Abstract
Background
Synthetic systems that use positive feedback have been developed to control human disease vectors and crop pests. The tTAV system, which has been deployed in several insect species, relies on a positive feedback circuit that can be inhibited via dietary tetracycline. Although insects carrying tTAV fail to survive until adulthood in the absence of tetracycline, the exact reason for its lethality, as well as the transcriptomic effects of an active positive feedback circuit, remain unknown.
Results
We engineered the tTAV system in Drosophila melanogaster and investigated the effects of tTAV genome integration locus on the whole fly transcriptome during larval and adult life stages in four transgenic fly strains using gene expression microarrays. We found that while there were widespread effects on the transcriptome, the gene expression differences after removal of tetracycline were not consistent between integration sites. No specific region of the genome was affected, no common set of genes or pathways, nor did the integration site affect the transcripts in cis.
Conclusion
Although the positive feedback tTAV system is effective at killing insect larvae regardless of where it is inserted in the genome, it does not exhibit a specific, consistent transcriptional signature. Instead, each insertion site is associated with broad, but different, transcriptional effects. Our results suggest that lethality may not be caused by a direct effect on transcription of a set of key genes or pathways. Instead, we propose that rather than a specific action of a tTAV protein, it is the stochastic transcriptional effects specific to each insertion site that contribute to the tTAV-induced mortality.
Electronic supplementary material
The online version of this article (10.1186/s12864-017-4385-z) contains supplementary material, which is available to authorized users.
Keywords: Drosophila melanogaster, tTAV, Vector control, Transcriptome, Microarrays, Tetracycline
Background
Synthetic gene circuits that rely on positive feedback have been developed to replace irradiation-based methods for sterile insect technique (SIT) [1–4]. SIT has been an effective control strategy for insect populations, for more than 60 years, where sterilized males of a particular species are released and mate with females from the target population [5, 6]. It is species-specific, environmentally friendly, and has been effective at controlling a wide variety of insects. Molecular techniques can potentially improve the efficiency and effectiveness of SIT through the development of simple synthetic gene circuits that confer lethality or allow selection of only male insects for release [1, 2, 4, 7, 8].
A synthetic system that relies on a positive feedback gene circuit has been deployed in the mosquito Aedes aegypti [1, 2, 9–11]. This system, called OXI513a, has moved beyond field trials and has been used to suppress targeted mosquito populations [9, 12]. Like all SIT systems, population suppression is achieved via the release of OX513a male mosquitoes that mate with local females to produce no or very few offspring that survive to adulthood.
The lethality system used in OXI513a relies on the product of a single gene, tetracycline-controlled TransActiVator (tTAV), which enhances its own expression [1–3]. Positive feedback is generated via basal expression of the tTAV activator protein, a fusion of the tetracycline-binding domain (tetR) and the herpes simplex virus transcriptional activator (VP16) [13]. In the absence of tetracycline, the tetR domain of the tTAV protein binds to one of several TetO sequences upstream from the tTAV-encoding region. Each binding of tTAV further increases its own expression, via its VP16 domain. However, when an organism is provided with sufficient dietary tetracycline, which functions as an inhibitor, the tetR domain binds the antibiotic. The feedback is suppressed and the expression of tTAV remains at the low levels established by the basal promoter (Fig. 1). The suppression means the dominant lethality is inhibited and the insects can be produced in large numbers for release.
Fig. 1.
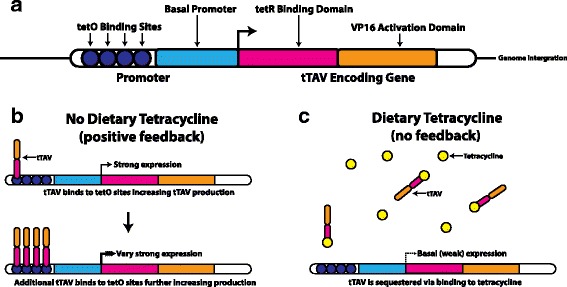
The TransActiVator (tTAV) feedback circuit. a Schematic of the tTAV system. b In the absence of tetracycline, basal levels of tTAV protein bind its own promoter at the TetO sites, thereby increasing tTAV protein production. This new protein binds the promoter and further increases expression. c In the presence of tetracycline, low levels of tTAV are sequestered and expression of tTAV mRNA remains at basal levels
In the absence of tetracycline, activation of the tTAV positive feedback system kills insects before they become reproductive, making this an effective control system in a post-release environment. However, unlike other genetic tTAV based systems that rely on control of gene expression induced lethality [4, 7, 14, 15], the exact reason for lethality of the positive feedback system remains unknown. Proposed mechanisms include tTAV toxicity, change in expression of critical transcripts, transcriptional squelching, wherein localised transcriptional machinery is titrated away, or overloading of the ubiquitin protease pathway due to high protein production [1, 3]. Complicating the understanding of this phenomenon is a very limited knowledge on the effects of synthetic positive feedback circuits on an organism’s transcriptome.
The tTAV system has been introduced into several insect species, but in some insertion sites the tTAV system is recessive lethal, even when not active, and therefore cannot be homozygous. A strain homozygous for the tTAV system was reported in A. aegypti and Ceratitis capitata (medfly) but not D. melanogaster [1, 3]. Moreover, a similar tTAV system that included an intron and multiple tTAV encoding regions, when introduced into Pectinophora gossypiella (pink bollworm) was predominantly recessive lethal, complicating the generation of a homozygous line [16].
The tTAV positive feedback systems function as expected in each insect species tested, in that tTAV expression is repressible with dietary tetracycline and lethal without it. However, the timing of lethality varied among species and even between insertion sites within the same species. In P. gossypiella, death reportedly occurred in the larval stage [16]. Whereas, of the three A. aegypti lines that displayed tetracycline repressible dominant lethality, two died in early larval stages, and the third died during the transition from late larvae to early pupae [2]. This insertion site-specific variation in the lethality timing was also noted in C. capitata [3].
Compared to animals grown on dietary tetracycline, tTAV expression is enhanced 36-fold in C. capitata third instar larvae and 48-fold in adults deprived of tetracycline for 4 days [3]. A. aegypti adults removed from 100 μg/mL tetracycline had a 150-fold increase tTAV expression after 4 days [1]. Finally, D. melanogaster adults had 46-fold and 69-fold increases after 1 and 4 days, respectively, after deprivation of 100 μg/mL tetracycline [1]. This highlights the responsiveness of the tTAV system, showing that the suppressive effect of residual tetracycline is quickly overcome. In each of these three species no impact on adult lifespan or health could be detected after removal of dietary tetracycline [1, 3]. Also, when adult mice carrying a tTAV-based system were no longer provided dietary tetracycline they lived for six months without observable ill effects [17]. It was proposed that this lack of lethality in adults may reflect transcriptional squelching or that interruptions to ubiquitin-dependent protein degradation may be less harmful to adults [3]. It may also be that the tTAV feedback circuit elicits a diminished response, at the transcriptomic level, in adults.
Understanding what factors contribute to the variance in tTAV-associated mortality is likely to inform the development of insect control systems. In the present study, the OXI513a tTAV feedback circuit was introduced and verified into D. melanogaster [1]. The tight tetracycline regulation of the system affords a convenient method to conduct a strain-by-strain assessment of the tTAV system and determine the transcriptomic effect, if any, of a positive feedback circuit. Transcriptomic analysis of four independent D. melanogaster tTAV insertion lines, in both adults and larvae, was conducted to examine the transcriptomic influence of the tTAV system.
Results
The tTAV lethality system in D. melanogaster
A tTAV positive feedback circuit, based on the LA513 plasmid, used in the development of A. aegypti OXI513a, [1], was developed for use in D. melanogaster. Using PhiC31 landing sites the above plasmid was integrated at specific widely used locations throughout the D. melanogaster genome (Table 1 and Methods). These landing sites represent a range of genomic locations that include each of the D. melanogaster chromosomes except the Y. Moreover, the chosen strains contain very well characterised homozygous landing sites. The tTAV heterozygous (technically hemizygous, but the term heterozygous is used here for convenience) stocks contained balancer chromosomes and, although viable when provided dietary tetracycline, failed to produce adult transgenic offspring in the absence of tetracycline. Lethality usually occurred prior to the third instar stage of larval development. Specifically, the onset of the lethality phenotype commenced part way through the second instar. Generally, all larvae would die prior the third instar but very rarely single third instar larvae would crawl up the side of control vials; none of these individuals would pupate prior to dying. Unlike tTAV in other organisms, not obvious difference in lethality timing between insertion sites was detected. As in previous tTAV systems, adults could be deprived of tetracycline without any observed ill effects. Of 10 integration loci tested, nine strains failed to produce homozygous offspring when raised on 100 μg/mL dietary tetracycline. Only cytological position 102D was viable as a homozygote. Increasing dietary tetracycline dosage to 200 μg/mL did not permit tTAV homozygosity or the establishment of a strain containing two tTAV integrations at both, adjacent, cytological positions 51C and 51D. However, crosses with 100 μg/mL dietary tetracycline between strains containing the tTAV integration at position 51C, on the second chromosome, and 76A2 or 86Fb, on the third chromosome, produced offspring containing both two tTAV integrations. Furthermore, an insertion at locus 19E7, on the X chromosome, produced viable male flies.
Table 1.
Details of tTAV stocks, insertion sites and viability. Viability is the ability to survive when grown on media containing 100 μg/mL tetracycline. The tTAV hemizygous stocks contained balancer chromosomes. All tTAV flies die in the absence of tetracycline
| Chromosome – Cytological Site | Viability |
|---|---|
| X – 19E7 | Hemizygous Viable / Male Viable / Homozygous Lethal |
| 2 L – 22A | Hemizygous Viable / Homozygous Lethal |
| 2 L – 28E7 | Hemizygous Viable / Homozygous Lethal |
| 2R – 43A1 | Hemizygous Viable / Homozygous Lethal |
| 2R – 51C | Hemizygous Viable / Homozygous Lethal |
| 2R – 51D | Hemizygous Viable / Homozygous Lethal |
| 3 L – 68E | Hemizygous Viable / Homozygous Lethal |
| 3 L – 76A2 | Hemizygous Viable / Homozygous Lethal |
| 3R – 86Fb | Hemizygous Viable / Homozygous Lethal |
| 4 – 102D | Hemizygous Viable / Homozygous Viable |
To ensure that the tTAV circuit behaved consistently, with respect to the previous systems, a series of survival tests were conducted. A two-generation crossing regime was used, wherein tTAV-102D males were crossed with ‘white eyed’ (w 1118) females, and their offspring were backcrossed to the same w 1118 line (Table 6 and Methods).
Table 6.
Fly stocks used
| Strain ID | Genotype | Origin |
|---|---|---|
| 19E7-tTAV | y1 w1118 PBac{y + -attP-9A, tTAV}VK00006 | Bloomington DSC line 9726 injected with tTAV construct |
| 22A–tTAV | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP,tTAV}ZH-22A | Bloomington DSC line 24,481 injected with tTAV construct |
| 28E7-tTAV | y1 w1118; PBac{y+-attP-3B, tTAV}VK00002 | Bloomington DSC line 9723 injected with tTAV construct |
| 43A1-tTAV | y1 w1118; PBac{y+-attP-9A, tTAV}VK00014 | Bloomington DSC line 9733 injected with tTAV construct |
| 51C–tTAV | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP, tTAV}ZH-51C | Bloomington DSC line 24,482 injected with tTAV construct |
| 51D–tTAV | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP, w+mc ,tTAV}ZH-51D/CyO | Bloomginton DSC line 24,483 injected with tTAV construct |
| 68E–tTAV | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP, tTAV}ZH-68E | Bloomington DSC line 24,485 injected with tTAV construct |
| 76A2-tTAV | y1 w1118; PBac{y+-attP-9A, w[+mc], tTAV}VK00013/TM1 | Bloomington line 9732 injected with tTAV construct |
| 86Fb-tTAV | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP, w+mc ,tTAV}ZH-86Fb/TM1 | Bloomington DSC line 24,749 injected with tTAV construct |
| 102D–tTAV | y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP, w+mc ,tTAV}ZH-102D | Bloomington DSC line 24,488 injected with tTAV construct |
| Y-hid | w1118/ hs-hid | VDRC line 60,001 |
| Ploen | Wild Caught in Plön, Germany | Isofemale line generated in the Reed group and grown for 10+ generations in the lab. |
| White Eyed | w1118/ hs-hid | Cross between male Y-hid and female Ploen flies selecting w1118 individuals |
Dietary concentrations of tetracycline, doxycycline, oxytetracycline, or chlorotetracycline varying over five orders of magnitude (0.01 to 100 μg/mL) were tested for their ability to rescue tTAV-102D in the aforementioned two-generation cross (Fig. 2a). Survival shows a clear response to dietary tetracycline concentration, with doxycycline capable of rescuing tTAV system lethality at a concentration almost an order of magnitude lower than other tetracycline homologs.
Fig. 2.
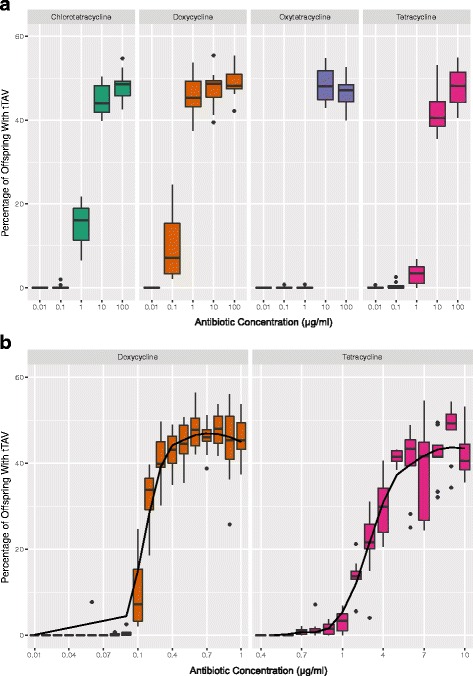
Characteristics of the tTAV circuit. The crossing scheme and media used are described in detail in the Methods, but, briefly, the homozygous 102D–tTAV line was crossed to a ‘white eyed’ w1118 line. Red-eyed wildtype flies, indicating the presence of the tTAV-system, were back crossed to the w1118 line and the percentage of tTAV flies in the F2 generation on various types and concentrations of antibiotic media was determined. Due to the use of tTAV heterozygotes, 50% survival represents the maximum percentage of the offspring that can inherit tTAV. Each data point is the median of 10 replicates. The data are presented as a standard boxplot with whiskers extending to the lowest or highest value within 1.5 times the IQR from the hinge. a Median survivorship for 4 members of the tetracycline class of antibiotics across dosages spanning 5 orders of magnitude. b Higher dosage resolution of median survivability for tetracycline and doxycycline, showing critical thresholds for antibiotic rescue. The IC50 values for doxycycline and tetracycline are 0.161 and 3.338, respectively
Both tetracycline- and doxycycline-induced survival had a similar, almost logarithmic dose response, despite the 10× difference in dosage. A more exact determination of the rescue threshold for tetracycline and doxycycline was obtained by repeating the above experiment with increased tetracycline dosage resolution (Fig. 2b). Tetracycline demonstrated a sharp decline in its capacity to rescue tTAV lethality between 5 μg/mL and 1 μg/mL whereas doxycycline demonstrated a similarity sharp decline between 0.4 μg/mL and 0.1 μg/mL.
Microarray analyses
To investigate the influence of the tTAV feedback circuit on gene activity, we assessed the changes in the transcriptome with and without tetracycline. This system provides an ideal internal control when examining flies raised with versus without tetracycline, as it allows us to differentiate transcriptional effects of the tTAV system in different genetic backgrounds and life stages (see Fig. 6 for the experimental design). We initially examined the transcriptional effects with and without tetracycline in a homozygous strain 102D–tTAV, but as we were unable to generate additional viable homozygous strains, we subsequently analysed three additional chromosome-balanced heterozygous tTAV strains. We also analysed a non-tTAV line. In each of the lines, we analysed two life stages, adults and second instar larvae, every time comparing gene activity with versus without tetracycline for each strain and life stage (Table 2). Due to the use of balancer chromosomes, wherein balancer homozygotes die as embryos, and the tTAV recessive lethality at these loci, the genotype of all offspring is known without the need for phenotypic selection.
Fig. 6.
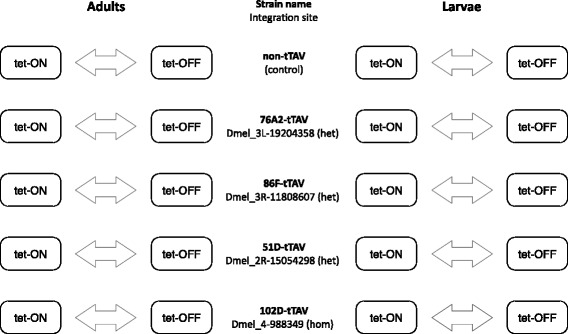
Diagram of experimental design. Two life stages (adults and larve) were tested in each of the 5 transgenic lines, 4 of them harbouring heterozygous integration of the tTAV constructs (het) and one homozygous (hom). All differential gene expression was assessed between tetracycline ON (tet-ON) and tetracycline OFF (tet-OFF) conditions (double-ended arrows) only within each single strain and life stage to ensure that observed effects are due to different treatments and not different genetic backgrounds
Table 2.
Fly Stocks used for Microarray
| Strain ID | Genotype | Bloomington ID | Genome Integration |
|---|---|---|---|
| non-tTAV | y1; Gr22biso-1 Gr22diso-1 cn1 CG33964iso-1 bw1 sp1; LysCiso-1 MstProxiso-1 GstD5iso-1 Rh61 | 2057 | |
| 51D–tTAV | y1 M{vas-int.Dm}ZH-2A wa; M{3xP3-RFP.attP, w[+mc], tTAV}ZH-51D/CyO | 24483a | Dmel_2R-15,054,298 |
| 76A2 - tTAV | y1 w1118; PBac{y + -attP-9A, w[+mc], tTAV}VK00013/TM1 | 9732a | Dmel_3L-19,204,358 |
| 86F - tTAV | y1 M{vas-int.Dm}ZH-2A wa; M{3xP3-RFP.attP, w[+mc], tTAV}ZH-86Fb/TM1 | 24749a | Dmel_3R-11,808,607 |
| 102D - tTAV | y1 M{vas-int.Dm}ZH-2A wa; M{3xP3-RFP.attP, w[+mc], tTAV}ZH-102D | 24488a | Dmel_4–988,349 |
adenotes progenitor strain ID
Expressed genes are shared among strains and within developmental stages
Overall, 10,950 genes were expressed in adults and 9947 in larvae, across all strains and treatments (Methods). Most expressed genes were shared among all strains in each developmental stage: 9554 genes of the 10,950 expressed in adults (87%) were expressed in all adult strains and treatments and 7784 of 9947 genes expressed in larvae (81%) were shared among all larvae strains and treatments (Table 3 and Fig. 3).
Table 3.
Number of expressed genes in each strain and stage
| non-tTAV | 102D–tTAV | 76A2-tTAV | 86F–tTAV | 51D–tTAV | |
|---|---|---|---|---|---|
| Adult Flies | 10,844 | 9986 | 10,784 | 10,831 | 10,575 |
| Larval Flies | 8881 | 9536 | 8715 | 8875 | 8720 |
Fig. 3.
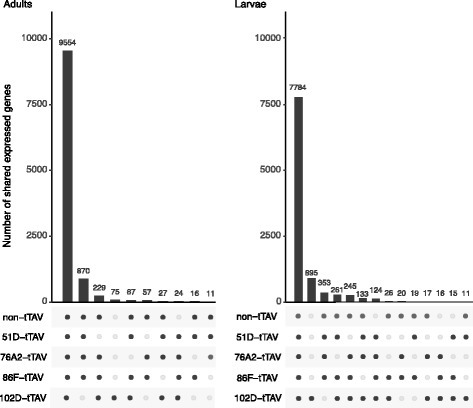
Number of shared expressed genes in all strains for adult and larval flies. Bar height represents the number of expressed genes and the black dots below indicate to which of the lines the genes belong. Sets with fewer than 10 genes are not shown. In both stages most of the genes are expressed in all five lines
Differentially expressed genes are not shared among different tTAV strains
We next analysed differential gene expression with and without tetracycline within each strain and developmental stage. We performed a moderated t-test for differential gene expression and set the false discovery rate (FDR) at 10% to identify differentially expressed genes (Methods).
At this cut-off, there were no genes differentially expressed due to dietary tetracycline in either larvae or adults in the non-tTAV control. Homozygous 102D–tTAV adults, however, stood out, with 2301 differentially expressed genes compared to 0, 1, and 3 in heterozygous adult tTAV strains. In contrast, in larvae, heterozygous strain 76A2-tTAV had 2116 differentially expressed genes compared to 115, 311, and 336 in the other larval strains (Table 4).
Table 4.
Number of differentially expressed genes in each strain and life stage at FDR = 10%
| non-tTAV | 102D–tTAV | 76A2-tTAV | 86F–tTAV | 51D–tTAV | |
|---|---|---|---|---|---|
| Adult Flies | 0 | 2301 | 0 | 3 | 1 |
| Larval Flies | 0 | 311 | 2116 | 115 | 336 |
Crucially, we detected little overlap in differentially expressed genes with vs. without tetracycline in any transgenic strains or life stages. Among adult tTAV flies, there were no shared differentially expressed genes. Among tTAV larvae, however, there were 31 differentially expressed genes common to all strains (Fig. 4).
Fig. 4.
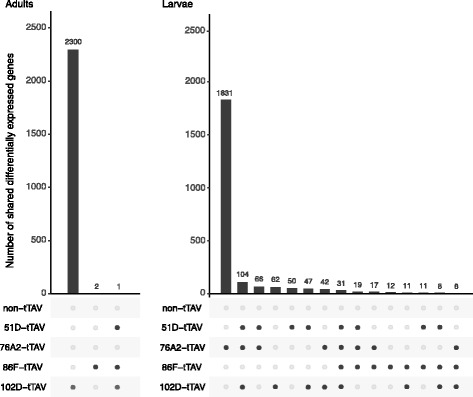
Number of shared differentially expressed genes in all transgenic strains for adult and larval flies. Bar heights represents the number of differentially expressed genes and black dots below indicate to which of lines genes belong. In both stages most of the differentially expressed genes are only present in a single line
When we investigated these 31 genes more closely, we found that 27 of them had opposite directions of expression change depending on the strain (in some strains a gene had higher expression without tetracycline and in other strains expression was higher with it). Only four genes showed a consistent pattern of expression change across all transgenic larval strains: crok (CG17218, FBgn0032421) had higher expression with tetracycline (i.e with tTAV system off), whereas Cyp6a17 (CG10241, FBgn0015714), olf186-F (CG11430, FBgn0041585), and Pex23 (CG32226, FBgn0052226) had higher expression without tetracycline (i.e. with tTAV system on). However, while these differences were statistically significant, they were very small: the range of fold changes in expression level with vs. without tetracycline in all larvae strains was between 0.72 and 1.74. Table 5 shows fold changes of expression levels and adjusted p-values for each of the four genes.
Table 5.
Shared differentially expressed genes show very small differences with versus without tetracycline. Fold change of expression levels and adjusted p-values for each of the four genes that showed consistent expression level change with versus without tetracycline in each transgenic larvae strains. Fold change is higher than 1 if expression increased off of tetracycline
| Crok | Cyp6a17 | olf186-F | Pex23 | |
|---|---|---|---|---|
| 102D–tTAV | ||||
| Fold Change | 0.81 | 1.34 | 1.10 | 1.03 |
| adj p-value | 0.05 | 0.05 | 0.03 | 0.02 |
| 76A2-tTAV | ||||
| Fold Change | 0.97 | 1.33 | 1.05 | 1.24 |
| adj p-value | 0.01 | 0.01 | 0.01 | <0.01 |
| 86F–tTAV | ||||
| Fold Change | 0.72 | 1.03 | 1.18 | 1.05 |
| adj p-value | 0.08 | 0.08 | 0.05 | 0.02 |
| 51D–tTAV | ||||
| Fold Change | 0.91 | 1.74 | 1.14 | 1.04 |
| adj p-value | 0.03 | 0.04 | 0.01 | <0.01 |
Differentially expressed genes in different strains do not belong to the same functional categories
We next investigated whether differentially expressed genes in each line and developmental stage were over-represented in similar functional categories. We observed very little overlap between compared groups, with gene ontology (GO) categories of fatty acid metabolism (GO:0006631, GO:0006635 and GO:0009062), C-acyltransferase activity (GO:0016408), protein deubiquitination (GO:0016579) and cellular lipid catabolic processes (GO:0044242) occurring in larvae 102D–tTAV and 51D–tTAV and mitochondrial part (GO:0044429) being common to 102D–tTAV adults and 76A2-tTAV larvae (Additional File 1: Table S1). We also analysed GO categories for the 31 genes that were differentially expressed in all four transgenic larvae strains. Among 14 categories found, three were related to ubiquitin-specific protease (GO:0004843) and to ubiquitinyl hydrolase activity (GO:0036459 and GO:0101005) (Additional File 2: Table S2).
Differences in gene expression are not correlated between strains
Given the limited consistency in the response to tetracycline treatment in various strains, we checked whether the gene expression differences with vs. without tetracycline in each strain might be correlated. Such correlation would indicate a consistent change in gene expression levels across many genes in each strain, irrespective of the significance of a small number of individual genes. We calculated all pairwise Pearson’s correlation coefficients of differences between expression levels with versus without tetracycline in each strain. We found that while all genome-wide correlations were statistically significant, including correlations with the non-tTAV strain (the largest p < 2 × 10−5), the sign of the correlation varied (Fig. 5).
Fig. 5.
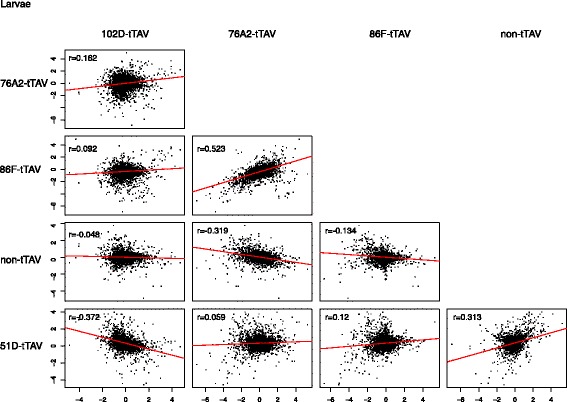
Genome-wide correlations between differences in expression levels with versus without tetracycline for each pair of tTAV larval strains. r is Pearson’s correlation coefficient. All correlations are statistically significant with the largest, p < 2 × 10–5
tTAV genomic insertion locus does not affect transcription of nearby genes
Given that we observed virtually no shared differentially expressed genes, no consistent functional categories of differentially expressed genes, nor correlations of gene expression differences with versus without tetracycline in different lines and life stages (Figs. 3, 4 and 5), we investigated whether the physical location of the transgene could explain these observations. As each of the strains had tTAV inserted in a different genomic location, tTAV transgene affecting genes in its physical proximity could be responsible for the observed pattern.
We tested for such cis-acting effects by analysing the distribution of differentially expressed genes in the genome with a sliding window approach. We constructed overlapping gene windows containing 10 genes each and overlapping a neighbouring window by 9 genes, thus ensuring single-gene resolution of the potential effects. We then counted windows in which the proportion of differentially expressed genes was above an arbitrarily chosen threshold of 30%. We observed no clear relationship between the insertion loci and location of differentially expressed genes. There were no windows with more than 3 differentially expressed genes in any strain or life stage, except in 102D–tTAV adults and 76A2-tTAV larvae, where 202 (out of 9529, 2%) and 387 (out of 7764, 5%) windows, respectively, showed more than 3 differentially expressed genes, consistent with the very large number of differentially expressed genes in these strains and life stages (Additional File 3: Figure S3 and Fig. 4).
To test whether the observed distribution of windows was statistically significant, we performed a permutation test, in which we randomly placed the genes along each chromosome or chromosome arm and re-calculated the number of windows in which the proportion of differentially expressed genes was above 30%, for 10,000 iterations. The only significant value we observed for these comparisons was for chromosome 2 L in larval strain 76A2-tTAV (p = 0.0338), potentially indicating that the distribution of 10-gene windows with more than 3 differentially expressed genes on chromosome 2 L in this strain and developmental stage was non-random. We note, however, that in this strain the transgene is located on chromosome 2R.
Discussion
Use of increasingly complex gene circuits for biotechnology and research applications necessitates understanding their influences on the systems in which they are placed. To this end, development of any genetic circuit, such as the tTAV system, in a well-characterized multicellular model organism like D. melanogaster, allows assessment of transcriptomic perturbations.
Although we detected insertion site variation, we found no large differences between insertion sites in timing of lethality, unlike those described in the A. aegypti or C. capitata [1–3]. A possible explanation for the reduced insertion site variation is that tTAV systems employ a D. melanogaster basal promoter. Although the use of heterologous promoters is routine, particularly in non-model organism transgenic work, significant genomic differences exist due to the large evolutionary distance between D. melanogaster and A. aegypti [18–20]. These differences may lead to less predictable transcription behaviour.
The tTAV system reported here responds to tetracycline analogues in a dose-dependent fashion (Fig. 2a & b) and doxycycline rescues tTAV lethality at a concentration almost 10 times lower than tetracycline (Fig. 2b). These results are very similar to those from other systems [4, 21], as expected given the behaviour of doxycycline in commercial TET systems, such as the Clontech TET-On system. The integration-site-specific recessive lethality of the tTAV system, even when inactivated via dietary tetracycline, coupled with the viable males with X chromosome integration and the viability of double heterozygotes, suggests an interaction more complex than simple dosage of the tTAV protein. Moreover, as double heterozygotes of close insertion sites, 51C and 51D, were also non-viable, a possible explanation is that the tTAV system is subject to transvection effects on gene expression [22]. However, extensive further testing, such as placing the tTAV promoter region and the coding region on separate chromosomes, would need to be conducted to confirm this. The timing of lethality to late larvae/early pupae implies disruptions of imaginal disc development [23, 24] or neurogenesis and behaviour [25]. Further elucidation of mechanisms involved in site-specific transcriptional effects might be achieved by quantifying tissue specificities of changes in gene expression in response to positive feedback (e.g., imaginal disc versus mushroom bodies).
To assess the impact of the tTAV system on the transcriptome, and to determine the cause of lethality, we mediated the activity of the tTAV system with dietary tetracycline and compared gene expression between on and off states. Although each of the conditions and strains tested had a similar number of expressed genes (Table 3 and Fig. 3), we identified 1–3 orders of magnitude difference between the numbers differentially expressed genes in larvae versus those in the adults of the same line (Table 4 and Fig. 4).
In the non-tTAV strain, we failed to detect any differentially expressed genes with a 10% FDR (Table 4). This is surprising, as several authors have reported that in D. melanogaster tetracycline disrupts mitochondrial function and leads to trans-generational effects [26, 27]. It has been shown that tetracycline can disrupt mitochondrial protein translation [28], which results in mitonuclear protein imbalance and inhibition of respiration [29]. Respiration inhibition in HEK293 cells disappeared after removal of doxycycline [29]. In addition, RT112 cells exposed to doxycycline were found to differentially express 9.5% of all expressed genes [29]. These findings, and numerous others, illustrate the need for caution in drawing biological conclusions when tetracycline is used for research purposes [30]. However, unlike the present study, many D. melanogaster studies did not subject their fly lines to several generations of growth on tetracycline prior to with-versus-without tetracycline experiments. It is possible that by this process we are removing much of the tetracycline-induced variability by selective killing of the D. melanogaster microbiota [28], or via mitochondrial damage that takes time to return to normal. Regardless of the reason for this muted response to tetracycline, it appears that any differential expression in our experiments is the result of tTAV activation and not to the use of tetracycline.
By examining genes that are differentially expressed in each insertion line, there may be the expectation that the lethal tTAV phenotype is due to change in expression of critical genes shared between the lines, or that a synthetic positive feedback loop has a standard transcriptional response. However, only larval samples showed genes that were differentially expressed for all insertion sites. Of the 31 differentially expressed genes only four had the same direction of differential expression and the fold change was, generally, extremely low (Table 5). In addition, regression analysis of differentially expressed genes in each strain found that all correlations were significant, but varied in direction (Fig. 5). It appears that not only does the insertion of tTAV have a limited effect on the transcriptome, but also any effect it does have is insertion site-specific with little overlap between sites.
It is possible that the four genes with similar differential expression in larval samples represent a group of core genes responsible for lethality. Two of these, crok and olf186-F, result in recessive lethality when mutated. At first glance, it might seem reasonable that tTAV lethality could be related to changes in expression of these genes, but this does not appear to be true. Although the differential expression is in the same direction for each of the four genes, the magnitudes of the changes are modest, and they differ between insertion lines (Table 5). Both recessive p-element insertions and RNAi knockdowns exist for crok and olf186-F, and both produce lethality as late as the pupa stage [31, 32]. This is in contrast to larval lethality seen in second to third instar stages for each tTAV line. This variation in phenotype and high variability in expression between insertion sites means crok and olf186-F do not offer a simple explanation for D. melanogaster lethality.
Another possible reason for tTAV lethality is that the feedback circuit overloads translational or protein catabolism machinery, but our data did not support this conclusion. We searched for previously identified transcriptional markers of translational stress to determine whether this is a viable hypothesis [33]. We checked whether Xbp1 (FBgn0021872), crc (FBgn0000370), CG7140 (FBgn0037147), CalX (FBgn0013995), MGST1 (FBgn0025814), CBS (FBgn0031148) and KrT95D (FBgn0020647) are among the differentially expressed genes in any of our strains or developmental stages at FDR = 10%. We only detected differential expression of crc, MGST1 and CG7140 in the adult 102D–tTAV strain; crc was also present in larval 102D–tTAV, 76A2-tTAV and 51D–tTAV, while MGST1 was present in larval 76A2-tTAV.
Cellular stresses are known to trigger specific pathway responses and by analysing differentially expressed pathways we sought to capture transcriptome responses shared across all insertion lines that might have been missed in single gene analyses. However, once again, our data did not reveal a clear set of cellular processes represented in all cell types. When GO analysis was performed on the 31 genes differentially expressed in all larval tTAV samples, ubiquitin protease/hydrolase categories were over-represented. Another hypothesis for tTAV lethality is that the circuit overloads the ubiquitin-tagged proteolysis pathway [1] and while the above observation might have been indicative of that, once again, it is unlikely to be the case. As mentioned above, only four of the 31 genes shared the same direction of differential expression, whereas one might have expected a general up-regulation of these pathways in response to overproduction of tTAV protein.
We also explored the hypothesis that activation of the tTAV circuit influences expression in specific genomic regions, either haphazardly or physically near the insertion site, but no evidence of a response was detected.
Although the tTAV system alters gene expression, depending on the integration site, there were no obvious localized effects of the integration. One might assume that the presence of a regulatory region with several activators bound, as is the case with an active tTAV, would potentially influence expression of neighbouring genes. However, after examining gene expression around integration sites we found no evidence of localised tTAV-induced differential expression.
Finally, tTAV protein may be toxic due to the behaviour of either the tetracycline-binding domain, tetR, or the activator domain, VP16. Early in vitro work on a fusion between the DNA binding domain of yeast GAL4 protein and VP16 demonstrated that this construct inhibited transcription of promoters lacking GAL4-binding domains [34–36]. In vivo work in Saccharomyces cerevisiae showed a link between transcriptional squelching of a reporter construct and dosage of VP16 [37]. This work also demonstrated that in addition to activation activity, VP16, the DNA-binding domain of the fusion protein was required for squelching. To check for a squelching phenotype, we examined differentially expressed genes for a general tendency toward down-regulation when the tTAV system was active. However we failed to detect a clear pattern of squelching, which strongly suggests that this squelching behaviour is likely not the cause of tTAV lethality.
Although we didn’t detect transcriptional depression, there could potentially be other mechanisms, or else our system is not sensitive enough to detect transient squelching. Several versions of VP16 have been developed that reduce activity and are tolerated at higher levels in HELA cells [38]. Additional study of these modified VP16 elements in our D. melanogaster system could further dissect potential squelching. Finally, it may also be worth investigating the behaviour of these modified VP16 elements in insect control systems that do not rely on tTAV positive feedback [7, 14, 15].
There are several reasons why it is difficult to categorically refute the proposed mechanisms of tTAV lethality. Our time point late in the second instar may fail to capture the precise event leading to death. Also it may be that lethality occurs due to a translational level event or is due to post-translational modification; hence our transcriptome analysis would miss these events. It may also be that lethality is related to highly tissue-specific expression. However, it is clear that tTAV activation has a large impact on the transcriptome and that this impact is specific to the tTAV genome integration locus. Given that there is very little overlap between the transcript profiles of each integration site and that there is no evidence for alternative explanations, we suggest that tTAV lethality is the result of integration site-based stochastic differential gene expression, perhaps due to tissue-specific expression patterns. Next, temporal and spatial expression patterns can be explored using specific promoters, such as those relating to the UAS/GAL4 system and alternative feedback systems. This will further illuminate the basis of tTAV lethality and will characterize cellular effects of transcriptional positive feedback.
Conclusion
Although variation due to genome insertion position is a well-characterised phenomenon, discussion has tended to focus on expression variability of the inserted construct and not on the entire transcriptome. This can be further complicated when the inserted construct is a circuit that can be activated. Here we show that the tTAV system, when activated, influences the whole transcriptome but that these expressions differences are unpredictable. Specifically, there was very little overlap in expression between any of the insertion sites. Furthermore, there was no discernible pattern in the types of transcripts affected, as assessed with GO analysis, nor were there any common genomic regions affected. Finally, expression differences within each strain did not appear to be localised to the insertion site. Our data suggest that the hypothesis that the lethality is caused by a direct effect on transcription of a set of key genes or pathways may be incorrect. Rather than a specific action of a tTAV protein, it is the stochastic transcriptional effects specific to each insertion site that contribute to the tTAV-induced mortality.
It is imperative to develop and characterize disease vector and crop pest control systems that are effective, targeted, consistent and cost-effective. Identifying transcriptomic underpinnings of cryptic lethality phenotypes can improve and refine the technology, helping it to gain public trust [39]. In addition, regulated systems, like tTAV, provide researchers with powerful tools to distinguish cis and trans effects in gene regulation at the whole transcriptome level.
Methods
Development of transformation plasmid
The tTAV system was synthesised using DNA 2.0, based on descriptions of the RIDL system [1–3]. This fragment was subsequently cloned into the multiple cloning site of pattB [40] and verified by sequencing. The full sequence of the plasmid is provided at 10.6084/m9.figshare.5700958.v1.
Fly strains
Strains of flies used in experiments described here can be found in Table 6. Transgenic flies were created by injection of the tTAV construct into previously described docking lines that represented a cross section of the D. melanogaster genome [40, 41]. Importantly, with respect to the difficulties of obtaining tTAV homozygous lines, the docking lines previously described are all homozygous viable. Potential transformants are screened for red eyes indicating complementation of the w 1118 from the w +mc marker present on the tTAV construct. All fly injections and transformant screening was performed by BestGene, Chino Hills, USA.
Fly rearing and media
Flies were incubated on standard Bloomington media at 24 °C under a 14 h light / 10 h dark cycle in either 50 mL vials or 300 mL bottles. Tetracycline media for stock maintenance was made by adding an appropriate volume of 100 mg/mL of tetracycline suspended in 99% ethanol to the surface of solid prepared food.
For survival tests, tetracycline media was made by adding an appropriate amount of antibiotic to achieve a final concentration of 100, 10 or 1 μg/mL and by diluting with unsupplemented media to the required concentration.
For microarray experiments, TET-On media was made by adding tetracycline to a final concentration of 100 μg/mL to cooled (>65 °C) media. Approximately 10 mL of this was added to 50 mL vials. TET-Off media was the same batch of food prior being supplemented with tetracycline.
tTAV survival tests
The tTAV circuit contains the red eye marker, w +mc , which allows it to be tracked when crossed with white eyed, w 1118 , virgins. A two-generation crossing system was used in all survival experiments. To aid collection of virgin females, a wild stock (‘Plön’) was crossed to ‘Y-hid’, which allows killing of male larvae via incubation at 37 °C for 30 min. Since tTAV is marked with a functional copy of the white gene, conferring red eyes, all individuals in the first generation were red-eyed and contained one copy of the tTAV circuit. Three males from this first generation were then backcrossed to 15 females from the ‘White Eyed’ line in 50-mL vials. This particular crossing regime was employed such that the second generation contained 50% tTAV / wild flies; thus, even when conditions did not permit survival of many tTAV flies, there were enough individuals to permit survival of the vial. Parental flies were removed after 5 days. Starting the day after the first offspring emerged, they were counted. Survivability is determined by counting both red- and white-eyed flies, adding these totals for each vial, and dividing the red-eyed files by the total. Each data point was derived from 10 biological replicates, but vials that failed produce any flies were removed from the analysis. Plots in Fig. 2 were made with ggplot2 [42].
Microarray flies and RNA extraction
The tTAV lines and non-tTAV control were maintained on TET-on media for 5 generations prior to commencing. Thirty flies, 15 male and 15 female, of the same age, from each of the strains in Table 2, were transferred to either TET-On or TET-Off media. Five days after transfer, adult flies were removed and 10 adult flies, 5 male & 5 female, were frozen at −80 °C. Ten larvae from each of these matings were collected at the late second instar stage, the last life stage normally seen prior to lethality, and frozen at −80 °C. Three biological replicates were produced for each combination of strain, life-stage, and media. RNA was prepared from frozen samples using Trizol and DNAse was treated using a PureLink RNA Mini Kit (Invitrogen).
Generating microarray data
Microarray experiments employed Agilent Drosophila Gene Expression Microarrays 4x44K and were scanned on an Agilent G2505C scanner (Agilent Technologies). Data collection was divided into two separate experiments. A pilot experiment for strain 102D consisting of two life stages in two conditions each with three biological replicates – a total of 12 samples. The remaining four strains were run as a separate experiment with a total of 48 samples. For each experiment, sample chip position was randomized to avoid genotype- and treatment-specific batch effects. Fig. 6 schematically shows the experimental design.
Normalisation of microarray data
Microarray data were normalized using the limma package [43] in R [44]. Specifically, the microarray background was corrected using the limma normexp function and data were normalized with limma quantile separately for each life stage. Following normalization, low intensity and control probes were removed as per the limma user guide (rev. 9 June 2015). Low intensity was defined as having at least 10% lower intensity than the 95th percentile of negative control probes on at least 3 arrays in each stage. The principal component analysis (stats package in R) was used to check for unusual groupings of samples that could indicate confounding effects and relative log expression analysis was used to check for sample outliers with regard to expression level distribution [45] (Additional File 4: Figure S1 and Additional file 5: Figure S2).
Annotation of microarray data
Probes were annotated using MEGABLAST [46]. All probe names and sequences were extracted from the array, and a total of 32,162 unique probes were used as input in a local MEGABLAST (ver. 2.2.31+) against the Ensembl Drosophila cDNA database of all known and predicted genes (downloaded on 15th January 2016). MEGABLAST settings used were:
blastn -task megablast -db db.fa -query query.txt -dust no -max_target_seqs 1 -outfmt “6 qseqid sseqid evalue pident stitle”.
Hits with 100% sequence identity, 92.3% of all 26,665 unique hits, and an e-value ≤1e-20 were retained (total 23,752) and Ensembl Gene IDs were extracted from the retained hit description field. Positional information for each gene was obtained using the BioMart database with the R package biomaRt [47]. Comparisons between our annotation and that of the manufacturer revealed that there were 5108 (22%) “mismatched” probes in our set. However, when we compared the overlap of probes after normalization and low intensity filtering, the “mismatched” probe fraction was reduced to at most 12%, indicating that almost half of the probes removed during filtering were those for which the manufacturer’s annotation differed from ours.
All further analyses were done on our set of MEGABLAST–annotated probes. After normalization and low intensity filtering, each sample was filtered to contain only probes present in our set.
Differentially expressed gene analysis
A moderated t test [43] was used to compare tet-on vs tet-off expression levels for each probe within each life stage and strain only, ie. the differentially expressed genes were all identified by comparing a single strain and life stage of transgenic flies in two different states: with tetracycline and without tetracycline. Therefore, any effects on transcriptome detected could only be due to the dietary tetracycline and not due to differences in genetic background. We used Benjamin-Hofberg correction for multiple testing using the limma package [43]. FDR thresholds at 1%, 5%, 10%, 15% and 25% were tested and produced similar overall pattern of gene expression differences, but 10% FDR was the lowest FDR tested for which we obtained large enough numbers of differentially expressed genes to enable analysis of GO categories and genome-wide positional effects and avoided potential false positives such as differentially expressed genes in non-tTAV strain (see Table S3 in Additional File 6).
Ensuring that we used a single probe per Ensembl Gene ID in the dataset further reduced the complexity. Multiple copies of identical probes with p values both under and over the threshold of 0.05 (moderated t-test) were removed completely. In cases where there were multiple probes with multiple p values assigned to a single Ensembl Gene ID, we retained only the probe with the lowest p value.
Common gene sets diagrams, principal component analysis, and genomic plots were generated using limma, UpSetR [48] prcomp and Bioconductor [47] packages. Sliding window analysis was run using a custom function. Plots and diagrams were made with ggplot2 [42].
Additional files
Gene ontology analysis of differentially expressed genes in transgenic strains. (DOCX 27 kb)
Gene ontology analysis of 31 differentially expressed genes shared among all transgenic strains in larvae. (DOCX 15 kb)
Proportion of differentially expressed genes (with vs without tetracycline) in 10-gene windows across genes commonly expressed in all adult (A, n = 9538) and larvae (B, n = 7773) strains. (DOCX 10589 kb)
Principal component analysis for adults and larvae from all strains. (DOCX 124 kb)
Relative log expression plots for adults and larvae from all heterozygous strains. (DOCX 1671 kb)
Number of differentially expressed genes at different levels of false discovery rate for all strains and stages. (DOCX 67 kb)
Acknowledgements
We thank Anita Möller for help with fly husbandry and Nicole Thomsen for preparing the microarrays. We thank Dr. Ania Lorenc for advice on analysis. We greatly appreciate the constructive comments on the manuscript offered by Prof. Nicholas Luscombe and Drs. David Rogers, Steven D. Aird and Violeta Lopez Huerta. Stocks were obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Vienna Drosophila Resource Center (VDRC, http://stockcenter.vdrc.at).
Funding
This work was supported by funds from the Max Planck Society and the Deutsche Forschungsgemeinschaft (RE-3062/2–1) awarded to FAR. JAD was supported by the Max Planck Society and the Okinawa Institute of Science & Technology Graduate University. JB was supported by the Max Planck Society and the University of Huddersfield. The funding bodies played no role in the design of these experiments, collection and analysis of the data or writing of the manuscript.
Availability of data and materials
Microarray data has been deposited in the ArrayExpress database with the ID number E-MTAB-6332.
Abbreviations
- FDR
False discovery rate
- GO
Gene ontology
- SIT
Sterile insect treatment
- tTAV
Tetracycline-controlled transactivator
Authors’ contributions
JAD & JB designed, performed, and interpreted the experiments and wrote the manuscript. RGR & FAR conceived and designed the tTAV D. melanogaster model. RGR built the D. melanogaster tTAV construct. All authors edited and approved the manuscript.
Ethics approval and consent to participate
Not applicable for Drosophila melanogaster work or collection.
Consent for publication
Not applicable.
Competing interests
RGR & FAR have a patent application, US14654450, relating to insect biotechnology.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12864-017-4385-z) contains supplementary material, which is available to authorized users.
Contributor Information
Jarosław Bryk, Email: j.bryk@hud.ac.uk.
R. Guy Reeves, Email: reeves@evolbio.mpg.de.
Floyd A. Reed, Email: floydr@hawaii.edu
Jai A. Denton, Email: jai.denton@oist.jp
References
- 1.Alphey L, Limited O. Expression system for insect pest control. US 20070056051 A1, US patent office. 2015. [Google Scholar]
- 2.Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong P, Epton MJ, Fu G, Scaife S, Hiscox A, Condon KC, et al. A dominant lethal genetic system for autocidal control of the Mediterranean fruitfly. Nat Biotechnol. 2005;23:453–456. doi: 10.1038/nbt1071. [DOI] [PubMed] [Google Scholar]
- 4.Horn C, Wimmer EA. A transgene-based, embryo-specific lethality system for insect pest management. Nat Biotechnol. 2002;21:64–70. doi: 10.1038/nbt769. [DOI] [PubMed] [Google Scholar]
- 5.Knipling EF. Possibilities of insect control or eradication through the use of sexually sterile males. J Econ Entomol. 1955;48:459–462. doi: 10.1093/jee/48.4.459. [DOI] [Google Scholar]
- 6.Krafsur ES. Sterile insect technique for suppressing and eradicating insect population: 55 years and counting. Journal of Agricultural Entomology. 1998;15:303–317. [Google Scholar]
- 7.Schetelig MF, Caceres C, Zacharopoulou A, Franz G, Wimmer EA. Conditional embryonic lethality to improve the sterile insect technique in Ceratitis Capitata (Diptera: Tephritidae) BMC Biol. 2009;7:4. doi: 10.1186/1741-7007-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science American Association for the Advancement of Science. 2000;287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- 9.Harris AF, Nimmo D, McKemey AR, Kelly N, Scaife S, Donnelly CA, et al. Field performance of engineered male mosquitoes. Nat Biotechnol. 2011;29:1034–1037. doi: 10.1038/nbt.2019. [DOI] [PubMed] [Google Scholar]
- 10.Nordin O, Donald W, Ming WH, Ney TG, Mohamed KA, Halim NAA, et al. Oral ingestion of transgenic RIDL Ae. Aegypti larvae has no negative effect on two predator Toxorhynchites species. PLoS One. 2013;8:e58805–e58807. doi: 10.1371/journal.pone.0058805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massonnet-Bruneel B, Corre-Catelin N, Lacroix R, Lees RS, Hoang KP, Nimmo D, et al. Fitness of transgenic mosquito Aedes Aegypti males carrying a dominant lethal genetic system. PLoS One. 2013;8:e62711–e62717. doi: 10.1371/journal.pone.0062711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho DO, McKemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey L, et al. Suppression of a field population of Aedes Aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis. 2015;9:e0003864–e0003815. doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schetelig MF, Handler AM. Strategy for enhanced transgenic strain development for embryonic conditional lethality in Anastrepha Suspensa. Proc Natl Acad Sci. 2012;109:9348–9353. doi: 10.1073/pnas.1203352109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schetelig MF, Targovska A, Meza JS, Bourtzis K, Handler AM. Tetracycline-suppressible female lethality and sterility in the Mexican fruit fly, Anastrepha Ludens. Insect Mol Biol. 2016;25:500–508. doi: 10.1111/imb.12238. [DOI] [PubMed] [Google Scholar]
- 16.Morrison NI, Simmons GS, Fu G, O’Connell S, Walker AS, Dafa’alla T, et al. Engineered repressible lethality for controlling the pink bollworm, a Lepidopteran Pest of cotton. Palli SR, editor. PLoS One 2012;7:e50922–e50910. [DOI] [PMC free article] [PubMed]
- 17.Shockett P, Difilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behura SK, Haugen M, Flannery E, Sarro J, Tessier CR, Severson DW, et al. Comparative genomic analysis of Drosophila Melanogaster and vector mosquito developmental genes. PLoS One. 2011;6:e21504–e21519. doi: 10.1371/journal.pone.0021504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieglaff DH, Dunn WA, Xie XS, Megy K, Marinotti O, James AA. Comparative genomics allows the discovery of cis-regulatory elements in mosquitoes. Proc Natl Acad Sci. 2009;106:3053–3058. doi: 10.1073/pnas.0813264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, ZJ T, et al. Genome sequence of Aedes Aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis Z, Matzen K, Neira Oviedo M, Nimmo D, Gray P, Winskill P, et al. Assessment of the impact of potential tetracycline exposure on the phenotype of Aedes Aegypti OX513A: implications for field use. PLoS Negl Trop Dis. 2015;9:e0003999–e0003915. doi: 10.1371/journal.pntd.0003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellert DJ, Truman JW. Transvection is common throughout the drosophila genome. Genetics. 2012;191:1129–1141. doi: 10.1534/genetics.112.140475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shearn A. Complementation analysis of late lethal mutants of Drosophila Melanogaster. Genetics. 1974;77:115–125. doi: 10.1093/genetics/77.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shearn A, Rice T, Garen A, Gehring W. Imaginal disc abnormalities in lethal mutants of drosophila. Proc Natl Acad Sci. 1971;68:2594–2598. doi: 10.1073/pnas.68.10.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM. Suppression of polyglutamine-mediated neurodegeneration in drosophila by the molecular chaperone HSP70. Nat Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 26.Ballard JWO, Melvin RG. Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in drosophila. Insect Mol Biol. 2007;16:799–802. doi: 10.1111/j.1365-2583.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Shea KL, Singh ND. Tetracycline-exposed drosophila melanogastermales produce fewer offspring but a relative excess of sons. Ecol Evol. 2015;5:3130–3139. doi: 10.1002/ece3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark-Walker GD, Linnane AW. In vivo differentiation of yeast cytoplasmic and mitochondrial protein synthesis with antibiotics. Biochem Biophys Res Commun. 1966;25:8–13. doi: 10.1016/0006-291X(66)90631-0. [DOI] [PubMed] [Google Scholar]
- 29.Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, et al. Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep. 2015;10:1681–1691. doi: 10.1016/j.celrep.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatzispyrou IA, Held NM, Mouchiroud L, Auwerx J, Houtkooper RH. Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 2015;75:4446–4449. doi: 10.1158/0008-5472.CAN-15-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilton A, Oshima K, Zare F, Byri S, Nannmark U, Nyberg KG, et al. Crooked, coiled and crimpled are three Ly6-like proteins required for proper localization of septate junction components. Development. 2010;137:2427–2437. doi: 10.1242/dev.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, et al. Genome-wide analysis of notch signalling in drosophila by transgenic RNAi. Nature. 2009;458:987–992. doi: 10.1038/nature07936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow CY, Wolfner MF, Clark AG. Using natural variation in drosophila to discover previously unknown endoplasmic reticulum stress genes. Proc Natl Acad Sci. 2013;110:9013–9018. doi: 10.1073/pnas.1307125110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill G, Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988;334:721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- 35.Sadowski I, Ma J, Triezenberg S. Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 36.Berger SL, Cress WD, Cress A, Triezenberg SJ, Guarente L. Selective inhibition of activated but not basal transcription by the acidic activation domain of VP16: evidence for transcriptional adaptors. Cell. 1990;61:1199–1208. doi: 10.1016/0092-8674(90)90684-7. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert DM, Heery DM, Losson R, Chambon P, Lemoine Y. Estradiol-inducible squelching and cell growth arrest by a chimeric VP16-estrogen receptor expressed in Saccharomyces Cerevisiae: suppression by an allele of PDR1. Mol Cell Biol. 1993;13:462–472. doi: 10.1128/MCB.13.1.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron U, Gossen M, Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 1997;25:2723–2729. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeves RG, Denton JA, Santucci F, Bryk J, Reed FA. Scientific standards and the regulation of genetically modified insects. Lehane MJ, editor. PLoS Negl Trop Dis 2012;6:e1502–e1515. [DOI] [PMC free article] [PubMed]
- 40.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venken KJT, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. Melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 42.Wickham H. ggplot2: elegant graphics for data analysis [internet] New York: Springer-Verlag; 2009. [Google Scholar]
- 43.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47–7. [DOI] [PMC free article] [PubMed]
- 44.RCT . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 45.Gentleman RCVHWIRDS. Bioinformatics and computational biology solutions using R and bioconductor: Springer; 2005. https://academic.oup.com/jee/article-abstract/48/4/459/2205947.
- 46.Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agarwala R, Schaffer AA. Database indexing for production MegaBLAST searches. Bioinformatics. 2008;24:1757–1764. doi: 10.1093/bioinformatics/btn322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durinck S, Moreau Y, Kasprzyk A, Davis S, De Moor B, Brazma A, et al. BioMart and bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics. 2005;21:3439–3440. doi: 10.1093/bioinformatics/bti525. [DOI] [PubMed] [Google Scholar]
- 48.Conway JR, Lex A, Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33:2938–40. doi: 10.1093/bioinformatics/btx364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene ontology analysis of differentially expressed genes in transgenic strains. (DOCX 27 kb)
Gene ontology analysis of 31 differentially expressed genes shared among all transgenic strains in larvae. (DOCX 15 kb)
Proportion of differentially expressed genes (with vs without tetracycline) in 10-gene windows across genes commonly expressed in all adult (A, n = 9538) and larvae (B, n = 7773) strains. (DOCX 10589 kb)
Principal component analysis for adults and larvae from all strains. (DOCX 124 kb)
Relative log expression plots for adults and larvae from all heterozygous strains. (DOCX 1671 kb)
Number of differentially expressed genes at different levels of false discovery rate for all strains and stages. (DOCX 67 kb)
Data Availability Statement
Microarray data has been deposited in the ArrayExpress database with the ID number E-MTAB-6332.


