine the symmetry shift of cortical activation in the regions of interest between two hemispheres, we used the laterality index, which was defined as (C – I)/(C + I), where C and I are the number of activated voxel counts in the region contralateral or ipsilateral to the affected hand movement (Kim et al., 1994; Carey et al., 2002). The laterality index ranged from 1.0 (total contralateral activation) to –1.0 (total ipsilateral activation) (Cramer et al., 1997; Carey et al., 2002).
Statistical analysis
The data are presented as the mean ± SD. Statistical Package for the Social Sciences (SPSS, version 17.0, SPSS, Chicago, IL, USA) was used to perform all the statistical analyses. A t-test was used to analyze the quantitative data: A paired-sample t-test was used to compare the upper limb function and fMRI data between the pre-training and post-training in each group. An independent-sample t-test was used to compare the upper limb function and fMRI data between the experimental and control groups both before and after training. The qualitative data were analyzed by applying a chi-square test. The statistical significance was set to P < 0.05.
Results
Demographic baseline of subacute stroke patients
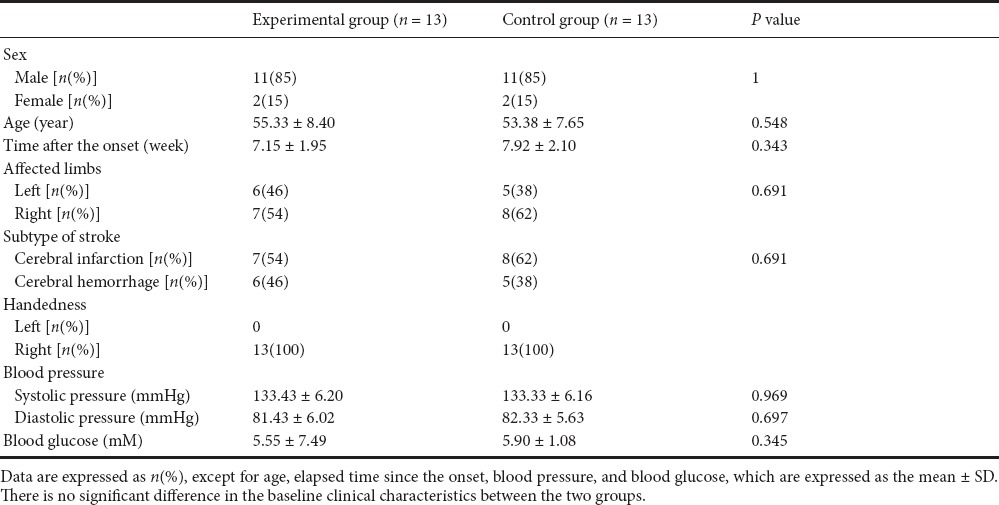
Twenty-six patients with subacute stroke were included. None of the patients dropped out of the study. Table 1 lists the patient clinical data. No significant difference was observed between the two groups in terms of their baseline clinical characteristics.
Table 1.
Demographic baseline clinical data of enrolled patients
Motor function of affected upper limbs in subacute stroke patients before and after Leap Motion-based virtual reality training
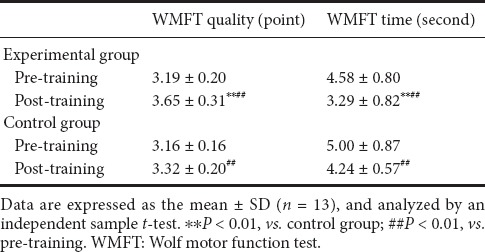
No significant difference in the WMFT assessment score was observed between the groups prior to the training (P > 0.05). After 4 weeks of training, significant improvements in the motor functions of the affected upper limbs were observed in all the participants of both groups, as measured by the WMFT quality (P < 0.01). The improvement was greater in the experimental group than in the control group (P < 0.01). After 4 weeks of therapy, the action performance time in the WMFT (WMFT time, s) had decreased significantly in both groups (P < 0.01). However, the WMFT time was significantly shorter in the experimental group than in the control group (P < 0.01; Table 2).
Table 2.
Effect of Leap Motion-based virtual reality training on motor functions of subacute stroke patients
Neural reorganization in subacute stroke patients before and after Leap Motion-based virtual reality training
The activated brain regions of interest during the thumb-to-palm opposition task, both before training and after training in the two groups, are shown in Figure 3. Before training, when using the affected hand, the bilateral or ipsilateral SMC, and the bilateral or unilateral SMA and cerebellum were mainly activated in both groups. After training, a shift in the SMC activation from the ipsilateral or bilateral to contralateral regions was found after intensive use of the paretic limb in both groups; these changes were more obvious in the experimental group than in the control group. There was no obvious evolving pattern of change in the SMA and cerebellum (Nair et al., 2007; Mintzopoulos et al., 2009). The mainly activated areas were the bilateral or ipsilateral SMC. The changes in the laterality index of the SMC and the activation intensity (T value) of the contralateral SMC before and after the training are shown in Table 3.
Figure 3.
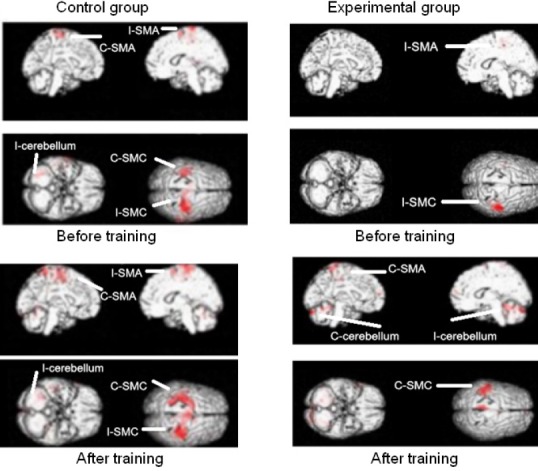
Activated brain regions of interest during the thumb-to-palm opposition task before and after training in the two groups.
Control group (n = 13): Conventional occupational therapy twice a day, each 45 minutes, 5 days per week for 4 weeks; Experimental group (n = 13): Leap Motion-based virtual reality training and conventional occupational therapy, once a day, each 45 minutes, 5 days per week for 4 weeks, separately. C: Contralateral (side opposite that of the paretic hand, ipsilesional); I: ipsilateral (same side as the paretic hand, contralesional); SMC: sensorimotor cortex; SMA: supplementary motor area. The activated brain regions are marked in red.
Table 3.
Effect of Leap Motion-based virtual reality training on activation intensity (T value) of contralateral SMC and laterality index of SMC of subacute stroke patients
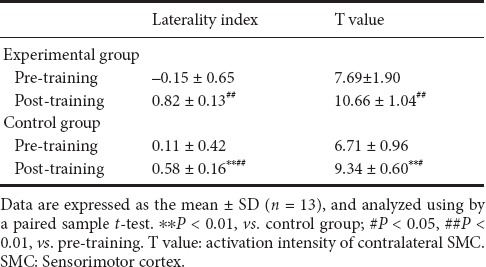
There was no significant difference in the activation intensity of the contralateral SMC and the laterality index of SMC between the groups before training (P > 0.05). After 4 weeks of training, both the activation intensity of the contralateral SMC and the laterality index of the SMC had increased in both the experimental group (P < 0.01) and control group (P < 0.05). The Leap Motion-based virtual reality training produced a greater increase in the activation intensity of the contralateral SMC in the laterality index of SMC in the experimental group than in the control group (P < 0.01).
Adverse effects of Leap Motion-based virtual reality training on subacute stroke patients
The patients of the two groups completed all the training sessions without any adverse effects. In addition, the Leap Motion-based virtual reality training was deemed to be more enjoyable than, and preferable to, conventional therapy by all patients of the experimental group.
Discussion
The present study evaluated the effect of a rehabilitation protocol incorporating Leap Motion-based virtual reality training and conventional occupational rehabilitation therapy on the cortical reorganization and motor function recovery of the affected upper limb of patients who had experienced a subacute stroke.
All the participants in both groups exhibited significant improvements in the motor function of their affected upper limb in terms of the WMFT quality score (WMFT quality) after training. The WMFT quality score for the experimental group (Leap Motion-based virtual reality training combined with conventional occupational therapy) indicated greater improvement than that attained by the control group (only conventional occupational rehabilitation training, of the same total training duration as the experimental group). Meanwhile, after training, the action performance time in the WMFT was significantly shorter for both groups, while the WMFT time for the experimental group was significantly shorter than that for the control group. These results indicate that Leap Motion-based virtual reality training could facilitate the recovery of the motor function and dexterity of a paretic upper limb. This finding is consistent with that reported in a previously published study (Iosa et al., 2015). Similarly, the present study found that Leap Motion-based virtual reality training was deemed to be more enjoyable than, and preferable to, the conventional therapy, because Leap Motion-based virtual reality training using interactive virtual reality games can provide task-oriented practice, as well as visual and auditory feedback regarding performance and gain, which further motivates and engages players to increase the rehabilitation intensity (Saposnik et al., 2010, 2011; Iosa et al., 2015; Laver et al., 2015; Tsoupikova et al., 2015). A greater amount of practice promotes better outcomes in the event of impairments. Therefore, the effect of Leap Motion-based virtual reality training should be associated with the principle of high-intensity, repetitive and task-orientated training (Barreca et al., 2003; Wolf et al., 2006; Dobkin, 2008; Langhorne et al., 2009; Saposnik et al., 2011; Laver et al., 2015).
The results of the present study indicate that Leap Motion-based virtual reality training not only facilitates the motor function recovery of paretic upper limbs, but also promotes neural reorganization, as evidenced by the fMRI scan.
After training, the laterality index of SMC increased in both the experimental group and control group, while the Leap Motion-based virtual reality training produced a greater increase in the laterality index of SMC in the experimental group than in the control group. Cortical activation by the affected hand movements in the experimental group was also more obviously reorganized from ipsilateral (before Leap Motion-based virtual reality) to contralateral (after Leap Motion-based virtual reality) activation in the laterality index than that in the control group. These findings agreed with previous reports that pointed to a shift in the SMC activation from the ipsilateral or bilateral to contralateral regions after the intensive use of the paretic limb in adults (Carey et al., 2002; Miyai et al., 2002, 2003). Intensive repeated practice might be necessary to modify neural organization according to previous reports (Kopp et al., 1999; Nudo, 2011). Repetitive practice of the affected limb may increase practice-induced neuroplasticity by generating effective synaptic potentiation (Liepert et al., 2000). There was a more obvious shift in the SMC between brain hemispheres in the experimental group than that in the control group after training. This phenomenon might be due to two mechanisms. On the one hand, interactive virtual reality games provide task-oriented practice and feedback regarding performance and gain, which further motivate and engage players to increase the rehabilitation intensity (Saposnik et al., 2010, 2011; Laver et al., 2015; Tsoupikova et al., 2015). The higher intensity of the practice could promote a more obvious shift in the SMC between brain hemispheres in the experimental group than that in the control group through practice-induced neuroplasticity. On the other hand, during virtual reality training, the patient observed and imitated the movement of the representation (avatar) of his/her own hand and carried out the tasks required of the games in the virtual world by controlling the avatar through hand-arm coordination in the real world. Therefore, some studies have suggested that virtual reality training might induce a reorganization mechanism of the imitation-dependent cortex neuroplasticity through mirror neural networks (Rizzolatti et al., 1999; Holden and Dyar, 2002; Kim et al., 2006).
After training, the activation intensity of the contralateral primary SMC of the mainly activated area also increased in both the experimental group and control group. The Leap Motion-based virtual reality training produced a greater increase in the experimental group than in the control group. A previous study revealed that the recovery of motor function is mainly due to the activation of the contralateral (ipsilesional) sensorimotor area at the subacute stage (Kim et al., 2006). The function of the primary SMC is to control the speed, extent, direction, and force of the movement. Our findings support the theory that the contralateral primary sensorimotor area continues to exert primary control over the affected upper limb after rehabilitation training.
In addition, activation in the SMA and cerebellum was also observed in both groups before and after training. The SMA is involved in both producing and mentally rehearsing movement sequences, while the cerebellum oversees the coordination of voluntary movements and motor learning. However, there was no obvious evolving pattern of change after training in these two areas in the present study. This observation was consistent with the results of previous studies (Nair et al., 2007; Mintzopoulos et al., 2009). The activation of the SMA and cerebellum induced by Leap Motion-based virtual reality training may play a compensatory role in neural reorganization.
In summary, our findings revealed that Leap Motion-based virtual reality training could facilitate cortical reorganization and might facilitate the motor function recovery of an affected upper limb in patients who had experienced a subacute stroke. The Leap Motion-based virtual reality training could be a promising and feasible adjuvant rehabilitation intervention to conventional rehabilitation therapy for promoting motor recovery in stroke patients.
Although the results are encouraging, some limitations of our study should be noted. First, the sample size is too small for an in-depth analysis, and therefore any future work should be planned with a larger number of patients. Second, the patients in this study all had mild to moderate impairments; thus, the results cannot be generalized for the rehabilitation of all patients after a stroke, especially those with severe impairments. Third, the long-term effects of Leap Motion-based virtual reality training were not evaluated in this study. Future studies should further investigate the changes 3 months after training to better understand the lasting effect.
Acknowledgments
We would like to thank the patients for their participation. We thank all physicians and nurses from Department of Neurorehabilitation, China Rehabilitation Research Center for their assistance in performing this study.
Footnotes
Funding: This study was financially supported by the Sub-Project under National “Twelfth Five-Year” Plan for Science & Technology Support Project in China, No. 2011BAI08B11; and the Research Project of China Rehabilitation Research Center, No. 2014-3.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by Ethics Committee of China Rehabilitation Research Center (approval number: 2012-045-1). The study has been registered in Chinese Clinical Trial Registry (registration number: ChiCTR-OCH-12002238). The study followed the Declaration of Helsinki and relevant ethical principles.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form, patients gave their consent for their images and other clinical information to be reported in the journal. Patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jixian Wang, Shanghai Jiao Tong University, China.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- 1.Baeck JS, Kim YT, Seo JH, Ryeom HK, Lee J, Choi SM, Woo M, Kim W, Kim JG, Chang Y. Brain activation patterns of motor imagery reflect plastic changes associated with intensive shooting training. Behav Brain Res. 2012;234:26–32. doi: 10.1016/j.bbr.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Bao X, Mao Y, Lin Q, Qiu Y, Chen S, Li L, Cates RS, Zhou S, Huang D. Mechanism of Kinect-based virtual reality training for motor functional recovery of upper limbs after subacute stroke. Neural Regen Res. 2013;8:2904–2913. doi: 10.3969/j.issn.1673-5374.2013.31.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreca S, Wolf SL, Fasoli S, Bohannon R. Treatment interventions for the paretic upper limb of stroke survivors: a critical review. Neurorehabil Neural Repair. 2003;17:220–226. doi: 10.1177/0888439003259415. [DOI] [PubMed] [Google Scholar]
- 4.Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- 5.Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 7.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 9.Día DV, Gutiérrez SSM. Biomechanics and motor control of human movement. XIKUA Boletín Científico de la Escuela Superior de Tlahuelilpan 1. 2013 [Google Scholar]
- 10.da Silva Cameirao M, Bermúdez I Badia S, Duarte E, Verschure PF. Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the rehabilitation gaming system. Restor Neurol Neurosci. 2011;29:287–298. doi: 10.3233/RNN-2011-0599. [DOI] [PubMed] [Google Scholar]
- 11.Dobkin BH. Training and exercise to drive poststroke recovery. Nat Clin Pract Neurol. 2008;4:76–85. doi: 10.1038/ncpneuro0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 13.French B, Thomas LH, Coupe J, McMahon NE, Connell L, Harrison J, Sutton CJ, Tishkovskaya S, Watkins CL. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2016;11:CD006073. doi: 10.1002/14651858.CD006073.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatica-Rojas V, Méndez-Rebolledo G. Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases. Neural Regen Res. 2014;9:888–896. doi: 10.4103/1673-5374.131612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bagg S, Bayley M, Dowlatshahi D, Dukelow S, Garnhum M, Glasser E, Halabi ML, Kang E, MacKay-Lyons M, Martino R, Rochette A, Rowe S, Salbach N, Semenko B, Stack B, et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11:459–484. doi: 10.1177/1747493016643553. [DOI] [PubMed] [Google Scholar]
- 16.Holden MK, Dyar T. Virtual Environment Training: A New Tool for Neurorehabilitation. J Neurol Phys Ther. 2002;26:62–71. [Google Scholar]
- 17.Iosa M, Morone G, Fusco A, Castagnoli M, Fusco FR, Pratesi L, Paolucci S. Leap motion controlled videogame-based therapy for rehabilitation of elderly patients with subacute stroke: a feasibility pilot study. Top Stroke Rehabil. 2015;22:306–316. doi: 10.1179/1074935714Z.0000000036. [DOI] [PubMed] [Google Scholar]
- 18.Jang SH, You SH, Hallett M, Cho YW, Park CM, Cho SH, Lee HY, Kim TH. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: an experimenter-blind preliminary study. Arch Phys Med Rehabil. 2005;86:2218–2223. doi: 10.1016/j.apmr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Jutai JW, Teasell RW. The necessity and limitations of evidence-based practice in stroke rehabilitation. Top Stroke Rehabil. 2003;10:71–78. doi: 10.1310/CRDA-PGFW-KHEL-20E1. [DOI] [PubMed] [Google Scholar]
- 20.Kim SG, Hendrich K, Hu X, Merkle H, Uǧurbil K. Potential pitfalls of functional MRI using conventional gradient-recalled echo techniques. NMR Biomed. 1994;7:69–74. doi: 10.1002/nbm.1940070111. [DOI] [PubMed] [Google Scholar]
- 21.Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH. Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology. 2006;67:330–333. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- 22.Kim YM, Chun MH, Yun GJ, Song YJ, Young HE. The effect of virtual reality training on unilateral spatial neglect in stroke patients. Ann Rehabil Med. 2011;35:309–315. doi: 10.5535/arm.2011.35.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong KH, Chua KS, Lee J. Recovery of upper limb dexterity in patients more than 1 year after stroke: Frequency, clinical correlates and predictors. NeuroRehabilitation. 2011;28:105–111. doi: 10.3233/NRE-2011-0639. [DOI] [PubMed] [Google Scholar]
- 24.Kopp B, Kunkel A, Muhlnickel W, Villringer K, Taub E, Flor H. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. Neuroreport. 1999;10:807–810. doi: 10.1097/00001756-199903170-00026. [DOI] [PubMed] [Google Scholar]
- 25.Kwakkel G, Wagenaar RC, Kollen BJ, Lankhorst GJ. Predicting disability in stroke--a critical review of the literature. Age Ageing. 1996;25:479–489. doi: 10.1093/ageing/25.6.479. [DOI] [PubMed] [Google Scholar]
- 26.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 27.Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2015:CD008349. doi: 10.1002/14651858.CD008349.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefebvre S, Dricot L, Gradkowski W, Laloux P, Vandermeeren Y. Brain activations underlying different patterns of performance improvement during early motor skill learning. Neuroimage. 2012;62:290–299. doi: 10.1016/j.neuroimage.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 29.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 30.Liu SW, Duan CQ. Interactive metronome applied in the rehabilitative treatment of the central nervous system. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:2619–2624. [Google Scholar]
- 31.Martin J, Rabin B, Kale A, DiSanto P. Motor retraining in virtual reality: a feasibility study for upper-extremity rehabilitation in individuals with chronic stroke. J Phys Ther Educ. 2011;25:20. [Google Scholar]
- 32.Merians AS, Tunik E, Adamovich SV. Virtual reality to maximize function for hand and arm rehabilitation: exploration of neural mechanisms. Stud Health Technol Inform. 2009;145:109–125. [PMC free article] [PubMed] [Google Scholar]
- 33.Mintzopoulos D, Astrakas LG, Khanicheh A, Konstas AA, Singhal A, Moskowitz MA, Rosen BR, Tzika AA. Connectivity alterations assessed by combining fMRI and MR-compatible hand robots in chronic stroke. Neuroimage 47 Suppl. 2009;2:T90-97. doi: 10.1016/j.neuroimage.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke. 2003;34:2866–2870. doi: 10.1161/01.STR.0000100166.81077.8A. [DOI] [PubMed] [Google Scholar]
- 35.Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, Kubota K. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol. 2002;52:188–194. doi: 10.1002/ana.10274. [DOI] [PubMed] [Google Scholar]
- 36.Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage. 2007;34:253–263. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:852–857. doi: 10.1016/0003-9993(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 38.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36:1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f. [DOI] [PubMed] [Google Scholar]
- 39.Nudo RJ. Neural bases of recovery after brain injury. J Commun Disord. 2011;44:515–520. doi: 10.1016/j.jcomdis.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orihuela-Espina F, Fernández del Castillo I, Palafox L, Pasaye E, Sánchez-Villavicencio I, Leder R, Franco JH, Sucar LE. Neural reorganization accompanying upper limb motor rehabilitation from stroke with virtual reality-based gesture therapy. Top Stroke Rehabil. 2013;20:197–209. doi: 10.1310/tsr2003-197. [DOI] [PubMed] [Google Scholar]
- 42.Page SJ, Levine P, Sisto SA, Johnston MV. Mental practice combined with physical practice for upper-limb motor deficit in subacute stroke. Phys Ther. 2001;81:1455–1462. doi: 10.1093/ptj/81.8.1455. [DOI] [PubMed] [Google Scholar]
- 43.Piron L, Tonin P, Atzori AM, Zucconi C, Massaro C, Trivello E, Dam M. The augmented-feedback rehabilitation technique facilitates the arm motor recovery in patients after a recent stroke. Stud Health Technol Inform. 2003;94:265–267. [PubMed] [Google Scholar]
- 44.Rizzolatti G, Fadiga L, Fogassi L, Gallese V. Resonance behaviors and mirror neurons. Arch Ital Biol. 1999;137:85–100. [PubMed] [Google Scholar]
- 45.Saposnik G, Levin M Outcome Research Canada (SORCan) Working Group. Virtual reality in stroke rehabilitation: a meta-analysis and implications for clinicians. Stroke. 2011;42:1380–1386. doi: 10.1161/STROKEAHA.110.605451. [DOI] [PubMed] [Google Scholar]
- 46.Saposnik G, Teasell R, Mamdani M, Hall J, McIlroy W, Cheung D, Thorpe KE, Cohen LG, Bayley M Stroke Outcome Research Canada (SORCan) Working Group. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke. 2010;41:1477–1484. doi: 10.1161/STROKEAHA.110.584979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sehatzadeh S. Effect of increased intensity of physiotherapy on patient outcomes after stroke: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15:1–42. [PMC free article] [PubMed] [Google Scholar]
- 48.Smeragliuolo AH, Hill NJ, Disla L, Putrino D. Validation of the Leap Motion Controller using markered motion capture technology. J Biomech. 2016;49:1742–1750. doi: 10.1016/j.jbiomech.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Szameitat AJ, Shen S, Conforto A, Sterr A. Cortical activation during executed, imagined, observed, and passive wrist movements in healthy volunteers and stroke patients. Neuroimage. 2012;62:266–280. doi: 10.1016/j.neuroimage.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Tarkka IM, Könonen M, Pitkanen K, Sivenius J, Mervaalat E. Alterations in cortical excitability in chronic stroke after constraint-induced movement therapy. Neurol Res. 2008;30:504–510. doi: 10.1179/016164107X252519. [DOI] [PubMed] [Google Scholar]
- 51.Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy: a new family of techniques with broad application to physical rehabilitation--a clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- 52.Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 53.Teasell R, Meyer MJ, McClure A, Pan C, Murie-Fernandez M, Foley N, Salter K. Stroke rehabilitation: an international perspective. Top Stroke Rehabil. 2009;16:44–56. doi: 10.1310/tsr1601-44. [DOI] [PubMed] [Google Scholar]
- 54.Teasell RW, Murie Fernandez M, McIntyre A, Mehta S. Rethinking the continuum of stroke rehabilitation. Arch Phys Med Rehabil. 2014;95:595–596. doi: 10.1016/j.apmr.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Tong Y, Forreider B, Sun X, Geng X, Zhang W, Du H, Zhang T, Ding Y. Music-supported therapy (MST) in improving post-stroke patients’ upper-limb motor function: a randomised controlled pilot study. Neurol Res. 2015;37:434–440. doi: 10.1179/1743132815Y.0000000034. [DOI] [PubMed] [Google Scholar]
- 56.Tsoupikova D, Stoykov NS, Corrigan M, Thielbar K, Vick R, Li Y, Triandafilou K, Preuss F, Kamper D. Virtual immersion for post-stroke hand rehabilitation therapy. Ann Biomed Eng. 2015;43:467–477. doi: 10.1007/s10439-014-1218-y. [DOI] [PubMed] [Google Scholar]
- 57.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9:e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X. Key points of diagnosis of cerebrovascular diseases. Zhonghua Shenjing Ke Zazhi. 1996;29:379–380. [Google Scholar]
- 59.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 60.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 61.Yavuzer G, Senel A, Atay MB, Stam HJ. “Playstation eyetoy games” improve upper extremity-related motor functioning in subacute stroke: a randomized controlled clinical trial. Eur J Phys Rehabil Med. 2008;44:237–244. [PubMed] [Google Scholar]
- 62.Yin CW, Sien NY, Ying LA, Chung SF, Tan May Leng D. Virtual reality for upper extremity rehabilitation in early stroke: a pilot randomized controlled trial. Clin Rehabil. 2014;28:1107–1114. doi: 10.1177/0269215514532851. [DOI] [PubMed] [Google Scholar]
- 63.Yu N, Xu C, Li H, Wang K, Wang L, Liu J. Fusion of haptic and gesture sensors for rehabilitation of bimanual coordination and dexterous manipulation. Sensors (Basel) 2016;16:E395. doi: 10.3390/s16030395. [DOI] [PMC free article] [PubMed] [Google Scholar]