Abstract
Background
The aim of this study was to compare intravoxel incoherent motion diffusion-weighted magnetic resonance imaging (IVIM DW MRI) to T1 mapping for characterization of hepatic alveolar echinococcosis (HAE).
Material/Methods
Eighteen HAE patients confirmed by surgery were examined with conventional MRI, IVIM DWI MRI with 10 b values (range: 0–1,000 sec/mm2), and longitudinal relaxation time (T1) mapping. Diffusion coefficient (D), perfusion fraction (f), pseudo-diffusion coefficient (D*), and T1 relaxation time were calculated in solid components, perilesional components, and background liver parenchyma of HAE patients. The correlation between T1 relaxation time and IVIM-derived parameters was assessed by using the Pearson correlation test.
Results
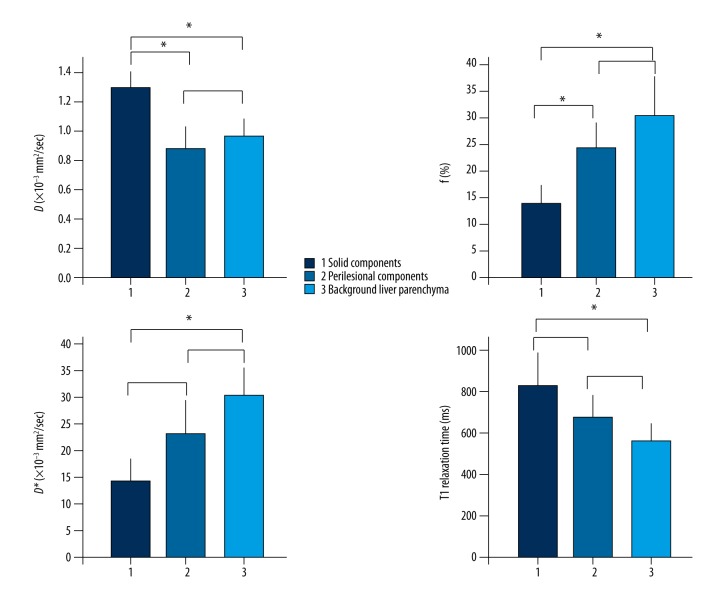
T1 relaxation times were significantly higher in solid components (820.58±331.24 ms) compared to background components (551.52±182.93 ms) of HAE patients (p<0.05). IVIM-derived D values were significantly higher in solid components (1.30±0.28×10−3 mm2/sec) compared to perilesional components (0.88±0.28×10−3 mm2/sec) and background liver parenchyma (0.97±0.27×10−3 mm2/sec) of liver parenchyma. There were significant differences in f values between solid components (13.70±7.66%), perilesional components (23.59±10.73%) and background liver parenchyma (30.78±10.18%). IVIM derived D* values were significantly lower in solid components (14.32±10.85×10−3 mm2/sec) than in background liver parenchyma (30.06±15.68×10−3 mm2/sec). Importantly, IVIM-derived f values were significantly correlated with T1 relaxation time: r=−0.337 (p<0.05).
Conclusions
Based on our image comparison, IVIM DWI MRI might be better than T1 mapping, and IVIM-derived f values might be a valuable index for characterization of HAE.
MeSH Keywords: Hereditary Angioedema Type III; Imaging, Three-Dimensional; Radiology Department, Hospital
Background
Human alveolar echinococcosis (AE) is a form of parasitic zoonosis caused by the larvae of the echinococcus multilocularis [1]. Most patients with AE are diagnosed at a late stage when the disease has advanced to unresectable hepatic lesions. Compared to medicinal treatment, radical resection is more effective for hepatic alveolar echinococcosis (HAE). A diagnosis of AE is based on the presence of at least two of the following findings: 1) typical lesion(s) detected by cross-sectional imaging; 2) antibodies against echinococcus species identified by immunoserology; 3) histopathologic features of echinococcus multilocularis; and 4) DNA of echinococcus multilocularis detected in a clinical specimen [2]. The definite diagnosis of HAE is based on the histopathological examinations and DNA analysis [2]. Among the conventional imaging methods, ultrasonography (US) is generally the first-line examination for detecting AE. For a diagnosis of AE, US is usually combined with computed tomography (CT) and/or magnetic resonance imaging (MRI) [3,4]. Magnetic resonance elastography (MRE) has been used for detection and staging of liver fibrosis [5], but is not common in the characterization of AE.
Diffusion weighted imaging (DWI) has been applied to diagnosis of diseases, including HAE, in abdominal organs [6–9]. In a comparative study between DWI and histopathological features of HAE, Ren et al., found a significant negative correlation between apparent diffusion coefficient (ADC) values and percentage of HAE fibrosis in the peripheral area [9]. In recent years, intravoxel incoherent motion diffusion weighted magnetic resonance imaging (IVIM DW MRI), which was first described by Le Bihan et al. [10], has been applied in the clinical diagnosis of HAE. This method separates micro-capillary perfusion from tissue diffusion. These IVIM-derived parameters are of value in evaluating HAE. Meanwhile, longitudinal relaxation time constant (T1) mapping has also been used as a diagnostic tool, especially in cardiovascular systems [11–13]. Lower T1 time has been associated with a greater degree of histologically defined interstitial fibrosis [13]. The degree of fibrosis can reflect parasitic viability of HAE, to a certain extent.
In this study, we performed and compared IVIM DW MRI to T1 mapping in the quantitative assessment of HAE patients, and evaluated the values of these two diagnostic methods for characterization of HAE.
Material and Methods
Patients
This study was approved by the Ethics Committee of First Affiliated Hospital of Xinjiang Medical University. Written informed consent was obtained from individual participants or their guardians. Between November 2014 and December 2015, a total of 26 patients, who were examined with MRI for suspected HAE, were identified. The inclusion criteria for HAE included: the size of a single lesion was greater than 2 cm; the patient had no history of any specific treatment; and the image quality met both IVIM DWI MRI and T1 mapping post-processing demands. Eight patients had to be excluded: one for pathological findings other than HAE, two for a history of previous drug therapy prior to MRI, and five for poor image quality. The final study population consisted of 18 patients (45±12 years old, range 23–68 years), including six males (50±11 years old, range 40–68 years) and 12 females (43±12 years old, range 23–64 years).
Conventional MRI
MRI scans were performed using a 1.5T MR scanner (Siemens Magnetom and Avanto). Patients were positioned supine on a spine coil and covered with a phased-array body coil. The scanning range was the upper abdomen. Our routine MRI protocol consisted of an axial T1-weighted image (repetition time (TR)/echo time (TE), 180.0 ms/4.78 ms; number of excitations, two), an axial fat-saturated fast spin echo T2-weighted image (TR/TE, 2,200.0 ms/90.0 ms; number of signals acquired, two).
IVIM DWI and post-processing
The concept of IVIM MRI was initially presented by Le Bihan et al. [10]. IVIM MRI is a method used to assess the influence of microcirculation on the apparent diffusion coefficient by using diffusion-weighted imaging (DWI). DWIs with free-breathing were obtained for this study using a single-shot echo-planar imaging pulse sequence in three perpendicular directions. A parallel imaging technique was used to decrease echo train length. We acquired 10 different b values (0, 50, 100, 150, 200, 250, 300, 500, 750 and 1,000 sec/mm2). Post-processing of the obtained DWI data were performed by using Medical Imaging Interaction Toolkit (MITK) diffusion software to acquire IVIM parameters of D, D*, and f. The echo attenuation in a single voxel could be written according to the following equation by Le Bihan et al. [10]:
where S(TE)1 is the mean signal intensity, S(0) is the mean signal intensity when b=0 sec/mm2, TE is the echo time, T2 is the spin-spin relaxation time, f is the fraction of the diffusion correlation to microcirculation, and D is the pure molecular diffusion parameter. D, D*, and f were estimated using a least-square nonlinear fitting on a pixel-by-pixel basis, automatically calculated by the MITK diffusion software.
T1 mapping and T1 relaxation time calculation
A three-dimensional (3D) dual flip angle gradient echo breath-hold sequence was performed to obtain T1 relaxation time. The imaging parameters of the T1 mapping were as follows: TR/TE 15/1.79; one signal acquired; flip angles of 5° and 26°; eight slabs; and acquisition time 42 seconds. The MRI data were transferred to a workstation (Siemens, Erlangen, Germany) to measure T1 relaxation time. Quantitative T1 relaxation time maps were derived automatically on a voxel-by-voxel basis and T1 was calculated by using nonlinear least squares fitting.
Regions of interest (ROI)
Identification of HAE and selection of region of interest (ROI) placement were performed by means of consensus of two attending radiologists, and used a high-resolution monitor on a picture archiving communication system (Syngo Plaza, Siemens). Conventional axial fat-saturated fast spin echo T2-weighted images were used as references to determine lesion areas on the corresponding T1 maps and IVIM parametric maps. IVIM parameters, including D, f, D*, and T1 relaxation time, were measured. The ROIs were manually placed on each lesion at the level of maximum transverse lesion diameter in the solid, perilesional, and background liver parenchyma. The perilesional area referred to an area of normal hepatic parenchyma near to the HAE focus.
Histologic analysis
The HAE lesion was detached from the liver and fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin & eosin (H&E) staining for the pathologic evaluation.
Statistical analysis
Statistical analysis of data was carried out using the SPSS 17 software package. Analysis of variance (ANOVA) variance analysis was used to compare parameter values (D, f, D*, and T1) among different components of HAE. Pearson correlation analysis was used to compare IVIM parameter values and T1 relaxation time. Significance was set at p value less than 0.05.
Results
IVIM DW MRI derived parameters
D, f, and D* were calculated using segmented bi-exponential analysis. Table 1 showed D, f, and D* values from IVIM bi-exponential fitting and T1 relaxation time of different components. The data in Table 1 were analyzed by ANOVA method to calculate p values for a single parameter between solid components (n=17), perilesional components (n=18), and background liver parenchyma (n=18); and then by the post-test of ANOVA for p value between two different components for determination of significant differences (Figure 1). IVIM-derived D values were significantly different (p<0.01) between the three components: solid components (1.30±0.28×10−3 mm2/sec), perilesional components (0.88±0.28×10−3 mm2/sec), and background liver parenchyma (0.97±0.27×10−3 mm2/sec) (Table 1). Comparison of individual pairs revealed that the D values of the solid components were significant higher than that of the perilesional components or the background liver parenchyma group (p<0.05) (Figure 1). There were significant differences in the f values between the solid components (13.70±7.66%), the perilesional components (23.59±10.73%), and the background liver parenchyma (30.78±10.18%) (p<0.01) (Table 1). Furthermore, the f value of the solid components was significant lower than that of the perilesional components or the background liver parenchyma (p<0.05) (Figure 1). IVIM-derived D* values were significantly different between the solid components (14.32 ± 10.85 ×10−3 mm2/sec), the perilesional components (22.94±12.51×10−3 mm2/sec) and the background liver parenchyma (30.06±15.68×10−3 mm2/sec) (p<0.01) (Table 1). The D values of solid components were significantly lower than that of the background liver parenchyma (p<0.05) (Figure 1).
Table 1.
D, f, D* values and T1 relaxation times of different component.
| D (×10−3 mm2/sec) | f (%) | D* (×10−3 mm2/sec) | T1 relaxation time (ms) | |
|---|---|---|---|---|
| Solid components (n=17) | 1.30±0.28 | 13.70±7.66 | 14.32±10.85 | 820.58±331.24 |
| Perilesional components (n=18) | 0.88±0.28 | 23.59±10.73 | 22.94±12.51 | 672.17±217.27 |
| Background liver parenchyma (n=18) | 0.97±0.27 | 30.78±10.18 | 30.06±15.68 | 551.52±182.93 |
| P | 0.000 | 0.000 | 0.004 | 0.010 |
D – diffusion coefficient; f – perfusion fraction; and D* – pseudo-diffusion coefficient. Numbers were shown in Mean ±SD. P values were calculated by ANOVA analysis.
Figure 1.
Comparison of parameters, diffusion coefficient (D), perfusion fraction (f), pseudo-diffusion coefficient (D*), and T1 relaxation times in different components of hepatic alveolar echinococcosis (HAE). The data in Table 1 were analyzed by ANOVA method to calculate p values for a single parameter between solid components, perilesional components, and background liver parenchyma (see Table 1), and then by the post-test of ANOVA for p value between pairs; * p<0.05.
T1 relaxation time
ANOVA analysis revealed that the values of T1 relaxation time between the solid components (820.58±331.34 ms), the perilesional components (627.17±217.27 ms), and the background liver parenchyma (551.52±182.93 ms) were significant different (p<0.05) (Table 1). The post-test of ANOVA further showed that T1 relaxation time of the solid component was significantly higher than that of the background liver parenchyma of HAE (p<0.05) (Figure 1).
Pearson correlation between IVIM derived parameters with T1 relaxation time
Pearson correlation statistics was used to analyze the possible correlation between the IVIM parameters D, f, D*, and T1 relaxation time. As shown in Table 2, IVIM-derived f values were significantly correlated with T1 relaxation time (r=−0.337, p<0.05).
Table 2.
Pearson correlation statistics between IVIM-derived parameters and T1 relaxation time.
| Variable 1 | Variable 2 | P value | Correlation (r) |
|---|---|---|---|
| D | T1 values | 0.166 | 0.235 |
| D* | T1 values | 0.125 | −0.214 |
| f | T1 values | 0.014 | −0.337* |
D – diffusion coefficient; f – perfusion fraction; and D* – pseudo-diffusion coefficient.
Image findings
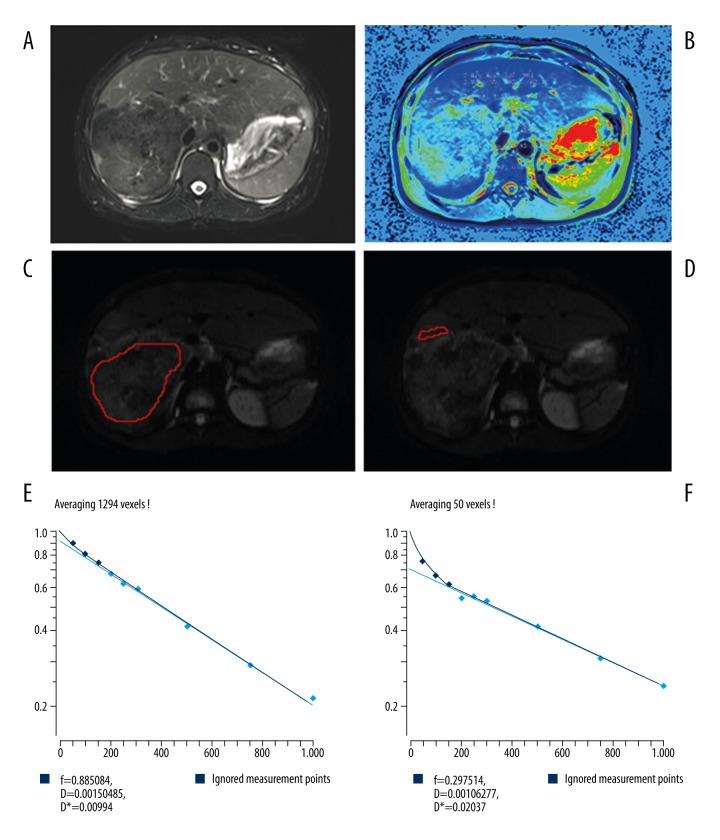
As an example, the IVIM MRI and T1 mapping of a 37-year-old female with HAE are shown in Figure 2. Hypo-dense liver mass with distinct irregular margins can be seen in HAE of right posterior lobe of the liver on fat-saturated fast spin echo T2-weighted image (Figure 2A). The mean value of T1 of the perilesional area was lower than that of the solid component of HAE (Figure 2B). The solid components and the perilesional components are shown in Figure 2C and 2D, respectively. Furthermore, the D value of the solid components was higher than that of perilesional components, the f value of the solid components was lower than that of the perilesional components, and the D* value of solid components was lower than that of the perilesional components (Figure 2E, 2F). Overall, in IVIM MRI, the number of AE liver lesions was 2.4±2.1 (range 1–8). The lesion size was 3.6 ± 3.3 cm (range 1.1–13.6 cm).
Figure 2.
Images of a 37-year-old female with hepatic alveolar echinococcosis (HAE). (A) Axial fat-saturated fast spin echo T2-weighted image; (B) T1 mapping with the region of interest (ROI) manually placed on the solid and perilesional components of HAE; (C) diffusion-weighted imaging (DWI) with b of 0 sec/mm2, and the ROI manually placed on the solid component; (D) DWI with b value=0 sec/mm2 and the ROI manually placed on the perilesional component of HAE; (E) the graph shows signal intensity decay of different b values on a solid component; (F) the graph shows signal intensity decay of different b values on a perilesional component.
H&E staining
In the histological examination of the biopsy specimen, H&E staining showed inflammatory fibrous zones between the coagulation of necrotic and normal liver tissues, indicating HAE (Figure 3).
Figure 3.
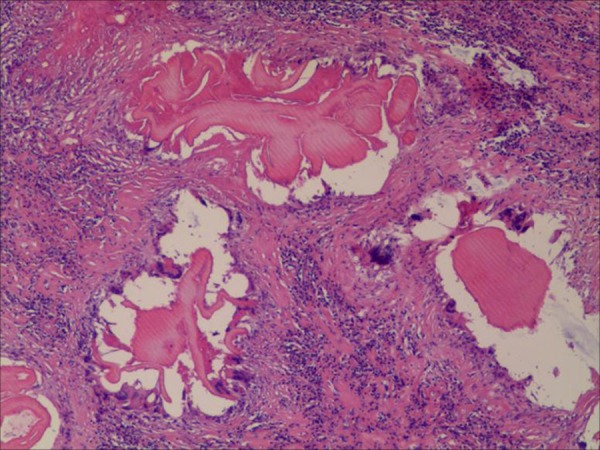
Histopathology of liver lesion. H&E staining showed inflammatory fibrous zone between the coagulation of necrotic and normal liver tissue. (H&E, magnification, 400×).
Discussion
Hepatic alveolar echinococcosis (HAE) is a relatively rare zoonotic helminthic disease with similarities to malignancy. In the present study, by using IVIM DWI MRI and T1 mapping as noninvasive tools for characterization of HAE, we were able to derive and compare the parameters of solid components, perilesional components, and background liver parenchyma. We found that IVIM-derived D and f values were better than T1 relaxation time generated from T1 mapping in the perilesional area of HAE. Between solid and perilesional components, IVIM-derived D and f values showed significant differences, but no statistical difference was shown in T1 relaxation time.
Ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) are also used in diagnosis and follow-up of HAE [14]. By measuring the hardness of HAE, US elastography is usually the first choice for early diagnosis in a clinic [3]. CT is useful in evaluating the stages of AE [4,14]. As a conventional imaging method, MRI is complimentary to CT in evaluating the invasion by AE in the abdominal and musculoskeletal systems, and more important, in the central nervous system. Although it is helpful in the determination of non-calcified suspicious lesions, MRI is not as successful as CT in showing calcifications pathognomonic for AE [15]. Magnetic resonance elastography (MRE) is a noninvasive MRI-based technique for quantitatively assessing the mechanical properties of tissues in vivo, and is used for diagnosis and staging of liver fibrosis [5]. This method might potentially be used in the diagnosis of AE.
The infiltration and inflammatory reaction zone of HAE in the perilesional area might not be obvious in conventional MRI. Our data showed that IVIM DWI distinguishes perilesional components and solid components, which might reflect parasitic viability of AE. The D value is considered the pure diffusion coefficient when microcirculation of blood in capillaries is excluded from motion forms [10]. In our study, the D values were 1.30±0.28×10−3 mm2/sec in solid components and 0.88±0.28×10−3 mm2/sec in perilesional components. The composition of solid components on MRI is reported to be mainly composed of granulomas, coagulation necrosis, and calcifications [8]. Thus, changes of the D values in our study were difficult to explain due to the complex composition of the solid components. Surprisingly, there was no significant difference in the D values between the perilesional components and the background liver parenchyma. From the IVIM DWI MRI, we observed relatively high signal intensity bands on the perilesional components in most cases, although there were no statistical differences. These results might be due to the narrow boundary zone, which might limit the ability to reach statistical significance. The IVIM-derived f value represents the fraction of perfusion linked to microcirculation [16]. Notably, in our study, there was significant difference in the f values between solid components, perilesional components, and background liver parenchyma. The f value of perilesional components was 23.59±10.73%, suggesting that the microcirculation of the perilesional components surrounding the lesion were more obvious than that of solid components and were inferior to background liver parenchyma. In some AE lesions, enhancement of the peripheral area is reported to be intense and long lasting, reflecting the development of a major neovascularization [17]. Our data suggested that IVIM derived D and f values might be used as indicators in assessing the perilesional characteristic of HAE lesions.
Pathologically, peri-parasitic development is associated with a massive granulomatous reaction and intense fibrogenesis [18]. We found that the T1 values of background liver parenchyma were lower than in solid components. Several studies have reported that T1 relaxation times in healthy people were lower than in patients with liver cirrhosis [19,20], which may reflect changes in relaxation time. This reason might also be an explanation for our T1 value results.
Conclusions
IVIM derived parameters might be preferable for accessing characterization of HAE when compared to T1 relaxation time.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by The National Natural Science Fund of China (number: 81260232)
References
- 1.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362(9392):1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 2.Kern P. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis. 2010;23:505–12. doi: 10.1097/QCO.0b013e32833d7516. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomot G, Vuitton DA, Harraga S, et al. Combined ultrasound and serologic screening for hepatic alveolar echinococcosis in central China. Am J Trop Med Hyg. 2002;66(1):23–29. doi: 10.4269/ajtmh.2002.66.23. [DOI] [PubMed] [Google Scholar]
- 4.Kantarci M, Bayraktutan U, Karabulut N, et al. Alveolar echinococcosis: Spectrum of findings at cross-sectional imaging. Radiographics. 2012;32(7):2053–70. doi: 10.1148/rg.327125708. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: Clinical applications. J Comput Assist Tomogr. 2013;37:887–96. doi: 10.1097/RCT.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh DM, Scurr E, Collins DJ, et al. Colorectal hepatic metastases: Quantitative measurements using single-shot echo-planar diffusion-weighted MR imaging. Eur Radiol. 2006;16(9):1898–905. doi: 10.1007/s00330-006-0201-x. [DOI] [PubMed] [Google Scholar]
- 7.Taouli B, Vilgrain V, Dumont E, et al. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: Prospective study in 66 patients. Radiology. 2003;226(1):71–78. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]
- 8.Becce F, Pomoni A, Uldry E, et al. Alveolar echinococcosis of the liver: Diffusion-weighted MRI findings and potential role in lesion characterisation. Eur J Radiol. 2014;83(4):625–31. doi: 10.1016/j.ejrad.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Ren B, Wang J, Liu W. [Comparative study between diffusion weighted imaging and histopathological features in hepatic alveolar echinococcosis]. Chinese Journal of Radiology. 2012;46:57–61. [in Chinese] [Google Scholar]
- 10.Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 11.Salerno M, Janardhanan R, Jiji RS, et al. Comparison of methods for determining the partition coefficient of gadolinium in the myocardium using T1 mapping. J Magn Reson Imaging. 2013;38(1):217–24. doi: 10.1002/jmri.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDiarmid AK, Broadbent DA, Higgins DM, et al. The effect of changes to MOLLI scheme on T1 mapping and extra cellular volume calculation in healthy volunteers with 3 tesla cardiovascular magnetic resonance imaging. Quant Imaging Med Surg. 2015;5(4):503–10. doi: 10.3978/j.issn.2223-4292.2015.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibley CT, Noureldin RA, Gai N, et al. T1 Mapping in cardiomyopathy at cardiac MR: Comparison with endomyocardial biopsy. Radiology. 2012;265(3):724–32. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Delabrousse É, Blagosklonov O, et al. Innovation in hepatic alveolar echinococcosis imaging: Best use of old tools, and necessary evaluation of new ones. Parasite. 2014;21:74. doi: 10.1051/parasite/2014072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulakci M, Kartal MG, Yilmaz S, et al. Multimodality imaging in diagnosis and management of alveolar echinococcosis: An update. Diagn Interv Radiol. 2016;22:247–56. doi: 10.5152/dir.2015.15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joo I, Lee JM, Yoon JH, et al. Nonalcoholic fatty liver disease: intravoxel incoherent motion diffusion-weighted MR imaging-an experimental study in a rabbit model. Radiology. 2014;270(1):131–40. doi: 10.1148/radiol.13122506. [DOI] [PubMed] [Google Scholar]
- 17.Bresson-Hadni S, Delabrousse E, Blagosklonov O, et al. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Intl. 2006;55:267–72. doi: 10.1016/j.parint.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 18.Bories C, Liance M, Bories PN, et al. No evidence for increased production of nitric oxide in C57BL/6J mice infected with Echinococcus multilocularis. Ann Trop Med Parasitol. 1996;90(6):641–44. doi: 10.1080/00034983.1996.11813095. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen C, Christoffersen P, Henriksen O, Juhl E. Prolonged T1 in patients with liver cirrhosis: An in vivo MRI study. Magn Reson Imaging. 1990;8(5):599–604. doi: 10.1016/0730-725x(90)90137-q. [DOI] [PubMed] [Google Scholar]
- 20.Heye T, Yang SR, Bock M, et al. MR relaxometry of the liver: Significant elevation of T1 relaxation time in patients with liver cirrhosis. Eur Radiol. 2012;22(6):1224–32. doi: 10.1007/s00330-012-2378-5. [DOI] [PubMed] [Google Scholar]