Abstract
Patient: Male, 70
Final Diagnosis: Spontaneous spinal epidural hematoma
Symptoms: Abdominal pain • chest pain • complete paraplegia
Medication: —
Clinical Procedure: Conservative management
Specialty: Nephrology
Objective:
Unusual clinical course
Background:
Spontaneous spinal epidural hematoma (SSEH) occurs in the spinal epidural space in the absence of traumatic or iatrogenic causes, and is considered to be a neurological emergency, as spinal cord compression may lead to neurological deficit. Prompt diagnosis of SSEH can be difficult due to the variety of presenting symptoms, which may resemble those of stroke. Patients who undergo hemodialysis (HD) are at risk of bleeding due to anticoagulation during dialysis and uremia. However, SSEH in HD patients undergoing HD has rarely been reported.
Case Report:
A 70-year-old Japanese man, who has been undergoing maintenance HD for the previous three years, was admitted to Kariya Toyota General Hospital, Aichi, Japan, with acute chest and abdominal pain, and with complete paraplegia. The patient denied any recent trauma or medical procedures. Magnetic resonance imaging showed an extensive hematoma in the thoracic and lumbar epidural space, extending from T8 to L5. The patient’s symptoms improved within three hours following hospital admission, and after three days without HD treatment, the SSEH decreased in size, and the patient successfully recovered without residual neurological deficits and without requiring surgery.
Conclusions:
The management of SSEH in patients undergoing HD can be difficult, due to anticoagulation during dialysis and uremia. Prompt diagnosis and close neurological monitoring are important for appropriate management. In patients whose symptoms improve within a short period, conservative management may be considered.
MeSH Keywords: Hematoma, Epidural, Spinal; Kidney Failure, Chronic; Renal Dialysis
Background
Spontaneous spinal epidural hematoma (SSEH) was first reported by Jackson in 1869 [1]. SSEH can occur at any age and occurs without a known episode of trauma, but the clinical associations that have been reported have included spinal arteriovenous malformations [2], anticoagulant therapy [3], anti-platelet therapy [4], bleeding disorders [5], and pregnancy [6]. The occurrence of SSEH is rare, with an estimated annual incidence of 0.1 per 100,000 persons [7]. However, SSEH presents as a neurological emergency as it can lead to irreversible neurological deficit.
Bleeding from the vertebral venous plexus (VVP) is considered to be the origin of SSEH because the VVP is a fragile structure and is valveless [8]. Increase in intra-abdominal or intrathoracic pressure has been reported to lead to bleeding from the VVP, during activities such as breath holding during diving [9], swimming [10], and sneezing [11]. Compression of the spinal cord and spinal nerve roots in the vertebral epidural space by the hematoma can lead to a variety of neurological signs and symptoms, which is why spinal epidural hematoma is sometimes misdiagnosed as a stroke [12], acute coronary syndrome [13], or as an ‘acute abdomen’ [14].
Rapid and accurate diagnosis is needed for appropriate management of SSEH. Although surgical approaches to the treatment of SSEH, including decompression laminectomy, may be considered in patients with a rapid clinical course, conservative management may be more appropriate in patients who show spontaneous recovery within a short period. However, there are no clear clinical guidelines for the management of SSEH occurring in different clinical contexts, such as in patients undergoing hemodialysis (HD).
At present, there have been few reported cases of SSEH in patients undergoing HD [15–21]. This report describes a case of a large SSEH in a patient undergoing HD, who initially presented with complete paraplegia and who successfully recovered with conservative management and without surgery.
Case Report
A 70-year-old Japanese man, who was undergoing HD for end-stage renal disease (ESRD) caused by diabetic nephropathy, was admitted to our hospital due to acute onset of back pain and weakness in the lower extremities. The patient had been undergoing four-hourly HD sessions, three times per week for the previous three years. During dialysis, low molecular weight heparin (LMWH), as an initial bolus of 1000 U followed by continuous infusion of 500 U/hr, was used as an anticoagulant. The patient’s last HD session, which was completed with no immediate complications, was one day prior to this emergency admission to the hospital.
The patient reported that his symptoms began while he was lying in bed at home, and described the sudden onset of chest and abdominal pain. The patient stated that he felt weakness and loss of sensation in the lower extremities, 10 minutes after the onset of the initial symptoms of chest and abdominal pain. In addition to a medical history of diabetes, he also had a past medical history of hypertension. However, he had no recent surgery and denied any recent traumatic event or change in his treatment. His blood pressure was well controlled, and he was not taking any oral anticoagulant or antiplatelet drugs and was not anticoagulated, other than during dialysis.
On admission to hospital, his vital signs included a body temperature of 36.7°C, blood pressure 203/86 mmHg, heart rate of 88 beats per minute, blood oxygen saturation level of 99% in normal air, and respiratory rate of 18 breaths per minute. Neurological examination showed the presence of complete paraplegia. Lower limb manual muscle test (MMT) score was 1 out of 5 (trace), and loss of sensation was detected below the level of T10 nerve supply. On rectal examination, there was no muscle tone in external anal sphincter muscle. The patient’s coagulation screen showed prothrombin time-international normalized ratio of 0.95, partial thromboplastin time of 29.5 seconds, and plasma fibrinogen of 387 mg/dL.
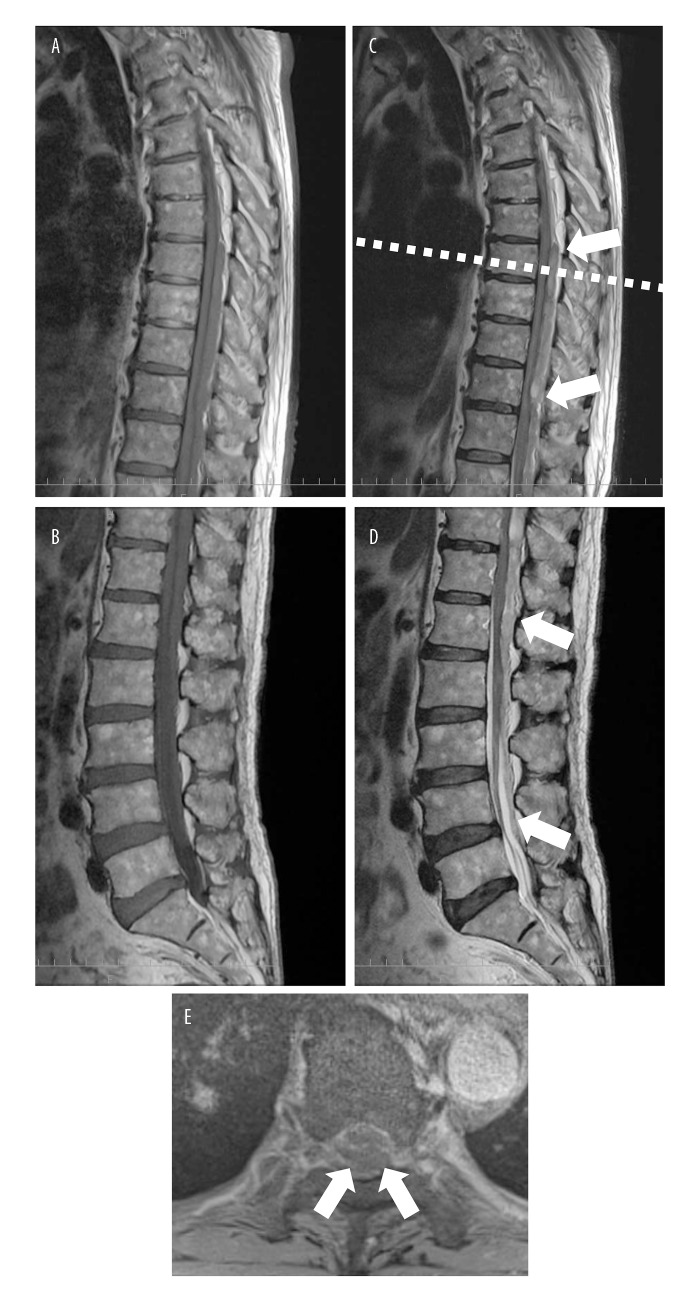
Magnetic resonance imaging (MRI) showed an extensive spinal epidural hematoma from the level of T8 to the level of L5 (Figure 1). Although emergency surgical intervention was considered, complete paraplegia was noted to improve gradually soon after the completion of MRI, about three hours after the onset of symptoms. The patient started to move his legs, and the lower extremity MMT score improved to 4 (good), his lower limb sensation, returned to normal, and repeat rectal examination showed that the muscle tone of the external anal sphincter muscle was good. Based on the improved clinical course, the patient was treated with bed rest and his next scheduled dialysis session, scheduled for the next day following admission, was canceled.
Figure 1.
Sagittal and transverse magnetic resonance imaging (MRI) of the thoracic and lumbar spine on the day of hospital admission. (A–D) The sagittal magnetic resonance image shows an extensive epidural hematoma at the T8–L5 level. (A, B) The hematoma is iso-hypointense on T1-weighted images. (C, D) The hematoma is iso-hyperintense on T2-weighted images (arrows). (E) The transverse magnetic resonance image of the spine captured at the level of the dotted line in image C shows compression of the spinal cord by the hematoma (arrows).
On the third day of hospital admission, the patient was restarted on his four-hourly HD sessions, three times per week, but with anticoagulation with nafamostat mesilate (NM), a synthetic serine protease inhibitor, by continuous infusion of 30 mg/hr. The first dialysis session was performed without any problems. The chest and abdominal pain gradually reduced until they had completely gone by day 5 following initial hospital admission, without requiring pain medications.
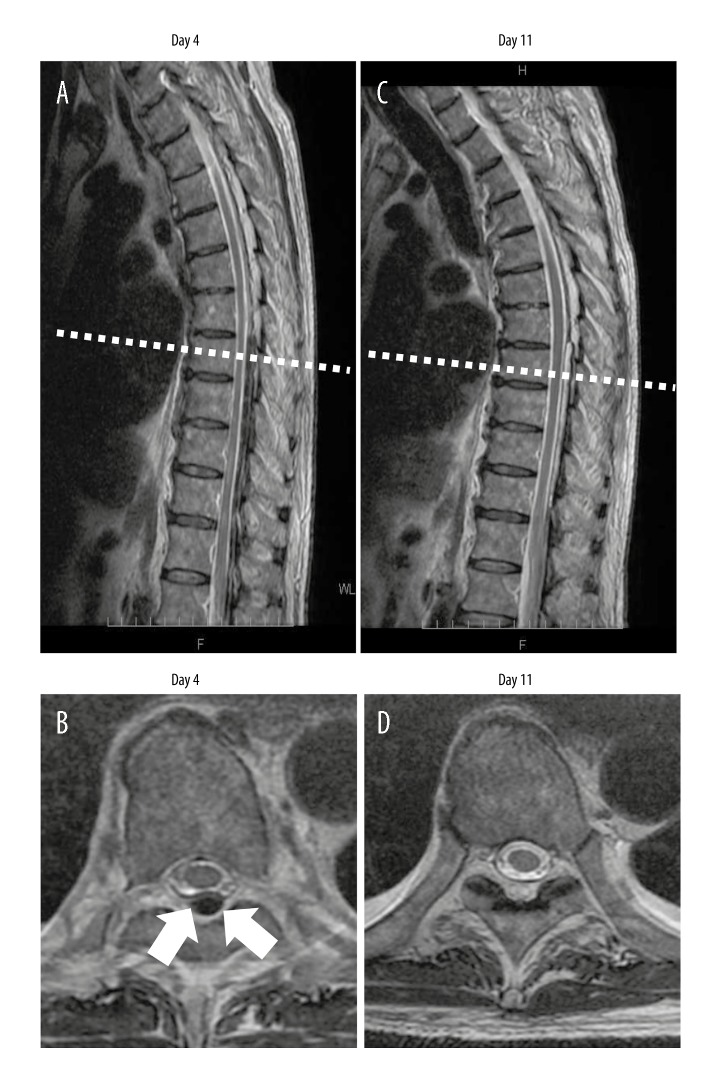
Figure 2 shows that comparison between the MRI images obtained on day 4 and day 11 following hospital admission, confirms that hematoma had reduced in size within a short period. The use of the anticoagulant, NM that had been used during dialysis, was switched back to LMWH on day 18. After ensuring that there was no exacerbation or recurrence of symptoms, the patient was discharged on day 19, without any neurological deficits. The patient’s lower limb MMT score was 5 (normal) at the time of hospital discharge.
Figure 2.
Follow-up magnetic resonance imaging (MRI) of the thoracic spine on day 4 and day 11. (A, B) MRI images on day 4. (C, D) MRI images on day 11. (A, C). The size of the epidural hematoma has decreased over time. (B, D) In transverse magnetic resonance images of the spine captured at the level of the dotted lines in images A and C respectively, there is a reduction in the size of the hematoma (arrows).
Discussion
In patients with ESRD, including diabetic nephropathy, the risk of bleeding is increased due to impaired hemostasis, which mainly results from platelet dysfunction due to uremia [22]. HD can also contribute to bleeding episodes by platelet activation and degranulation occurring during dialysis [23]. Also, anticoagulants are used during dialysis to prevent thrombosis in the HD circuit. LMWH has now replaced the use of unfractionated heparin (UHF) and is associated with fewer bleeding episodes and a reduced incidence of heparin-induced thrombocytopenia, compared with UFH [24,25]. In this case, we selected the use of NM as the anticoagulant used during dialysis, immediately following the resolution of the episode of SSEH, because NM is characterized by a short half-life. There have been no clinical trials that have investigated the safety of NM in patients undergoing HD with a spinal epidural hematoma. However, Yang et al. have reported that NM should be considered in HD patients who suffer from intracerebral hemorrhage, as this form of anticoagulant was associated with a significant reduction in the intracranial hematoma volume when compared with heparin [26].
When SSEH occurs in patients undergoing HD, the management may be difficult because not only are patients undergoing HD anticoagulated, but they also have an increased risk of bleeding due to uremia. Review of the published literature in the English language (using the PubMed database) has identified four cases of SSEH in patients undergoing HD [15–18]. A review of the published literature in the Japanese language (using the Igaku Chuo Zasshi database) has identified six cases of SSEH in patients undergoing HD, reported since 1990 [19–21] (Table 1). In all of the cases, acute pain at the hematoma site was the initial symptom. In these cases, the duration of HD varied from one year to 16 years; duration of HD was not shown to be a risk factor for the development of SSEH; in three cases (30%), SSEH occurred during dialysis (Table 1).
Table 1.
Previously reported cases of spontaneous spinal epidural hematoma in hemodialysis patients since 1990 in the English and Japanese literature, including the present case.
| Case number [Reference] | Literary language | Reported year | Age/Sex | Duration of HD | Onset | Underlying diseases | Symptoms | Location of the hematoma | Treatment (Operative interval) | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 [15] | English | 1999 | 67/F | 9 years | During dialysis | HTN AP ADPKD |
Back pain followed by complete sensory and motor loss | T8–L2 | Surgery (10 h) | Complete recovery |
| Case 2 [16] | English | 2002 | 61/M | 1 year | Off dialysis | DM | Back pain followed by muscle weakness, hypoesthesia below the level T6, anesthesia below the level T10, and absence of sphincter tone | T5–L1 | DDAVP administration and surgery (21 h) | Recovery; however, death due to infection 2 months after operation |
| Case 3 [17] | English | 2003 | 47/F | 3 years | N/A | DM | Back pain followed by muscle weakness in lower limbs (1/5*), sensory impairment below the level T4 and positive Babinski reflex on both sides | T3–S1 | Surgery (>48 h) | Improvement in muscle strength to 3/5* 1 month after surgery |
| Case 4 [18] | English | 2009 | 77/F | 3 years | Off dialysis | HTN | Neck pain and back pain followed by paresthesia, bilateral lower limb weakness (2/5*), and right upper limb weakness (0/5*) | C2–T9 | Surgery (immediately after few hours) | Good recovery in lower limbs (4/5*) and poor recovery in right upper limb |
| Case 5 [19] | Japanese | 1992 | 60/M | 16 years | Off dialysis | CGN | Neck pain followed by quadriplegia and paresthesia below the level C4, bilateral positive Babinski reflex and Wartenberg reflex and positive knee and ankle clonus | C3–T1 | Surgery (20 h) | Incomplete recovery; however, the patient could walk using crutches |
| Case 6 [19] | Japanese | 1993 | 47/F | 12 years | During dialysis | N/A | Neck pain followed by complete paralysis in lower limbs and partial paralysis in upper limbs | C3–T2 | Surgery (10 h) | Persistent partial paralysis in upper and lower limbs Ventilator-assistance needed |
| Case 7 [20] | Japanese | 1993 | 61/M | 15 years | During farm work | N/A | Neck pain followed by incomplete paralysis, paresthesia in upper and lower limbs, ankle clonus positive bilaterally, exaggeration of DTR and fasciculation in lower limbs | C3–C6 | Surgery (29 h) | Impaired skill movement, persistence of numbness distal to wrist joints and inguinal regions |
| Case 8 [20] | Japanese | 1996 | 54/F | 1 year | During dialysis | HCC | Systemic pain followed by incomplete paralysis in upper limbs, complete flaccid paralysis in lower limbs, bladder and rectal disturbance and decreased DTR | C5–T1 | Conservative management | Improvement in paralysis starting 11 days after onset, full recovery 4 months after onset Disappearance of hematoma disappeared 2 months after onset |
| Case 9 [20] | Japanese | 2000 | 63/M | 4 years | N/A | DM | Stiff neck, neck pain, chest pain followed by complete quadriplegia and absence of DTR | C2–C5 | Surgery (30 h) | Only slight improvement in right upper limb Death due to decubitus infection and worsening general status |
| Case 10 [21] | Japanese | 2009 | 61/F | N/A | N/A | DM | Neck pain and back pain followed by quadriplegia (right side: 3/5*, left side: 0–1/5*), paresthesia and absence of DTR and sphincter tone | C2–T2 | Surgery (18 h) | Upper and lower limb weakness (4/5*) and paresthesia remaining on the left side |
| Case 11 [The present case] | Japanese | 2017 | 70/M | 3 years | Off dialysis | DM HTN |
Chest pain and abdominal pain followed by complete hemiplegia and absence of sphincter tone | T8–L5 | Conservative management | Complete recovery |
Motor strength was evaluated based on the manual muscle test score. F – female; M – male; HD – hemodialysis; N/A – not available; HTN – hypertension; AP – angina pectoris; ADPKD – autosomal dominant polycystic kidney disease; DM – diabetes mellitus; CGN – chronic glomerulonephritis; HCC – hepatocellular carcinoma; DTR – deep tendon reflex; DDAVP – 1-desamino-8-arginine vasopressin.
The use of anticoagulants during dialysis could be one of the risk factors for the development of SSEH in patients undrgoing HD. On the review of previous case reports, conservative management was used in only one case (10%) [20] (Table 1). In this case, the length of complete recovery was about four months, and conservative management was selected due to patient comorbidities, including hepatocellular carcinoma [20]. Importantly, in most of the previous cases published in the English and Japanese literature, patients had neurological deficits even following early surgical intervention within 24 hours after the onset of symptoms [15,16,18,19,21].
This is the first reported case of SSEH in a patient undergoing HD who showed spontaneous recovery within a short period, of days. The findings of this case report indicate that recovery from SSEH may occur and may be associated with improved clinical outcome when compared with surgical intervention. However, there are no guidelines for the management of SSEH in patients undergoing HD, and further clinical studies are required. In this case, spontaneous recovery was due to decompression of the spinal cord as the hematoma extended in the epidural space, and then resolved. However, in 1996, Groen et al. reviewed 333 cases of SSEH in patients undergoing surgery and reported that there was no correlation between post surgical outcomes and patient sex, age, hematoma size, or hematoma position [27]. These authors also reported that the mortality rate was high in patients with cervical and cervicothoracic lesions [27].
In 2011, a study by Fedor et al. reviewed 107 cases of SSEH in patients who had undergone surgery since 1996 and reported that age, past medical history of hypertension, anticoagulant use, and hematoma size were not significant post-surgical prognostic factors for SSEH [28]. Also, these authors noted that early surgery, within 24 hours of the onset of symptoms resulted in improved clinical outcomes when compared with surgery after 24 hours of the onset of symptoms (Frankel grade was 0.60 points greater), although the findings were not statistically significant [28]. In 2004, Groen reported that the mean hematoma extent was significantly greater in the conservatively managed group compared with the surgical group among patients with SSEH [29]. The author speculated that spreading of the hematoma along the epidural space led to spinal cord decompression and subsequent symptomatic improvement [29]. Recently, Raasck et al. have reviewed the management and clinical outcomes for patients with SSEH in a review of 65 previously reported cases, which comprised 50 cases that were treated surgically and 15 cases that were treated conservatively [30]. The authors found that surgical management was more likely to be selected in high-risk cases and that surgical interval was a significant prognostic factor [30]. Also, conservative management, which was likely to be selected in low-risk cases, was effective, even in some high-risk cases [30]. In the current case, surgery was considered due to the presence of severe neurological symptoms on initial presentation, but conservative management was chosen as the patient’s symptoms improved remarkably within three hours.
Conclusions
Although SSEH is a rare condition, including in patients undergoing HD, early diagnosis and close neurological monitoring are important to improve clinical outcome. Although treatment strategies for SSEH remain unclear, immediate surgical management should be considered, particularly in patients with severe neurological manifestations and to prevent irreversible neurological deficit. However, as this case has shown, conservative management may be considered in patients with SSEH undergoing maintenance HD if neurological symptoms start to improve spontaneously within a short period, and if anticoagulation during diaylsis is closely monitored.
Acknowledgments
The authors are grateful to the biomedical technicians of Kariya Toyota General Hospital, Aichi, Japan, who support the management of patients on dialysis.
Footnotes
Conflicts of interest
None.
References:
- 1.Jackson R. Case of spinal apoplexy. The Lancet. 1869;2392:5–6. [Google Scholar]
- 2.Cabral AJ, Barros A, Aveiro C, Vasconcelos R. Spontaneous spinal epidural hematoma due to arteriovenous malformation in a child. BMJ Case Rep. 2011;2011:3875. doi: 10.1136/bcr.02.2011.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandvig A, Jonsson H. Spontaneous chronic epidural hematoma in the lumbar spine associated with Warfarin intake: A case report. Springerplus. 2016;1:1832. doi: 10.1186/s40064-016-3546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung JH, Hong JT, Son BC, Lee SW. Clopidogrel-induced spontaneous spinal epidural hematoma. J Korean Med Sci. 2007;3:577–79. doi: 10.3346/jkms.2007.22.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn DK, Jung WS, Lee JI. Hemophilia A in a senior patient: A case report of spontaneous spinal epidural hematoma as first presentation. Asian Spine J. 2015;3:452–55. doi: 10.4184/asj.2015.9.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P, Xin XT, Lan H, et al. Spontaneous cervical epidural hematoma during pregnancy: a case report and literature review. Eur Spine J. 2011;(Suppl 2):S176–79. doi: 10.1007/s00586-010-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtås S, Heiling M, Lönntoft M. Spontaneous spinal epidural hematoma: Findings at MR imaging and clinical correlation. Radiology. 1996;2:409–13. doi: 10.1148/radiology.199.2.8668786. [DOI] [PubMed] [Google Scholar]
- 8.Nathoo N, Caris EC, Wiener JA, Mendel E. History of the vertebral venous plexus and the significant contribution of Breschet and Batson. Neurosurgery. 2011;5:1007–14. doi: 10.1227/NEU.0b013e3182274865. [DOI] [PubMed] [Google Scholar]
- 9.Tremolizzo L, Patassini M, Malpieri M, et al. A case of spinal epidural hematoma during breath-hold diving. Diving Hyperb Med. 2012;2:98–100. [PubMed] [Google Scholar]
- 10.Fleager K, Lee A, Cheng I, et al. Massive spontaneous epidural hematoma in a high-level swimmer: A case report. J Bone Joint Surg Am. 2010;17:2843–46. doi: 10.2106/JBJS.I.01604. [DOI] [PubMed] [Google Scholar]
- 11.Štětkářová I, Jelínková L, Janík V, Peisker T. Spontaneous spinal epidural hematoma after abrupt sneezing with prompt recovery of severe paraparesis. Am J Emerg Med. 2014;12:1555.e3–5. doi: 10.1016/j.ajem.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Akimoto T, Yamada T, Shinoda S, et al. Spontaneous spinal epidural hematoma as a potentially important stroke mimic. J Cent Nerv Syst Dis. 2014;6:15–20. doi: 10.4137/JCNSD.S13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed N, Shahid M, Haque M, et al. Spontaneous spinal epidural hematoma mimicking acute coronary syndrome. Quant Imaging Med Surg. 2015;6:925–27. doi: 10.3978/j.issn.2223-4292.2015.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nirupam N, Pemde H, Chandra J. Spinal epidural hematoma in a patient with hemophilia B presenting as acute abdomen. Indian J Hematol Blood Transfus. 2014;(Suppl. 1):54–56. doi: 10.1007/s12288-013-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Koiwa F, Tayama H, et al. A case of acute spontaneous epiduaral haematoma in a chronic renal failure patient undergoing hemodialysis: Successful outcome with surgical management. Nephrol Dial Transplant. 1999;10:2499–501. doi: 10.1093/ndt/14.10.2499. [DOI] [PubMed] [Google Scholar]
- 16.Sung JM, Hsieh CC, Yu CY, Huang JJ. Acute spontaneous spinal epidural hematoma in a hemodialysis patient with a bleeding tendency. Nephron. 2002;2:358–60. doi: 10.1159/000058423. [DOI] [PubMed] [Google Scholar]
- 17.Ziyal IM, Aydin S, Inci S, et al. Multilevel acute spinal epidural hematoma in a patient with chronic renal failure-case report. Neurol Med Chir (Tokyo) 2003;8:409–12. doi: 10.2176/nmc.43.409. [DOI] [PubMed] [Google Scholar]
- 18.Deger SM, Emmez H, Bahadirli K, et al. A spontaneous spinal epidural hematoma in a hemodialysis patient: A rare entity. Intern Med. 2009;24:2115–18. doi: 10.2169/internalmedicine.48.2335. [DOI] [PubMed] [Google Scholar]
- 19.Hinoi T, Tanaka I, Haruta N, et al. [A case of acute spinal epidural hematoma in a chronic hemodialysis patient] J Jpn Soc Dial Ther. 1993;12:1801–5. [in Japanese] [Google Scholar]
- 20.Ishii T, Watanabe S, Iwanaga S, et al. [A case of spinal epidural hematomana during maintenance dialysis therapy] J Jpn Soc Dia Ther. 2000;3:209–17. [in Japanese] [Google Scholar]
- 21.Yoshimatsu H, Wakioka T, Yoshida K, et al. [Clinical results of spontaneous spinal epidural hematoma in cervical area] Orthopedics & Traumatology. 2009;2:237–40. [in Japanese] [Google Scholar]
- 22.Lutz J, Menke J, Sollinger D, et al. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;1:29–40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 23.Daugirdas JT, Bernardo AA. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 2012;2:147–57. doi: 10.1038/ki.2012.130. [DOI] [PubMed] [Google Scholar]
- 24.Shen JI, Winkelmayer WC. Use of safety of unfractionated heparin for anticoagulation during maintenance hemodialysis. Am J Kidney Dis. 2012;3:473–86. doi: 10.1053/j.ajkd.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davenport A. The rationale for the use of low molecular weight heparin for hemodialysis treatment. Hemodial Int. 2013;(Suppl. 1):S28–32. doi: 10.1111/hdi.12086. [DOI] [PubMed] [Google Scholar]
- 26.Yang JW, Han BG, Kim BR, et al. Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing hemodialysis with intracerebral hemorrhage. Ren Fail. 2009;8:668–75. doi: 10.3109/08860220903180616. [DOI] [PubMed] [Google Scholar]
- 27.Groen RJ, van Alphen HA. Operative treatment of spontaneous spinal epidural hematomas: A study of the factors determining postoperative outcome. Neurosurgery. 1996;3:494–508. doi: 10.1097/00006123-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Fedor M, Kim ES, Ding K, et al. Spontaneous spinal epidural hematoma: A retrospective study on prognostic factor and review of the literature. Korean J Spine. 2011;4:272–82. doi: 10.14245/kjs.2011.8.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: A review of the literature and a compression with operative cases. Acta Neurochir (Wien) 2004;2:103–10. doi: 10.1007/s00701-003-0160-9. [DOI] [PubMed] [Google Scholar]
- 30.Raasck K, Habis AA, Aoude A, et al. Spontaneous spinal epidural hematoma management: A case series and literature review. Spinal Cord Ser Cases. 2017;3:16043. doi: 10.1038/scsandc.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]