Abstract
Background
The aim of this study was to investigate the optimal vitamin D status in the middle-aged and elderly population residing in Shanghai, China.
Material/Methods
A total of 1,829 males and postmenopausal females older than 45 years of age in the Changfeng community of Shanghai were included in this study. The optimal vitamin D level was determined according to the suppression of parathyroid hormone (PTH) and the highest bone mineral density (BMD). Locally weighted scatter plot smoothing (LOWESS) was performed to study the correlations of 25(OH)D with PTH and BMD in the lumbar spine and total hip, adjusting for gender, age, weight, use of calcium and vitamin D supplements, eGFR, smoking status, and alcohol consumption.
Results
The mean serum 25(OH)D concentration was 48.0±19.2 nmol/L for the whole study population. The circulating PTH was maximally suppressed by the serum 25(OH)D of 55 nmol/L in the total population (60 nmol/L for males and 50 nmol/L for females). The 25(OH)D concentrations corresponding to the highest BMD at lumbar spine (L1–L4) and total hip were 53 nmol/L and 75 nmol/L, respectively, for the whole population. These values were also higher in males than females.
Conclusions
The optimal 25(OH)D concentration of 55 nmol/L is sufficient to maintain the bone health and metabolic status in middle-aged and elderly individuals living in Shanghai. Males probably need higher vitamin D concentration than females. There are differences between vitamin D status based on lumbar spine BMD and total hip BMD.
MeSH Keywords: Bone Density, Parathyroid Hormone, Vitamin D
Background
Vitamin D is a well-known regulatory factor in calcium and phosphorus metabolism, which plays a critical role in bone and mineral homeostasis. Moreover, vitamin D has also been shown to exert extra-skeletal actions in addition to its classical roles. Vitamin D deficiency has been linked to many chronic illnesses, including common cancers [1], autoimmune diseases [2,3], infectious diseases [3], and cardiovascular diseases [4,5]. Therefore, in recent years, vitamin D has attracted increased attention. In fact, vitamin D deficiency is very prevalent among adults worldwide. Studies have reported that 20–100% of elderly men and women in the United States, Canada, and Europe suffer from vitamin D deficiency, with 25-hydroxyvitamin D [25(OH)D] less than 50 nmol/L [6–11]. In China, the prevalence rates of vitamin D deficiency [25(OH)D <50 nmol/L] and insufficiency [50≤ 25(OH)D <75 nmol/L] among middle-aged and elderly individuals have been reported to be 69.2% and 24.4%, respectively, indicating that only approximately 6% of this population has sufficient vitamin D levels [12].
Although the serum 25(OH)D level has been used to reflect a person’s vitamin D status [6], there is still no consistent standard for the optimal vitamin D status. The optimal serum 25(OH)D level can be defined by several criteria, including the maximal suppression of circulating parathyroid hormone (PTH), greatest calcium absorption, highest bone mineral density (BMD), reduced bone loss rates, reduced falling rates, and reduced fracture rates [13]. Previous studies have reported that the serum 25(OH)D level with the greatest inhibiting effects on PTH levels varies from 30–99 nmol/L [14–17]. The Institute of Medicine (IOM) guidelines have recommended 50 nmol/L as adequate levels of 25(OH)D to achieve bone health in general population [18]. On the other hand, however, the Endocrine Society guidelines recommend a target 25(OH)D concentration of 75 nmol/L [19].
The cutoff point for optimal vitamin D status is influenced by many factors, such as the residential latitude, current season, as well as an individual’s skin color, age, sex, genetics, renal function, mobility level, calcium intake, and phosphate and magnesium status [19,20]. Controversy persists regarding a standard cutoff point (such as 50 nmol/L or 75 nmol/L), because a single cutoff point may not be suitable for all populations (e.g., young versus old, and men versus women). Moreover, elevated standard cutoff point would lead to increased prevalence of vitamin D deficiency, which may result in aggressive treatment with vitamin D supplements in enlarged population and thereby increase the risk of vitamin D intoxication in some people.
It is well accepted that the middle-aged and elderly populations (> 45 years of age) are at high risk of vitamin D deficiency [21–24]. In this study, the vitamin D levels of residents greater than 45 years old from the Changfeng community of Shanghai, China, were investigated. According to these community-based data, chronic diseases among the middle-aged and elderly participants were also studied [25]. The optimal vitamin D levels in this population were determined based on the PTH levels combined with BMD, and a reference for the clinical treatment of vitamin D deficiency in adults living in Shanghai, China, was thereby provided.
Material and Methods
Study population
Consecutive numbering 2,223 males and females who were subjected to the vitamin D level detection, from May 2010 to June 2011, were initially screened. The exclusion criteria were as follows: primary hyperparathyroidism (n=3), Paget’s disease (n=0), bone tumors (n=53), and estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (n=265). There were 129 premenopausal females, who were also excluded. Finally, a total of 1,829 subjects were included in this study.
In the subgroup analysis, 184 healthy subjects were identified with normal bone mass (T score ≥−1.0 SD) and normal metabolic status, and some subjects were excluded, i.e., those with abnormal glucose metabolism (according to the American Diabetes Association 2010 criteria [22]), hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, taking antihypertensive medications, and with diagnosed hypertension), metabolic syndrome (according to the diagnostic criteria of the International Diabetes Foundation published in 2005 [23]), overweight status (24 ≤BMI <28 kg/m2), and obesity (BMI ≥28 kg/m2; according to the diagnostic criteria of the 2003 Working Group on Obesity in China [24]). Interviews, physical examinations, and BMD scans were carried out at the Changfeng Community Health Service Center. The study was approved by the ethics committee of Zhongshan Hospital, Fudan University, and was conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Patient interviewing
All the included participants were interviewed by trained investigators using a standardized questionnaire, and the data regarding their medical history, medication history, and health-related behaviors (lifestyle habits) were obtained. Information regarding current drug usage that would affect vitamin D levels was also obtained from these participants, including calcium, vitamin D, and bisphosphonate.
Physical examination
Weight and height were measured for each patient. Body mass index (BMI) was calculated as the weight divided by the height squared (kg/m2). Resting blood pressure was measured three times, and the mean value was used for analysis. Waist circumference was measured midway between the lowest rib margin and the iliac crest. Hip circumference was measured at the widest level over the greater trochanters.
Laboratory assessments
Blood samples were obtained from each patient after a fasting period of at least 10 hours. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), creatinine (Cr), and liver enzyme levels were measured using a Model 7600 Automated Bio-Analyser (Hitachi, Tokyo, Japan). Low density lipoprotein cholesterol (LDL-C) level was calculated using the Friedewald equation [26]. Fasting blood glucose (FBG) and 2-hour post-load plasma glucose (PPG) levels following a 75-g oral glucose challenge for participants without diabetes were measured with the glucose oxidase method.
Serum 25(OH)D and PTH levels were measured using a Roche Cobas E601 electro chemiluminescence system with complete reagents (Roche Diagnostics, Basel, Switzerland). Intra- and inter-batch variation coefficients for 25(OH)D and PTH were below 10% and 5%, respectively. The eGFR was estimated based on the serum Cr concentration using the Modification of MDRD formulation, i.e., eGFR (mL/min/1.73 m2)=186× Scr−1.154×age−0.203×0.742 (if female)×1.233 (if Chinese) [27].
BMD measurement
All participants underwent BMD measurement at the lumbar spine (L1–L4) and the total hip, using a Lunar iDXA (GE Healthcare, Milwaukee, WI, USA). Data for BMD and T score were obtained for each patient.
Statistical analysis
Data were expressed as mean ±SD. Statistical analysis was performed using the SAS 9.2 software (Cary, NC, USA). Normally distributed continuous variables between male and female subjects were compared using independent-sample t test, while skewed variables were compared using the Mann-Whitney U-test.
Measurements for 25(OH)D and PTH levels were divided into the following four groups according to seasons: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). Differences among the seasons were tested by one-way analysis of variance (ANOVA), and post hoc comparison was performed with least squares difference test.
Locally weighted scatter plot smoothing (LOWESS) was performed to study the correlations of 25(OH)D with PTH and BMD in lumbar spine and total hip, adjusting for gender, age, weight, using of calcium and vitamin D supplements, eGFR, smoking status, and alcohol consumption. All statistical analyses were two tailed; p<0.05 was considered statistically significant.
Results
Basic information and clinical characteristics of participants
Totally 1,829 participants were finally analyzed in the study, including 845 males and 984 postmenopausal females. Basic information and clinical characteristics of these participants are listed in Table 1. The mean age of these participants was 64.2±9.9 years. In the subgroup analysis, the 25(OH)D levels were analyzed, with normal bone mass (n=1,179) and for healthy subjects (normal bone mass and normal metabolism status; n=184). Our results showed that the mean 25(OH)D level in these participants was 48.0±19.2 nmol/L. In particular, the mean 25(OH)D level for male patients (51.9±20.1 nmol/L) was significantly higher than the postmenopausal female participants (44.7±17.9 nmol/L) (p<0.001). Moreover, the prevalence of 25(OH)D level <50 nmol/L was 60.4% over the whole population, which was 90.7% for the prevalence of 25(OH)D level <75 nmol/L. On the other hand, the mean PTH level in male participants (40.1±17.1 pg/mL) was significantly lower than the postmenopausal participants (41.8±18.9 pg/mL) (p=0.045). In addition, the BMD at the lumbar spine for male participants (1.15±0.18 g/cm2) was significantly higher than that the postmenopausal females (0.99±0.17 g/cm2) (p<0.001). Similar results were obtained for the BMD at hip (0.86±0.13 and 0.77±0.15 g/cm2 for male and postmenopausal female participants, respectively) (p<0.001).
Table 1.
Basic information and clinical characteristics of study subject.
| Total | Male | Female | P | |
|---|---|---|---|---|
| N | 1829 | 845 | 984 | – |
| Age (years) | 64±10 | 64±10 | 64±10 | 0.600 |
| Height (cm) | 161.4±8.4 | 167.6±6.3 | 156.2±6.2 | <0.001 |
| Weight (kg) | 63.5±10.8 | 68.6±9.8 | 59.2±9.6 | <0.001 |
| BMI (kg/m2) | 24.3±3.3 | 24.4±3.1 | 24.2±3.5 | 0.288 |
| Waist (cm) | 84.2±9.6 | 86.4±8.9 | 82.3±9.8 | <0.001 |
| Hips (cm) | 93.4±7.2 | 93.8±6.5 | 93.0±7.7 | 0.024 |
| SBP (mmHg) | 137±20 | 138±19 | 136±20 | 0.079 |
| DBP (mmHg) | 77±11 | 79±11 | 76±11 | <0.001 |
| FBG (mmol/l) | 5.6±1.6 | 5.7±1.8 | 5.4±1.3 | <0.001 |
| 2hPBG (mmol/l) | 7.7±3.4 | 7.8±3.7 | 7.7±3.2 | 0.284 |
| TC (mmol/l) | 5.1±0.9 | 4.8±0.9 | 5.4±0.9 | <0.001 |
| TG (mmol/l) | 1.8±1.3 | 1.8±1.3 | 1.8±1.2 | 0.894 |
| HDL-C (mmol/l) | 1.4±0.4 | 1.3±0.3 | 1.5±0.4 | <0.001 |
| LDL-C (mmol/l) | 2.9±0.8 | 2.8±0.7 | 3.1±0.8 | <0.001 |
| ALT (U/L) | 19±14 | 20±12 | 19±16 | 0.048 |
| AST (U/L) | 21±9 | 22±7 | 21±10 | 0.657 |
| Cr (μmol/l) | 68±15 | 78±12 | 59±9 | <0.001 |
| eGFR (ml/min/1.73 m2) | 97±19 | 95±19 | 98±20 | 0.003 |
| 25(OH)D (nmol/l) | 48.0±19.2 | 51.9±20.1 | 44.7±17.9 | <0.001 |
| PTH (pg/ml) | 41.0±18.1 | 40.1±17.1 | 41.8±18.9 | 0.045 |
| BMD at lumbar spine (g/cm2) | 1.06±0.19 | 1.15±0.18 | 0.99±0.17 | <0.001 |
| BMD at total hip (g/cm2) | 0.82±0.15 | 0.86±0.13 | 0.77±0.15 | <0.001 |
| 25(OH)D <50 nmmol/l (%) | 60.4% | 52.4% | 67.3% | <0.001 |
| 25(OH) <75 nmmol/l (%) | 90.7% | 86.6% | 94.1% | <0.001 |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; FBG – fasting blood glucose; 2hPBG – 2-hour postprandial blood glucose; TC – total cholesterol; TG – triglyceride; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; PTH – parathyroid hormone; BMD – bone mineral density; ALT – alanine aminotransferase; AST – aspartate aminotransferase; Cr – creatinine; eGFR – estimated glomerular filtration rate.
Relationship between 25(OH)D and PTH levels
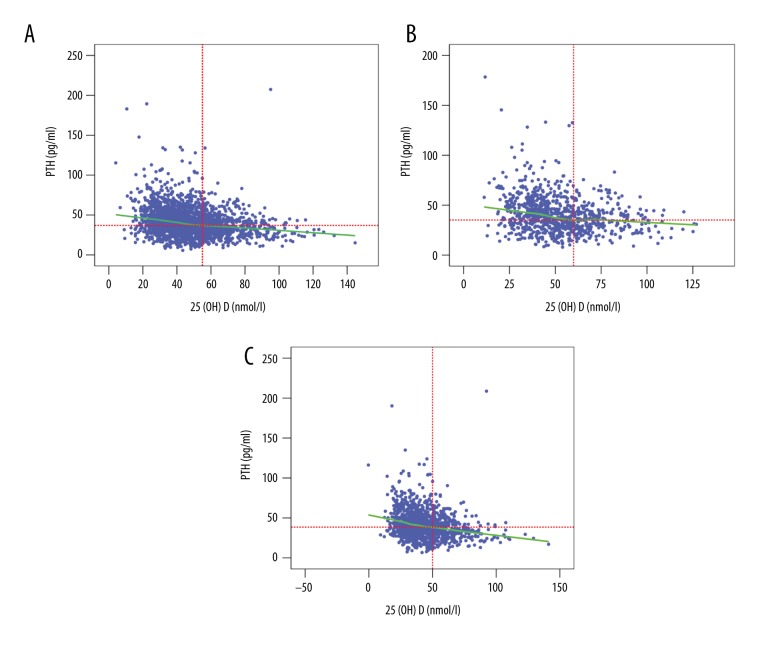
Relationship between the levels of 25(OH)D and PTH in the total population, males, and females was analyzed. As shown in Figure 1, the LOWESS plots showed an inverse relationship between the levels of 25(OH)D and PTH. However, no plateau was observed in the PTH level. Our results indicated that the circulating PTH concentration was maximally suppressed when the serum 25(OH)D was 55 nmol/L (Figure 1A) in all participants, 60 nmol/L in male participants (Figure 1B), and 50 nmol/L in female participants (Figure 1C), after adjusting for gender, age, weight, calcium, and vitamin D supplementation, eGFR, smoking status, and alcohol consumption.
Figure 1.
LOWESS plots for circulating PTH and serum 25(OH)D levels. (A) All study participants. (B) Males. (C) Females.
Relationship between 25(OH)D and BMD levels
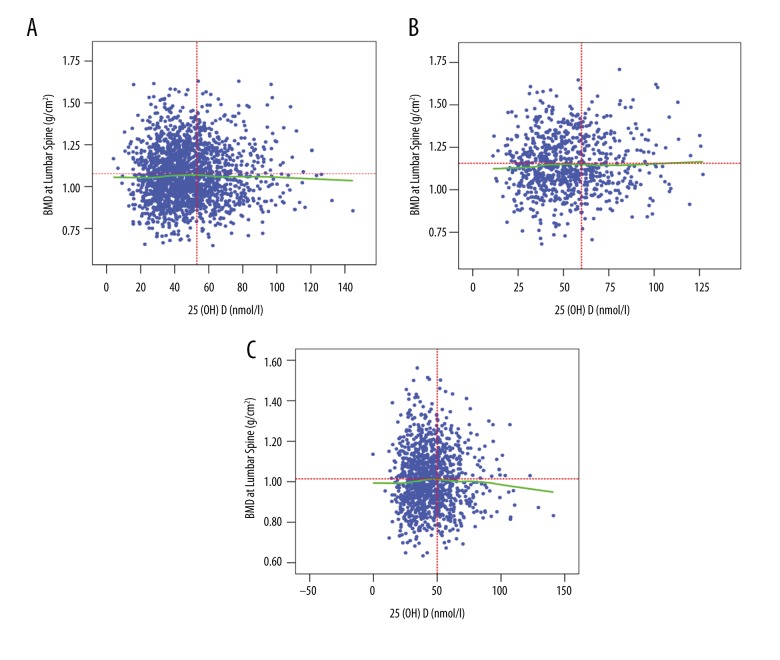
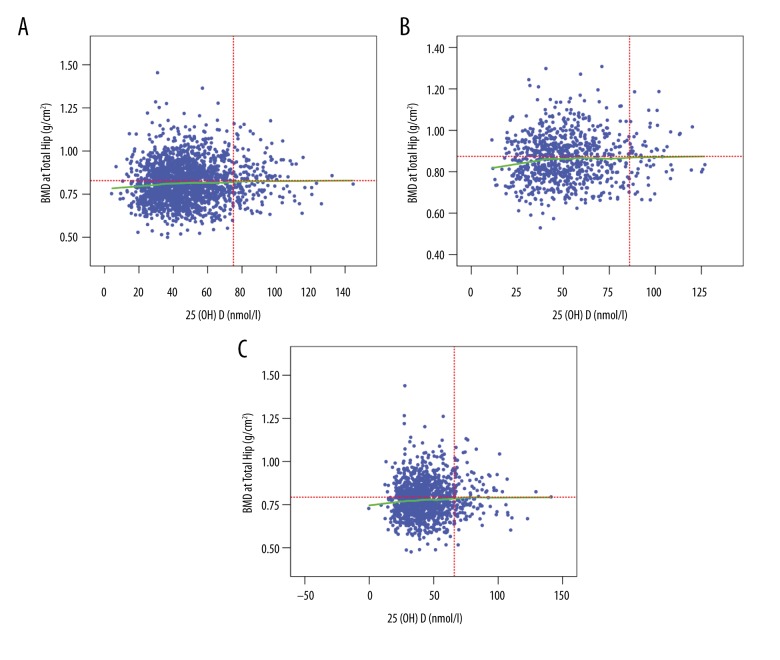
BMD measurements for the lumbar spine (L1–L4) and total hip were analyzed separately. Our results demonstrated that the highest BMD at the lumbar spine occurred at the serum 25(OH)D concentration of 53 nmol/L for the overall study population (Figure 2A), while this concentration was 60 nmol/L for the male participants (Figure 2B) and 48 nmol/L for the postmenopausal females (Figure 2C), after adjusting for gender, age, weight, calcium, and vitamin D supplementation, eGFR, smoking status, and alcohol consumption. The highest BMD at the hip occurred at the serum 25(OH)D concentration of 75 nmol/L for the overall study population (Figure 3A), while the concentrations were 86 nmol/L and 66 nmol/L for males (Figure 3B) and postmenopausal females (Figure 3C), respectively, also after adjusting for the confounding factors aforementioned.
Figure 2.
LOWESS plots for BMD in the lumbar spine and serum 25(OH)D levels. (A) All participants. (B) Males. (C) Females.
Figure 3.
LOWESS plots for BMD in the total hip and serum 25(OH)D levels. (A) All participants. (B) Males. (C) Females.
25(OH)D levels in subjects with normal bone mass and normal metabolism status
To further explore the optimal 25(OH)D level, 1,179 participants with normal bone mass (T score ≥ −1.0) (721 males and 458 postmenopausal females) were identified. As shown in Table 2, the mean 25(OH)D level in the population was 49.1±19.1 nmol/L. Moreover, the mean 25(OH)D level in male participants (51.9±20.1 nmol/L) was higher than postmenopausal female subjects (44.6±16.6 nmol/L) (p<0.001).
Table 2.
Basic information and clinical characteristics of subpopulation with normal BMD.
| Total | Men | Women | P | |
|---|---|---|---|---|
| N | 1179 | 721 | 458 | – |
| Age (years) | 64±10 | 64±10 | 62±9 | <0.001 |
| Height (cm) | 163.8±7.9 | 167.9±6.1 | 157.5±5.9 | <0.001 |
| Weight (kg) | 66.6±10.2 | 69.7±9.4 | 61.9±9.6 | <0.001 |
| BMI (kg/m2) | 24.8±3.2 | 24.7±3.0 | 24.9±3.5 | 0.235 |
| Waist (cm) | 85.7±9.3 | 87.1±8.6 | 83.5±9.9 | <0.001 |
| Hips (cm) | 94.3±7.0 | 94.3±6.2 | 94.4±8.0 | 0.915 |
| SBP (mmHg) | 137±19 | 139±19 | 135±19 | 0.004 |
| DBP (mmHg) | 78±11 | 80±11 | 76±11 | <0.001 |
| FBG (mmol/l) | 5.6±1.7 | 5.8±1.9 | 5.5±1.3 | 0.001 |
| 2hPBG (mmol/l) | 7.8±3.5 | 7.9±3.7 | 7.7±3.2 | 0.478 |
| TC (mmol/l) | 5.0±0.9 | 4.8±0.9 | 5.3±0.9 | <0.001 |
| TG (mmol/l) | 1.9±1.4 | 1.8±1.3 | 1.9±1.5 | 0.101 |
| HDL-C (mmol/l) | 1.3±0.3 | 1.2±0.3 | 1.4±0.3 | <0.001 |
| LDL-C (mmol/l) | 2.8±0.8 | 2.7±0.8 | 3.0±0.8 | <0.001 |
| ALT (U/L) | 20±12 | 20±12 | 20±12 | 0.540 |
| AST (U/L) | 21±7 | 21±7 | 21±7 | 0.874 |
| Cr (μmol/l) | 71±15 | 79±12 | 60±10 | <0.001 |
| eGFR (ml/min/1.73 m2) | 96±19 | 95±18 | 98±20 | 0.013 |
| 25(OH)D (nmol/l) | 49.1±19.1 | 51.9±20.1 | 44.6±16.6 | <0.001 |
| PTH (pg/ml) | 40.0±16.4 | 40.0±17.1 | 40.0±15.3 | 0.952 |
| BMD at lumbar spine (g/cm2) | 1.17±0.14 | 1.19±0.15 | 1.13±0.11 | <0.001 |
| BMD at total hip (g/cm2) | 0.87±0.14 | 0.88±0.12 | 0.85±0.16 | <0.001 |
| 25(OH)D <50 nmmol/l (%) | 58.6% | 52.9% | 67.5% | <0.001 |
| 25(OH) <75 nmmol/l (%) | 90.1% | 86.6% | 95.4% | <0.001 |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; FBG – fasting blood glucose; 2hPBG – 2-hour postprandial blood glucose; TC – total cholesterol; TG – triglyceride; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; PTH – parathyroid hormone; BMD – bone mineral density; ALT – alanine aminotransferase; AST – aspartate aminotransferase; Cr – creatinine; eGFR – estimated glomerular filtration rate.
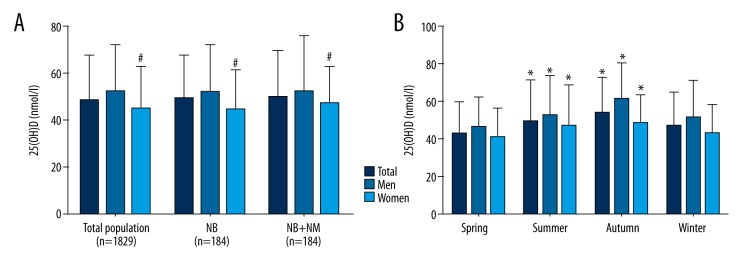
On the other hand, 184 healthy participants with normal bone mass and normal metabolic status were identified, excluding those with abnormal glucose metabolism (according to the American Diabetes Association 2010 criteria [28]), hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, use of antihypertensive medications, and diagnosed hypertension), metabolic syndrome (according to the diagnostic criteria of the International Diabetes Foundation published in 2005 [29]), overweight status (24≤ BMI <28 kg/m2), and obesity (BMI ≥28 kg/m2; according to the diagnostic criteria of the 2003 Working Group on Obesity in China [30]). In this group, the mean 25(OH)D level was 49.7±20.2 nmol/L. Moreover, the mean 25(OH)D level in male participants (52.5±23.3 nmol/L) was marginally higher than postmenopausal female subjects (47.0±16.3 nmol/L) (p=0.064) (Figure 4A).
Figure 4.
Analysis of the mean 25(OH)D levels in participants. (A) Mean 25(OH)D levels in different subgroups according to health status. Compared with males, # p<0.001. (B) Mean 25(OH)D levels in different seasons. Compared with spring, * p<0.001. NB – normal bone mass; NB+NM – normal bone mass and normal metabolism status.
Analysis of 25(OH)D levels for different seasons
The 25(OH)D levels in different seasons were then investigated. Our results showed that the mean 25(OH)D levels in the spring, summer, autumn, and winter for the whole study population were 43.4±16.3, 49.6±22.0, 54.3±18.5, and 47.0±18.2 nmol/L, respectively. Moreover, the 25(OH)D levels were higher in summer and fall compared to winter and spring (p<0.001). This trend also was observed for both genders (Figure 4B).
Discussion
The significance of vitamin D has been emphasized in public health contexts because vitamin D deficiency, which is independently associated with an increased risk of mortality in the general population [31], is a great threat to elderly people [32,33]. Previous studies have reported that vitamin D deficiency is related to a higher risk of metabolic bone diseases, cancers [1], autoimmune diseases [2,3], infectious diseases [3], and cardiovascular diseases [4,5]. However, the optimal vitamin D level for bone health is still controversial.
In this cross-sectional study, vitamin D deficiency is highly prevalent in the middle-aged and elderly population from Shanghai, China, especially in the postmenopausal females. The optimal vitamin D status for bone health in a population greater than 45 years of age in Shanghai were explored, and the relationship between serum vitamin D and PTH levels combined with BMD measurements at the lumbar spine (L1–L4) was investigated. Our results indicated that the optimal 25(OH)D level was 55 nmol/L for the overall population, 60 nmol/L for males, and 50 nmol/L for postmenopausal females. Therefore, male participants in this age range likely needed higher vitamin D concentration than female participants to achieve the same BMD level. The optimal cutoff point value of 55 nmol/L for the whole study population was determined based on the relationship between vitamin D and PTH concentrations, which was close to the level recommended by the IOM guidelines (50 nmol/L) [18], however lower than the target of 75 nmol/L recommended by the Endocrine Society guidelines [19]. In order to maintain the vitamin D-related metabolic balance, the maximal suppression of circulating PTH means not only the greatest calcium absorption, but also the synthesis and metabolic balance of vitamin D (i.e., the balance between synthetic pathways mediated by PTH and degradative pathways mediated by fibroblast growth factor-23, FGF-23). Alshayeb et al. [27] have found that raising the serum 25(OH)D levels with cholecalciferol treatment to above 50 nmol/L could also increase the degradation of 25(OH)D [34]. Therefore, they suggest that the 25(OH)D level of 50 nmol/L is sufficient for maintaining bone health, and the target of 75 nmol/L is relatively too high. Sai et al. [35] defined vitamin D insufficiency as the serum 25(OH)D concentration below 75 nmol/L based on serum PTH suppression, however this result was not supported by a literature review of 70 studies. Therefore, they conclude that vitamin D insufficiency should be redefined as the serum 25(OH)D concentration below 50 nmol/L. In addition, Nakamura et al. [36] investigated the cutoff point of serum 25(OH)D concentration in relation to elevated serum PTH concentrations in 582 elderly Japanese women. The study concluded that the serum 25(OH)D for elderly Japanese women should be maintained at 40 nmol/L or higher.
The relationship between the serum 25(OH)D concentration and BMD was also investigated. BMD is a noninvasive and effective way to evaluate bone health, and therefore has been considered as another way to estimate the optimal 25(OH)D level. It is well accepted that lower vitamin D levels are associated with bone health problem (e.g., increased risks of fractures and falls) [19,37]. Moreover, previous studies have shown that addition of vitamin D to calcium supplementation has long-term beneficial effects on BMD in the elderly [38–39]. Our data indicated a similar 25(OH)D concentration optimal to achieve the highest BMD in the lumbar spine (L1–L4) and maximal suppression of circulating PTH (53 nmol/L and 55 nmol/L, respectively) in the overall study population. To further explore the optimal 25(OH)D level in the middle-aged to elderly population, 1,179 participants with normal bone mass were analyzed. In this population, the mean level of 25(OH)D was 49.1±19.1 nmol/L, indicating the 25(OH)D level of 50 nmol/L, which basically met the demand for bone health. In a previously study concerning 320 elderly females with an average age of 77.2±4.6 years and mean 25(OH)D level of 44.3±12.9 nmol/L, Zhu et al. [40] found that the vitamin D supplementation added no extra short-term skeletal benefit superior to the calcium citrate supplementation. Similarly, Aloi et al. [41] conducted a study regarding 208 healthy black postmenopausal females aged 50–75 years old with the mean 25(OH)D level of 47 nmol/L, and they did not observed any effect of vitamin D3 supplementation on bone loss or bone turnover markers. These results indicated that increasing the 25(OH)D level to greater than 50 nmol/L might not improve bone health and the level of 50 nmol/L would be suitable for maintaining bone health in elderly people.
Increasing evidence has indicated the extra skeletal health effects of vitamin D, such as effects on the immune system, infectious diseases, autoimmune diseases, diabetes, cardiovascular disease, and cancers [33,34]. To exclude the potential confounding factors related to the extra skeletal effects of vitamin D, 184 healthy participants with normal bone mass and normal metabolic status were further analyzed. Interestingly, the mean 25(OH)D level in this subgroup was 49.7±20.2 nmol/L, which was close to 50 nmol/L. Thus, the reasonable 25(OH)D level for healthy population would be approximately 50 nmol/L. It seems that the 25(OH)D level of 50 nmol/L is sufficient for maintaining the basic metabolic health [35,36].
Interestingly, our results showed that the 25(OH)D level corresponding to the highest BMD in the lumbar spine (L1–L4) was basically equivalent to that associated with the maximal suppression of circulating PTH. However, the 25(OH)D level corresponding to the highest BMD in the total hip was 75 nmol/L, which was significantly higher than the lumbar spine (L1–L4). A recent study examined 675 iliac crest biopsies from German adults (401 males, mean age of 58.2 years; and 270 females, mean age of 68.2 years) for structural histomorphometric parameters. They have concluded that the dose of vitamin D supplementation should ensure that the circulating levels of 25(OH)D reach a minimum threshold of 75 nmol/L to maintain the skeletal health [42]. In this study, the same sites of the participants were examined, and consistent conclusion was addressed. Thus, there might be differences between vitamin D status based on lumbar spine BMD and total hip BMD. In order to improve the hip BMD, the circulating levels of 25(OH)D needed to be increased to above 75 nmol/L. A previous study reported that vitamin D status may be less related to the BMD of the total hip compared with that of the lumbar spine [43]. Moreover, another study found that vitamin D supplementation increases BMD to a lesser extent in the total hip than the lumbar spine [44]. These differences may be caused by the fact that other mechanical factors (i.e., physical activity and weight) would exert greater effects on the total hip BMD than on the lumbar spine BMD. Moreover, sex hormone deficiency has also been shown to make the lumbar spine more susceptible to vitamin D insufficiency [43].
Our results indicated that the optimal vitamin D level in males was significantly higher than the postmenopausal females, which was consistent with previous findings [20]. In another study 4,276 people (1,926 males and 2,350 females), aged 40 years and younger were selected from 16 administrative districts of South Korea [45]. The study showed that there are gender-dependent skeletal effects in this population, and thus serum 25(OH)D level should be maintained higher in males to ensure BMD [45]. Moreover, a previous study reported gender-based differences in the serum 25(OH)D and glycemic measurements [46]. Vitamin D deficiency is associated with reduced insulin production in participants with type 2 diabetes, which is mainly observed in males. In line with this, our results suggested that, compared with females, males might need higher circulating levels of vitamin D to maintain the same BMD and normal metabolic state. The underlying mechanism is not entirely clear. Some studies have indicated that males have lower fat content than females with the same BMI, which means less vitamin D is stored in fat and more stays in the blood after cutaneous synthesis [47,48]. In addition, previous studies showed that lower 25(OH)D concentrations were associated with lower SHBG levels and higher free testosterone levels in both males and females, and lower estradiol and higher DHEA levels in females, independent of adiposity and lifestyle. But there was no significant association between 25(OH)D and total testosterone in males [49,50]. Therefore, the difference of sex hormone may also represent an important factor for the different vitamin D status in females and males. The sex hormones and lifestyle were not addressed in the present study. Further in-depth studies are still needed to determine whether vitamin D supplementation influences the sex hormone levels. Shanghai is located on the eastern coast of China at subtropical latitude of 31°N. The city experiences four distinct seasons. Compared with summer and autumn, the vitamin D levels in our study population were lower in winter and spring. Vitamin D synthesis in the skin is mediated by ultraviolet B irradiation (290–315 nm). The effect of the short wavelength would be diminished by changes in the zenith angle over the year, which might lead to reduced vitamin D synthesis mediated by ultraviolet radiation [19,51]
There are also some limitations about our study. First, the adults under the age of 45 years or premenopausal females were not included. Second, our study population resided only in Shanghai, which does not represent the entire nation. Further studies are still needed in other regions. Third, the potential reasons for the differences in circulating vitamin D levels and the actually physiological requirement for vitamin D are still needed to be studied. In addition, detailed information about exercise and diet of the participants was absent.
Conclusions
In summary, in this study, the optimal vitamin D status in middle-aged and elderly individuals in terms of different vitamin D functions and different health status were investigated. Our results indicated that the optimal 25(OH)D level for the whole population was 55 nmol/L. Moreover, the optimal vitamin D status based on lumbar spine BMD was more reliable than that based on total hip BMD. Furthermore, males likely need more vitamin D than females to maintain normal BMD and metabolic state. Our findings provide valuable reference for the treatment of vitamin D deficiency among middle-aged and elderly people in Shanghai, China.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by grants from the National Key Basic Research Program of China (Grant No. 2012CB524906 to X. Gao http://www.973.gov.cn/Default_3.aspx), and the Major Project of Subject Construction of Shanghai Bureau of Health (Grant No. 12GWZX0103 to X. Gao and Grant No. 2013ZYJB0802 to X. Gao). The Shanghai Changfeng Study has also received great support from the Changfeng Health Center, the Health Bureau of Putuo District, and the committees of all the sub-communities of Changfeng
References
- 1.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 2.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 Suppl):1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 3.Gibney KB, MacGregor L, Leder K, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46(3):443–46. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 4.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, O’Keefe JH, Bell D, et al. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Chapuy MC, Schott AM, Garnero P, et al. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab. 1996;81(3):1129–33. doi: 10.1210/jcem.81.3.8772587. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 9.Lips P, Hosking D, Lippuner K, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J Intern Med. 2006;260(3):245–54. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 11.Greene-Finestone LS, Berger C, de Groh M, et al. 25-Hydroxyvitamin D in Canadian adults: Biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22(5):1389–99. doi: 10.1007/s00198-010-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L, Yu Z, Pan A, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32(7):1278–83. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–16. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 14.Aloia JF, Talwar SA, Pollack S, et al. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr. 2006;84(3):602–9. doi: 10.1093/ajcn/84.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644–50. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 16.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 17.Peacock M. Effects of calcium and vitamin D insufficiency on the skeleton. Osteoporos Int. 1998;8(Suppl 2):S45–51. doi: 10.1007/pl00022733. [DOI] [PubMed] [Google Scholar]
- 18.Ross AC, Manson JE, Abrams SA, et al. The 2011 dietary reference intakes for calcium and vitamin D: What dietetics practitioners need to know. J Am Diet Assoc. 2011;111(4):524–27. doi: 10.1016/j.jada.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153–58. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 21.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–38. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konradsen S, Ag H, Lindberg F, et al. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47(2):87–91. doi: 10.1007/s00394-008-0700-4. [DOI] [PubMed] [Google Scholar]
- 23.Chan R, Chan D, Woo J, et al. Not all elderly people benefit from vitamin D supplementation with respect to physical function: Results from the Osteoporotic Fractures in Men Study, Hong Kong. J Am Geriatr Soc. 2012;60(2):290–95. doi: 10.1111/j.1532-5415.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 24.Kurasawa K. [Aging and vitamin D]. Clin Calcium. 2006;16(7):1122–27. [in Japanese] [PubMed] [Google Scholar]
- 25.Gao X, Hofman A, Hu Y, et al. The Shanghai Changfeng Study: A community-based prospective cohort study of chronic diseases among middle-aged and elderly: Objectives and design. Eur J Epidemiol. 2010;25(12):885–93. doi: 10.1007/s10654-010-9525-6. [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 27.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese participants with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 28.Association AD. Standards of medical care in diabetes – 2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Guo LF, Guo T, et al. Association of body composition with bone mineral density in northern Chinese men by different criteria for obesity. J Endocrinol Invest. 2015;38(3):323–31. doi: 10.1007/s40618-014-0167-5. [DOI] [PubMed] [Google Scholar]
- 31.Hutchinson MS, Grimnes G, Joakimsen RM, et al. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: The Tromso study. Eur J Endocrinol. 2010;162(5):935–42. doi: 10.1530/EJE-09-1041. [DOI] [PubMed] [Google Scholar]
- 32.Kuchuk NO, Pluijm SM, van Schoor NM, et al. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244–50. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 33.Ardawi MS, Sibiany AM, Bakhsh TM, et al. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos Int. 2012;23(2):675–86. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- 34.Alshayeb H, Showkat A, Wall BM, et al. Activation of FGF-23 mediated vitamin D degradative pathways by cholecalciferol. J Clin Endocrinol Metab. 2014;99(10):E1830–37. doi: 10.1210/jc.2014-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011;96(3):E436–46. doi: 10.1210/jc.2010-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Nashimoto M, Tsuchiya Y, et al. Threshold value of serum 25-hydroxyvitamin D concentration in relation to elevated serum parathyroid hormone concentrations in elderly Japanese women. J Bone Miner Metab. 2006;24(5):395–400. doi: 10.1007/s00774-006-0699-7. [DOI] [PubMed] [Google Scholar]
- 37.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu K, Devine A, Dick IM, et al. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: A five-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93(3):743–49. doi: 10.1210/jc.2007-1466. [DOI] [PubMed] [Google Scholar]
- 39.Islam MZ, Shamim AA, Viljakainen HT, et al. Effect of vitamin D, calcium and multiple micronutrient supplementation on vitamin D and bone status in Bangladeshi premenopausal garment factory workers with hypovitaminosis D: A double-blinded, randomised, placebo-controlled 1-year intervention. Br J Nutr. 2010;104(2):241–47. doi: 10.1017/S0007114510000437. [DOI] [PubMed] [Google Scholar]
- 40.Zhu K, Bruce D, Austin N, et al. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone-related chemistry in elderly women with vitamin D insufficiency. J Bone Miner Res. 2008;23(8):1343–48. doi: 10.1359/jbmr.080327. [DOI] [PubMed] [Google Scholar]
- 41.loia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165(14):1618–23. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priemel M, von Domarus C, Klatte TO, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 participants. J Bone Miner Res. 2010;25(2):305–12. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 43.Mezquita-Raya P, Munoz-Torres M, Luna JD, et al. Relation between vitamin D insufficiency, bone density, and bone metabolism in healthy postmenopausal women. J Bone Miner Res. 2001;16(8):1408–15. doi: 10.1359/jbmr.2001.16.8.1408. [DOI] [PubMed] [Google Scholar]
- 44.Dawson-Hughes B, Harris SS, Krall EA, et al. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am J Clin Nutr. 1995;61(5):1140–45. doi: 10.1093/ajcn/61.4.1140. [DOI] [PubMed] [Google Scholar]
- 45.Lim JS, Kim KM, Rhee Y, Lim SK. Gender-dependent skeletal effects of vitamin D deficiency in a younger generation. J Clin Endocrinol Metab. 2012;97(6):1995–2004. doi: 10.1210/jc.2011-3098. [DOI] [PubMed] [Google Scholar]
- 46.Esteghamati A, Aryan Z, Nakhjavani M. Vitamin D deficiency is associated with insulin resistance in nondiabetics and reduced insulin production in type 2 diabetics. Horm Metab Res. 2015;47(4):273–79. doi: 10.1055/s-0034-1389903. [DOI] [PubMed] [Google Scholar]
- 47.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–93. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 48.Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 49.Wang N, Zhai H, Zhu C, et al. Combined association of vitamin D and sex hormone binding globulin with nonalcoholic fatty liver disease in men and postmenopausal women: A cross-sectional study. Medicine (Baltimore) 2016;95(4):e2621. doi: 10.1097/MD.0000000000002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao D, Ouyang P, de Boer IH, et al. Serum vitamin D and sex hormones levels in men and women: The Multi-Ethnic Study of Atherosclerosis (MESA) Maturitas. 2017;96:95–102. doi: 10.1016/j.maturitas.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–78. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]