Abstract
Melanins are ubiquitous catecholic pigments, formed in organelles called melanosomes within melanocytes, the function of which is to protect skin against harmful effects of UV radiation. Melanosomes within melanoma cells are characteristically abnormal, with fragmented melanin and disrupted membranes. We hypothesize that the disruption of melanosomal melanin might be an early event in the etiology and progression of melanoma, leading to increased oxidative stress and mutation. In this report, we examine the effect of a combination of UV treatment and metal ion exposure on melanosomes within melanocytes, as well as their ability to act as pro-oxidants in ex situ experiments, and assay the effects of this treatment on viability and cell cycle progression. UVB exposure causes morphologic changes of the cells and bleaching of melanosomes in normal melanocytes, both significantly enhanced in Cu(II) and Cd(II)-treated cells, as observed by microscopy. The promoted bleaching by Cu(II) is due to its ability to redox cycle under oxidative conditions, generating reactive oxygen species; verified by the observed enhancement of hydroxyl radical generation when isolated melanosomes were treated with both Cu(II) ions and UVB, as assayed by DNA clipping. Single-dose UVB/Cu treatment does not greatly affect cell viability or cell cycle progression in heavily pigmented cells, but did so in an amelanotic early stage melanoma cell line.
INTRODUCTION
Melanin and melanoma
The incidence of melanoma has been increasing dramatically in recent years, presumably due to global changes in the protective ozone layer, but treatment is largely limited to surgery and is effective only when diagnosis is achieved early in the disease (1). Melanomas are among the most drug-resistant cancers; other than surgery, there is no widely accepted treatment for metastatic melanoma available (2,3). It has been suggested that melanin itself might be both a source of drug resistance as well as a target for chemotherapy (4–6).
It has often been noted that melanogenesis itself is a source of reactive oxygen species (ROS) and oxidative stress in melanoma (7,8). In normal melanocytes, black melanin particles are generated from the successive oxidation of tyrosine by tyrosinase (9), contained within suborganelles called melanosomes. As will be demonstrated, melanoma melanosomes are poorly formed with malformed membranes and granulized melanin (10–12). These structural differences are significant, as melanosomal compartmentalization protects the cell from the highly reactive small-molecule catechols that are generated during melanogenesis. Thus structural irregularities likely lead to release of ROS into the cytosol (4,13). Much data suggest that transformed melanocytes have higher levels of free radicals and ROS (13–16), for instance the elevation of redox-responsive signaling pathways in melanoma, e.g. NFB and APE/Ref1, is markedly elevated in human melanoma in response to oxidative stress (171–20). Glutathione depletion is selectively toxic for melanoma cells both in vitro and in vivo (21). There is also clinical evidence for oxidative stress during human melanoma pathogenesis. Picardo et al. (22) found a deficiency of antioxidants in melanocytes from melanoma patients compared to control individuals. Pavel et al. (23) showed that disturbed melanin synthesis and chronic oxidative stress was present in dysplastic nevi, the first transformative step toward melanoma in some cases. Thus disruption of melanin formation may be an early and distinguishing pathogenic event in melanomagenesis. Consequences of the resulting ongoing intracellular stress are gradual depletion of antioxidants and activation of redox-sensitive transcription factors that enhance antiapoptosis and drug resistance.
Melanin abnormalities are well characterized in cutaneous melanoma, the debilitation of eyesight by macular degeneration, the bleaching of the substantia nigra in Parkinson’s disease, as well as the common graying of hair (12). Ultrastructural studies of human melanosomes indicate differences in the quantity and quality among melanocytes of normal skin and malignant melanoma; for instance, melanocytes generally have few melanosomes while malignant melanoma contain many (11). It has been recognized for some time that the process of melanosomal genesis is altered early in melanoma progression, including abnormal disposition of melanin (10) and a loss of membrane integrity (12). In the cancer cells, melanin is deposited on such filaments in an irregular, patchy and often incomplete fashion.
We, and others, have postulated that the functional effect of melanin dysregulation is its evolution into a pro-oxidant, characterized in both synthetic melanins and melanoma cell cultures. For instance, melanoma of several types demonstrated a pro-oxidant response to peroxide stress, which was quenched by catalase but increased by superoxide dismutase, implying a high flux of superoxide generated by the stress 18); neither normal melanocytes nor other cancer cell lines showed a similar pro-oxidant behavior. In a subsequent study, the formation of hydroxyl radical by synthetic melanin and the melanin within melanoma cells under oxidative stress was measured using the radical trap 5,5-dimethyl-1-pyrroline-N-oxide, and by DNA clipping assays. Several previous studies demonstrated the ability of melanin to induce DNA damage (6,24–27). Thus the exposed melanin within melanoma cells may be related to the observed high rates of DNA mutations seen in these tumors (28).
Metals, melanoma and UV
Epidemiologic data suggest a complex role for sunlight in melanoma etiology and pathogenesis (29,30), with ongoing controversy regarding the relative role of UVA (320–400 mm) and UVB (290–320 mm) in the pathogenesis of human melanoma (31). A striking feature of melanoma is the general inability to detect thymine dimers or other classical UVR-induced mutations in primary or metastatic melanomas, even in genes of interest. (30,32). For the pathogenesis of melanoma, the absence of thymine dimers suggests either that UVR is not involved or that the UVR effect is indirect via undiscovered mechanisms.
There are several lines of evidence that suggest that some substances that bind melanin contribute to the etiology and pathogenesis of human melanoma. The elegant works of Pavel et al. (23,33) have implicated chronic oxidative stress and metals in the formation of dysplastic nevi. Extensive occupational epidemiologic data implicate heavy metal ions (and other substances that bind melanin) in the etiology and pathogenesis of melanoma (29). These include studies from the occupational epidemiologic literature (printers, electrical workers) as well as recent long-term studies of patients with metal-on-metal hip replacements (34). Melanoma tumors have unusually high concentrations of Cu and other metals, even higher than normal melanocytes which themselves accumulate metal ions (35,36). Many metal ions induce oxidative stress in biologic systems, and activate and inhibit a wide range of signaling pathways (37). It has been shown that divalent Zn and Cd salts show toxicity to melanoma at concentrations several orders of magnitude lower than that which affects melanocytes (38,39). The mechanism through which combinations of these factors may influence melanocyte transformation is not well understood. For example, welders have an excess risk of developing intraocular melanoma and malignant melanoma; UVR produced by the welding arcs may combine with exposure to the welding fumes, which contain heavy metals, to increase the risk (40–42).
In this study, we test the effect of Cu uptake and UVB on the melanosomes in human melanoma and melanocyte cells. Cu is a critical cofactor for the function of tyrosinase, the rate-limiting enzyme for melanin synthesis and therefore is found in abundance in melanosomes in melanocytes and melanoma cells. We hypothesized that as the melanosome becomes disorganized during melanoma progression, Cu uptake by the melanin may result in pro-oxidant redox cycling (43). For these reasons, we examined the effect of both Cu and UVB on melanin integrity (in situ) and reactivity (ex situ) as well as viability in the host cells.
MATERIALS AND METHODS
Melanocyte and melanoma cultures
Normal human melanocytes were isolated from an individual neonatal foreskin (44,45). The foreskin was placed in 0.25% trypsin at 4°C overnight. The next day 2–3 mL newborn calf serum was added and the tissue was gently scraped a few times. Both tissue and serum were transferred to a centrifuge tube containing melanocyte growth media and vortexed vigorously. The supernatant was transferred to a new centrifuge tube and centrifuged at 1200 rpm for 5 min. The supernatant was aspirated; the cell pellet was suspended in melanocyte growth media and cultivated in a 25 cm tissue culture flask. Melanocytes were grown in MCDB 153 medium (Aldrich) supplemented with 2% heat-inactivated fetal bovine serum, 150 U mL^sup -1^ penicillin, 0.15 mg mL^sup -1^ streptomycin, 10 μg mL^sup -1^ insulin, 0.15% bovine pituitary extract, 2.0 HIM CaCl^sub 2^, 0.1 mM isobutyl-1-methyl-xanthine and 10 ng mL^sup -1^ phorbol myristate-13-acetate. The cells were maintained at 37°C in a humidified incubator with 5% CO2 atmosphere.
The highly pigmented human melanoma cell line MNT1 was a generous gift from Dr. Vincent Hearing (Laboratory of Cell Biology, National Cancer Institute), and cells were grown in DMEM medium supplemented with 20% heat-inactivated fetal bovine serum, 10% AIM-V medium, 20 mM Hepes, 0.5% antibiotic-antimycotic solution, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, 30 ng mL^sup -1^ gentamicin and 3.7 μg mL^sup -1^ sodium bicarbonate. For viability comparison, cell line WM3211 was used, which is a radial growth primary melanoma, cultured in RPMI-1640 from Invitrogen.
UVB irradiation
Melanocytes were plated in plastic tissue culture dishes and were maintained in melanocyte growth media as described above. In metal uptake experiments the media contained CuCl^sub 2^ or ZnCl^sub 2^ at 10 μM, CdCl^sub 2^ at 0.1 μM. For electron microscopy samples, the cultured cells were exposed to 25 mJ cm^sup -2^ UVB radiation three times a week for 3 weeks. Prior to UVB exposure, the media was aspirated and the cells washed with sterile phosphate-buffered saline (PBS). Sufficient amount of PBS was added to the dish to cover the cells and the cells were irradiated. Immediately after irradiation, the PBS was replaced with fresh growth media. UVB radiation was generated and measured in a UV Stratalinker 2400 from Stratagene (La Jolla, CA). The instrument includes an internal photodetector, which was equipped with closely spaced array of five UVB fluorescent lamps, providing continuous emission spectrum with a sharp peak at 312 nm. The radiation output was initially calibrated using digital radiometer DRC-100 from Spectronics Corporation (Westbury, NY).
Isolation of melanosomes
Isolation was performed according to the literature procedure (46). Briefly, cells were washed with ice-cold 10 mM sodium phosphate, 0.15 M NaCl, pH 7.4 buffer and scraped. Cells were homogenized by 20 strokes in a Dounce homogenizer at 4°C in 0.25 M sucrose in 10 mM Hepes, 1 mM EDTA, pH 7.2 that contained 5 mM benzamidine and 20 μg mL^sup -1^ aprotinin. The homogenate was centrifuged at 1200 g for 10 min to remove nuclei and unbroken cells. For the DNA clipping assay, the resulting supernatant was transferred to a clean tube and centrifuged at 16 000 g for 15 min yielding a crude melanosomal pellet.
Electron microscopy
Samples were washed in 0.1 M pH 7.4 sodium cacodylate buffer and fixed overnight in 2% glutaraldehyde in 0.1 M cacodylate buffer pH 7.4. The samples were washed with buffer and postfixed with 1% osmium tetroxide for 1 h. Specimens were dehydrated through a series of 30%, 50%, 70%, 85%, 95% and 100% ethanol and immersed in propylene oxide. After 20 min the propylene oxide was replaced with 50% resin and the samples were held under vacuum overnight. The next day, the 50% resin was replaced with 100% resin, transferred to capsules and polymerized at 56°C overnight. Ultra-thin sections were cut with a Richert-Jung ultramicrotome and collected on formvar-coated slot grids. After drying at room temperature overnight, the sections were stained with uranyl acetate and lead citrate to enhance contrast and dried at room temperature again. The ultra-thin sections were examined with a Philips CM-10 transmission electron microscope.
Cell cycle analysis
The cells were harvested by trypsinization and washed twice with ice-cold PBS solution. Cells were fixed in 1 mL 70% ethanol at −20°C overnight. The following day, cells were washed twice with ice-cold PBS solution and re-suspended in 1 mL propidium iodide (PI) buffer (PBS pH 7.4, 0.1% triton-X 100, 0.1 M EDTA, 0.05 mg mL^sup -1^ RNase A, 50 μg mL^sup -1^ PI). After 30 min incubation at room temperature, cell cycle distribution was analyzed by flow cytometry using a Becton-Dickinson FACScan with Cell Quest software.
Cell viability analysis
The percent apoptotic and necrotic cells were assayed after short photolysis and metal ion exposures as previously described (6). Briefly, the melanocytes were trypsinized, washed twice in PBS and resuspended in binding buffer at a concentration of 1 ×10^sup 6^ cells mL^sup -1^, of which 100 μL was incubated with 5 μL of Annexin V (AV) conjugated to FITC (Molecular Probes, Eugene, OR) and 75 μM (10 μL) PI for 15 min at room temperature. Cells were then analyzed by flow cytometry using a Becton-Dickinson FACScan with Cell Quest software. The proportion of apoptotic cells was estimated by the percentage of cells that stained positive for AV while remaining impermeable to PI (AV+/PI−); necrosis was defined as positive stain with both AV and PI (AV+/PI+); and viability was defined as AV−/PI−.
Plasmid DNA preparation
To prepare DNA, the plasmid pCMV containing Escherichia coli cells was plated out onto nutrient LB agar medium containing ampicillin. Plasmid isolation and detection were carried out according to the QIAGEN plasmid purification handbook using QIAGEN Plasmid Midi Kit. The purified DNA was diluted in 100 μL^sup -1^ pH 7.0 phosphate buffer to a final concentration of 30 ng μL^sup -1^. Melanosomes were added to DNA solution for 24 h. The next day DNA solutions were concentrated using DNA clean &concentrator(TM)-5 kit from Zymo Research. Gel electrophoresis of plasmid DNA was carried out in 1% molecular biology grade agarose gel containing ethidium bromide at 110 V for 45 min. The bands were illuminated with UV light and photographed.
RESULTS AND DISCUSSION
Ultrastructural characterization of melanosomes in darkly pigmented human melanocytes and melanoma Several maturation stages of melanosomes have been described (47–49), and are illustrated in an electron micrograph (EM) of a normal melanocyte cell (Fig. 1). Stage I are the so-called premelanosomes characterized by spherical forms which contain dense spots and occasional filaments. Stage II melanosomes are more ellipsoidal with observable filaments (intralumenal striations) that exhibit periodicity perpendicular to the long axis of the organelle. In stage III melanosomes, deposition of melanin is seen on the filaments but its organization is still evident; mature melanosomes are stage IV, the pigment completely fills the organelle and obscures the underlying filaments.
Figure 1.
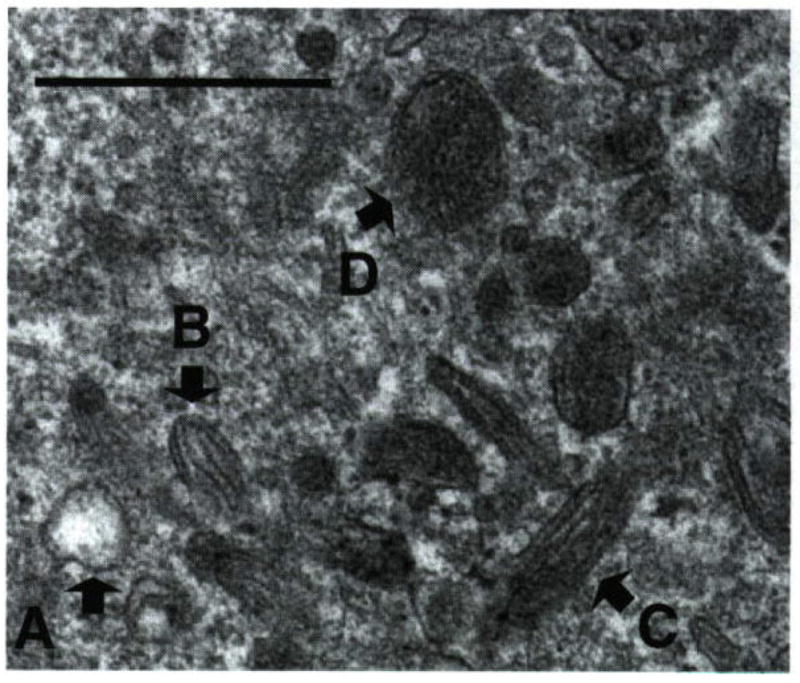
Stages of melanosomes in darkly pigmented normal melanocytes. Electron micrograph of normal human melanocyte showing different stages of melanosomes (Bar = 0.5 μm). Melanocyte cells were cultured in 10 mm tissue culture dishes and harvested and fixed for electron microscopy as described in the text. (A) Stage I melanosomes lack pigment but are membrane delimited; (B, C) stage III melanosomes with visible intraluminal fibers that run the length of the organelle but differing melanin deposition; (D) stage IV melanosome in which the intraluminal fibers are completely masked.
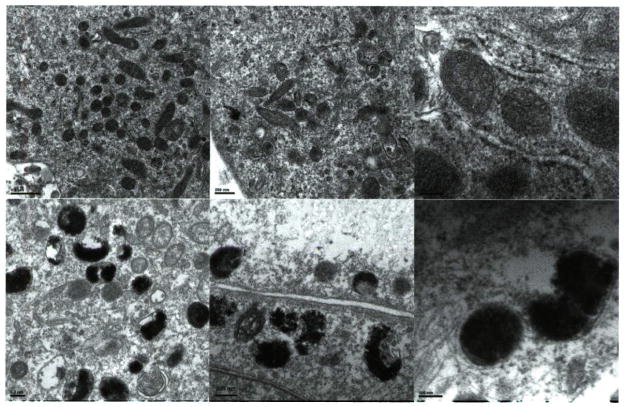
By contrast, the melanosomes within the MNT1 cells display malformed melanosomes (Fig. 2), characterized by large occlusions in pigmentation, often with damaged or absent membranes. The melanin within these abnormal melanosomes is granular with fragments sometimes evident outside of the melanosome. When filaments are observed, as in the analogous stage 2 or 3 melanosomes, they lack organized orientation, some in loose coils that give spiral rather than compact structures. Similar abnormalities are observed in melanosomes isolated from other melanoma cell lines, as characterized by our laboratory and others (10,11).
Figure 2.
Electron micrographs (EM) of melanosomes from melanocytes and melanoma. EM showing the melanosomes within normal human melanocytes (top) and within metastatic melanoma cell lines MNT1 (bottom). Magnifications: left, 6600× (bars = 500 nm); center, 8900× (bars = 200 nm); right, 2100× (bars = 100 nm).
Effect of UVB and metal ions on melanocyte morphology
Exposure of melanocytes to UVR has been shown to generate morphological changes (50), and to induce cell cycle arrest (51,52). We examined the effect of divalent Cu, Zn and Cd ions in the growth media on this behavior. Normal human melanocytes in culture are dendritic with small cytoplasm (Fig. 3). Treatment of the cells with UVB radiation led to increased dendricity and enlargement of the cell body. Addition of ZnCl^sub 2^ had no further effect. While addition of CuCl^sub 2^ at 10 μM to the growth medium alone did not cause observable change in the melanocyte morphology, those grown in Cu-containmg media and irradiated by UVB showed an increased cell body volume and dendricity. A similar but more dramatic effect was observed after irradiation of melanocytes in 0.10 μM CdCl^sub 2^. The phenotypic features of these treated melanocytes resemble melanocytes grown from dysplastic nevi grown in culture without any additions or treatments (53).
Figure 3.
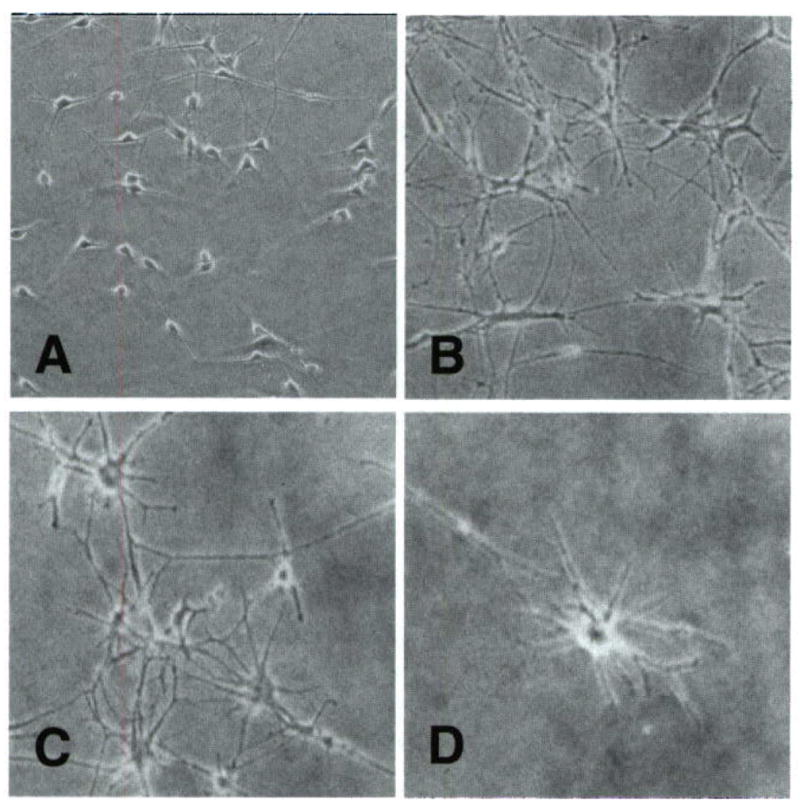
Morphological changes induced by UV and divalent metal ions in normal human melanocytes. Microscope images of: (A) untreated normal human melanocytes in culture, (B) the same treated with 25 mJ cm−2 UVB three times a week for 3 weeks; (C) the same maintained in 10 μM CuCl2 containing growth media, both showing increased dendricity and enlargement of the cell bodies; (D) the same maintained in 0.1 μM CdCl2 containing growth media showing high dendricity and enlargement.
Effect of UVB and metal ions on melanosomes in situ
The cumulative effects of divalent Cu, Cd and Zn in combination with UVB on melanocyte melanosomes were characterized using electron microscopy. Normal human melanocytes stage II melanosomes had the classic oval shape with regular internal filaments along the length of the organelle, stage III and IV melanosomes were oval or rounded in different sizes, and exhibited melanin deposition in a homogeneous manner which is similar to that reported by others (47).
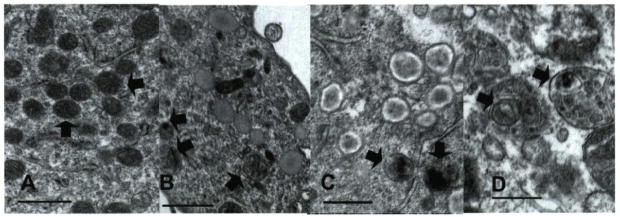
In UVB-treated melanocytes, several mildly bleached melanosomes were observed (Fig. 4B). In these cells melanin deposition in stage III and IV melanosomes was heterogeneous and granular. In melanocytes maintained in medium containing Cu(II) and treated with 3 ×3 treatments of UVB radiation, many highly bleached melanosomes were observed (Fig. 4C). These melanosomes appeared almost white on their edges and somewhat pigmented in the core of the organelle. Melanin deposition in the majority of stage III and IV melanosomes in these cells was highly heterogeneous and granular. The Zn(II) and UVB-treated cells show somewhat less pigmentation, but the melanosomes appear normal. The increased bleaching of melanosomes in Cu(II)-treated melanocytes is consistent with the previous finding by Korytowski and Sarna that Cu accelerates oxidative bleaching in synthetic melanin via production of ROS and peroxide (54).
Figure 4.
Electron micrographs (EM) of melanocytes treated with UV and divalent metal ions. (A) EM of untreated melanocytes; (B) melanocytes in culture treated with 25 mJ cm−2 UVB three times a week for 3 weeks; (C) same with 10 μM CuCl2 in growth media; (D) same as (B) but with 0.1 μM CdCl2 in growth media. Open arrows point to (A) normal stage IV melanosomes; (B, C) bleached, granulated melanosomes; (D) phagocytized melanosomes (bars = 0.5 μm).
The effect of Cd(II) and UVR was most dramatic. Cd is quite toxic to melanocytes even at very low concentrations. To maintain viablility, the melanocytes were treated at only 0.1 μM CdCl^sub 2^ and given the same sequence of UV treatments as above. Transmission electron microscopy image of these cells showed dramatic changes in melanosomal structures, many were misshapen with some bleaching evident. Some early-stage melanosomes were phagocytized within lysozymes (Fig. 4D) (55). There is granular melanin as well as disruption of the intralumenal striations organization in melanosomes, leading to the “loose coils” of pigmented filaments also observed in melanoma. Bleaching of the melanin is evident, but not enhanced over that in the absence of Cd. In all these long-term UVB experiments, cell attrition was a problem, especially with the Cu- and Cd-treated cells; images were only obtained on viable cells remaining after the treatments. Issues of toxicity will be addressed below.
The granular melanosomes observed upon UVB radiation of melanocytes resemble the fragmented pigment observed in unirradiated melanoma cells (Fig. 5). Melanoma cells are under constitutive intracellular oxidative stress (56), and granular melanosomes have been repeatedly observed in these cells (11). Observation of higher degree of granularity in Cu-treated cells upon UVR is likely an indication of enhanced melanin oxidation, and therefore a reduced redox buffering capacity of the pigment. The disruption of melanosomal organization by Cd mimics that seen in melanoma, and we speculate that these changes may be an indication of a different pathogenic feature of melanoma, not observed in the Cu-treated cells.
Figure 5.
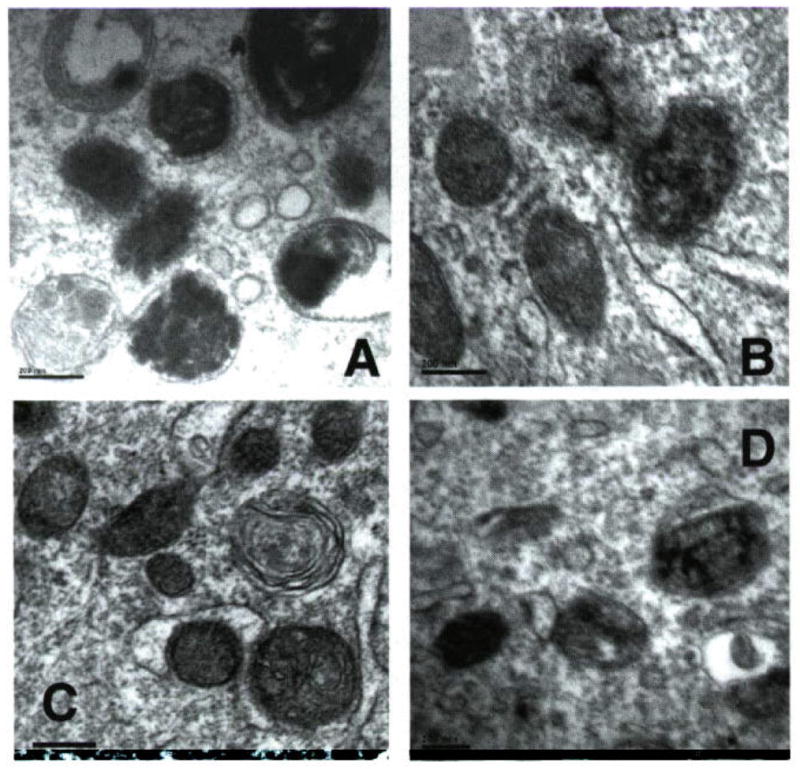
Electron micrograph (EM) comparison of melanoma melanosomes and UV and Cu- or Cd-treated melanocytes. EM of: (A) MNT1 melanoma cells, showing disrupted melanin deposition and granularity; (B) melanocytes treated with UVB showing some bleaching of melanin; (C) same treated with Cd(II) showing extensive disruption of melanin deposition; (D) same with Cu(II) showing extensive bleaching and granularized melanin. Conditions as described in Fig. 4 (bars = 0.2 μm).
Ex situ effect of UVB and Cu on melanin reactivity
The loss of regulation of melanosomes within melanoma should have dramatic consequences, as melanin precursors are particularly toxic species. ROS generation by isolated melanins has been well documented; they react in air to form a flux of Superoxide, and under certain conditions, peroxide and hydroxyl radicals (54,57). For example, melanin initiates lipid oxidations via superoxide-base mechanism, which is much accelerated in the presence of Fe ions (8). To assay this prooxidant behavior, a DNA clipping was used. This method characterizes the formation of hydroxyl radicals, which can clip supercoiled plasmid DNA and impair its movement during gel electrophoresis. Typically, both nicked and cleaved DNA will travel slower on the gel than the supercoiled form, giving two distinct bands above the native DNA. Both synthetic melanins and those derived from melanoma cell cultures have shown positive assays for this reactivity (6).
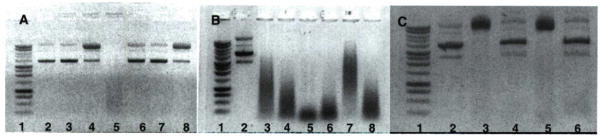
To compare the effect of dysfunctional melanin formation on oxidative reactivity, melanosome fractions were purified from both a highly pigmented human melanocyte and melanoma MNT1 cells. Quantification of the melanin is difficult, but approximately equivalent amounts isolated were from equivalent cultures (ca 10^sup 6^ cells), and final concentrations normalized by melanin absorbance at 500 nm. Some melanin samples were treated with Cu salt solution overnight and washed with PBS before assay. The plasmid DNA was then added to the samples and exposed to ROS generated by the glucose/glucose oxidase reaction. The following day, the DNA samples were purified and examined by agarose gel electrophoresis (Fig. 6). The MNT1 melanosomes showed dramatic reactivity under all conditions, cleaving the DNA into small fragments that show as smears on the gels. The fragmentation is also enhanced after UVB, and more so by Cu treatment. Importantly, normal melanosomes did not clip DNA; however, treatment with glucose/glucose oxidase or UVB alone caused minor clipping of the DNA, but UVB and Cu treatment combined to give strong clipping, consistent with the UVB-induced generation of peroxide or ROS by melanin (58).
Figure 6.
Reactive oxygen species reactivity of melanosomes from melanocytes and melanoma. Agarose gel electrophoresis of plasmid DNA showing different levels of clipping induced by melanosomes separated from: (A) dark human melanocytes (isolated from 106 cells, ca 0.8 mg/well); (B) melanoma cell line MNT1 (from 106 cells, ca 0.8 mg/well); (C) MNT1 melanoma (from 105 cells, ca 0.08 mg/well). Conditions for lanes in (A) and (B). from left: (1) DNA ladder; (2) untreated plasmid DNA; (3) DNA exposed to untreated melanosomes; (4) DNA exposed to Cu-treated melanosomes (200 μM CuCl2 overnight and spun down and washed with PBS the day after); (5) DNA exposed to Cu-treated melanosomes in the presence of glucose/glucose oxidase; (6) DNA exposed to melanosomes in the presence of glucose/glucose oxidase; (7) DNA exposed to 50 mJ cm−2 UVB-irradiated melanosomes; (8) DNA exposed to Cu and UVB-irradiated melanosomes. Conditions for lanes in (C), from left: (1) DNA ladder; (2) untreated plasmid DNA; (3) same exposed to untreated melanosomes; (4) same in the presence of azide (0.2 μL of 2 M NaN3); (5) DNA exposed to Cu-treated melanosomes; (6) same in the presence of azide.
To confirm that the dramatic clipping and fragmentation of the DNA by MNT1 melanosomes is caused by hydroxyl radicals, the melanosome samples were diluted 10-fold to attenuate the reactivity and to some, sodium azide was added. The azide anion is a known scavenger of hydroxyl radical, and if in excess should neutralize the radical’s damaging effects (59). As shown in Fig. 6C, the presence of azide effectively inhibits the clipping of supercoiled DNA by MNT1 melanosomes both with and without Cu treatment. These results are consistent with the enhanced bleaching of the UVB/Cu-treated melanocytes, via melanin-induced formation of ROS, of which hydroxyl radical is the most damaging form.
Effect of UVB and Cu on cell cycle and viability
There is much evidence that normal cellular melanin neutralizes the inflammatory response to radiation (60,61) and may help suppress hydrogen peroxide oxidative stress (18). Isolated melanins have also been shown to efficiently scavenge singlet oxygen (62) and Superoxide anion (63,64). These antioxidant abilities of melanin are likely important in its role in protecting cells from sun-induced damage. However, there is also much evidence for the photochemical generation of ROS by melanin (57), and implication of its toxicrty in diseases such as Parkinson’s (65). Previous studies on synthetic and sepia melanin have shown that its oxidation, enhanced by the binding of redox active metal ions, results in a substantial increase in the production of ROS (6,57,66,67). To investigate the short-term effect of the UVB/Cu-generated ROS on the host cells, assays of viability and cell cycle progression were performed. In these experiments, human melanocytes were treated with or without Cu-containing media for 24 h; certain cells were then washed and exposed to a single dose of UVB. The cells were replenished with growth media and after a short incubation period, trypsinized and fixed for cell cycle and viability analysis by flow cytometry.
The application of increasing doses of both Cu and UVB radiation to untreated melanocytes up to sunburn dose leads to little change in viability or cell cycle progression (Figs. 6 and 7). At a higher concentration, Cu appeared to increase G2 arrest to a minor degree. Likewise, treatment of heavily pigmented melanoma MNT1 by both Cu and UVB had only a small effect on its growth and viability. There was a small but consistent decrease in viability with increasing UVB, driven by an increase in apoptosis; but the change amounted to less than 10% of the MNT1 cells.
Figure 7.
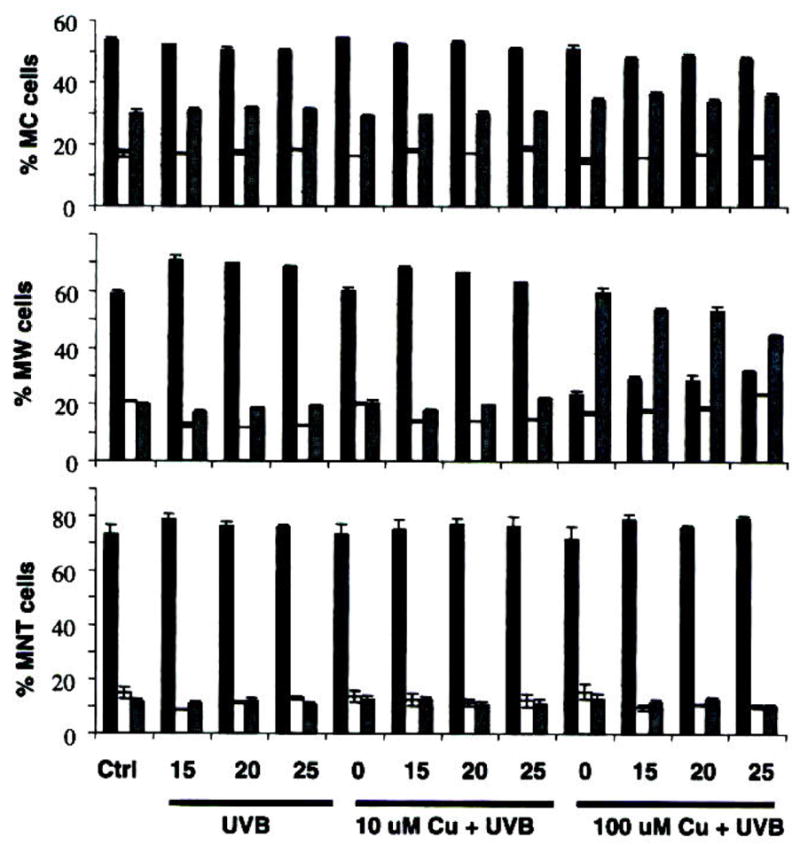
Cell cycle progression with UVB/Cu treatment. Columns represent percentage cells in G1 (black), S (white) and G2 (grey); top graph is normal human melanocytes (MC), middle is primary melanoma WM 3211 and bottom is metastatic melanoma MNT1. Cells were first treated with 10 or 100 μM Cu(II) in the media. After 24 h the cells were exposed to 15, 20 or 25 mJ cm−2 UVB, as indicated. The cells were collected after 24 h and fixed before assay. Values given as the mean ± SD derived from a minimum of three sets of experiments.
A major difference was observed between the cell cycle phases of the different cell lines, with MNT1 cells being dominantly in the G1 phase, and normal melanocytes more evenly distributed between G1, S and G2 phases. The melanocytes showed a small increase in G2 arrest at high Cu concentration, not seen for MNT1 cells, but overall, the single Cu/UVB treatment had little effect on the cell cycle.
As melanocytes and MNT1 cells seemed well protected against both UVR and Cu, a relatively amelanotic primary melanoma cell line WM3211 was tested under similar treatments. The WM3211 melanoma cells are phenotypically similar to in vitro melanocytes in that they are slow-growing and exhibit horizontal growth in the primary lesion. For these cells, applied UVB radiation causes an increase in G1 cell cycle arrest at all doses; but Cu at high dose completely shut off G2 progression. Viability was also much more affected by both treatments-increasing UVB doses led to a decrease in viability of >25%, mainly representing increased apoptosis. High doses of Cu led to a viability decrease of ca 15%; its combination with UVB appears additive rather than synergistic. The greater sensitivity of this cell type might reasonably be attributed to its lack of pigment (Fig. 8).
Figure 8.
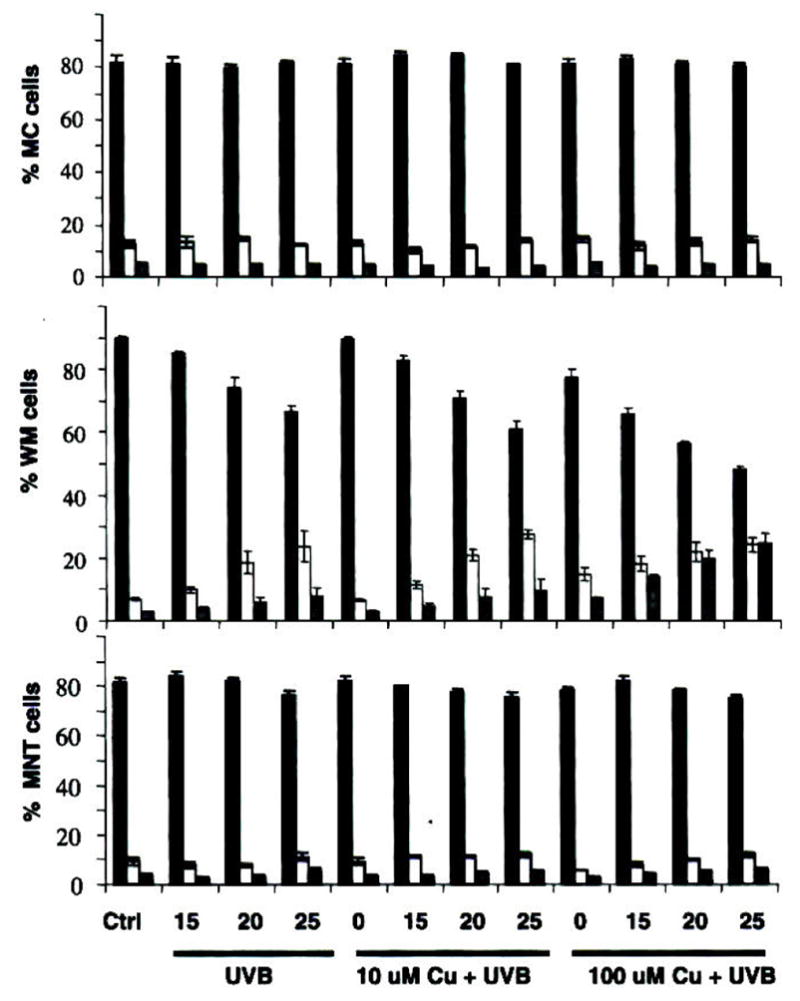
Cell viability with UVB/Cu treatment. Columns represent percentage cells as viable (black), apoptotic (white) and necrotic (grey); top graph is normal human melanocytes (MC), middle is primary melanoma WM 3211 and bottom is metastatic melanoma MNT1. Cells were first treated with 10 or 100 μM Cu(II) in the media. After 24 h the cells were exposed to 15, 20 or 25 mJ cm−2 UVB, as indicated. The cells were then collected after 24 h for assay. Values given as the mean ± SD derived from a minimum of three sets of experiments.
CONCLUSIONS
One of the most puzzling clinical observations in dermatology is that nonmelanoma skin cancers such as squamous cell carcinomas occur with high frequency in albinos of all ethnicities, but the occurrence of cutaneous melanoma in these individuals is exceedingly rare (68,69). Melanocytes are still present but they do not make melanin. Why is this? We propose that without melanin melanomas do not occur. The immediate reply is, of course, “If so, then why don’t darkly pigmented individuals develop melanomas at a high frequency?” There have been many well-established and well thought out answers to this question, as summarized recently by Wood et al. (70). The relative resistance of dark-skinned humans to UVR induction of melanoma compared with light-skinned humans likely involves contributions from increases in melanosome size leading to lower fluxes of oxidants from larger melanin aggregates in darker skins (36), increased melanosome/melanin levels in upper epidermal layers protecting underlying melanocytes (71) and the lack of pheomelanin, a more powerful photosensitizer than eumelanin, in dark-skinned individuals (72). Most recently a differential role of keratinocytes obtained from different ethnic skin in determining melanocyte response to UVR has also been identified (73).
Clearly the amount and nature of melanin plays an important role in responses to UVR and other environmental cues, both genetic and extrinsic, and hence in melanoma susceptibility. Our results and those of others suggest that the oxidation of melanin by UVR, enhanced by the binding of certain metals, might be an early event in the pathogenesis of melanoma. The melanosomal abnormalities observed in metal/UVB-treated melanocytes are very similar to those observed in metastatic melanoma; thus the conversion of melanin to a pro-oxidant generator of ROS may be a key feature promoting carcinogenesis.
Acknowledgments
This work was supported by the Chao Family Comprehensive Cancer Center, University of California at Irvine (CA62230, P.M.), an NIH-NCI institutional training grant (5T32 CA09054, S.G.) and American Cancer Society Research Scholar grant (RSG-03-251-01, P.J.F.). We thank Dr. Vincent Hearing for the gift of MNT1 cell line, Dr. Raymond Boissy for helpful comments and Rita Liu for assistance with cell imaging.
Footnotes
This invited paper is part of the Symposium-in-Print: Melanins.
References
- 1.Keilholz U. Biochemotherapy of melanoma. Forum. 2003;13:158–165. [PubMed] [Google Scholar]
- 2.Kleeberg UR. Wishful thinking, unicentric empiricism and the everyday world of the medical melanomologist. Melanoma Res. 1997;7:S143–S149. [PubMed] [Google Scholar]
- 3.Helmbach H, Rossmann E, Kern MA, Schadendorf D. Drug resistance in human melanoma. Int J Cancer. 2001;93:617–622. doi: 10.1002/ijc.1378. [DOI] [PubMed] [Google Scholar]
- 4.Jimbow K, Iwashina T, Alena F, Yamada K, Pankovich J, Umemura T. Exploitation of pigment biosynthesis pathway as a selective chemotherapeutic approach for malignant melanoma. J Invest Dermatol. 1993;100:231S–238S. [PubMed] [Google Scholar]
- 5.Prota G, d’Ischia M, Mascagna D. Melanogenesis as a targeting strategy against metastatic melanoma: A reassessment. Melanoma Res. 1994;4:351–358. doi: 10.1097/00008390-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Farmer PJ, Gidanian S, Shahandeh B, Di Bilio AJ, Tohidian N, Meyskens FL. Melanin as a target for melanoma chemotherapy: Pro-oxidant effect of oxygen and metals on melanoma viability. Pigment Cell Res. 2003;16:273–279. doi: 10.1034/j.1600-0749.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson ES, Jenkins ND, Todd JM. Relationship between Superoxide dismutase and melanin in a pathogenic fungus. Infect Immun. 1994;62:4085–4086. doi: 10.1128/iai.62.9.4085-4086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotomatsu A, Tanaka M, Hirai S. Synthetic melanin and ferric ions promote superoxide anion-mediated lipid peroxidation. FEBS Lett. 1994;342:105–108. doi: 10.1016/0014-5793(94)80481-8. [DOI] [PubMed] [Google Scholar]
- 9.Crippa R, Horak V, Prota G, Sworonos P, Wolfram L. Chemistry of melanins. In: Brossi A, editor. Alkaloids. Academic Press; New York: 1989. pp. 253–323. [Google Scholar]
- 10.Curran RC, McCann BG. The ultrastructure of benign pigmented naevi and melanocarcinomas in man. J Pathol. 1976;119:135–146. doi: 10.1002/path.1711190303. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes AR, Seki Y, Fitzpatrick TB, Stern RS. Melanosomal alterations in dysplastic melanocytic nevi. A quantitative, ultrastructural investigation. Cancer. 1988;61:358–369. doi: 10.1002/1097-0142(19880115)61:2<358::aid-cncr2820610227>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Borovansky J, Mirejovsky P, Riley PA. Possible relationship between abnormal melanosome structure and cytotoxic phenomena in malignant melanoma. Neoplasma. 1991;38:4–10. [PubMed] [Google Scholar]
- 13.Riley PA, Cooksey CJ, Johnson CI, Land EJ, Latter AM, Ramsden CA. Melanogenesis-targeted anti-melanoma pro-drug development: Effect of side-chain variations on the cytotoxicity of tyrosinase-generated ortho-quinones in a model screening system. Eur J Cancer. 1997;33:135–143. doi: 10.1016/s0959-8049(96)00340-1. [DOI] [PubMed] [Google Scholar]
- 14.Ito S, Palumbo A, Prota G. Tyrosinase-catalyzed conjugation of dopa with glutathione. Experientia. 1985;41:960–961. doi: 10.1007/BF01970033. [DOI] [PubMed] [Google Scholar]
- 15.Zareba M, Bober A, Korytowski W, Zecca L, Sarna T. The effect of a synthetic neuromelanin on yield of free hydoxyl radicals generated in model systems. Biochim Biophys Acta. 1995;127:343–348. doi: 10.1016/0925-4439(95)00058-c. [DOI] [PubMed] [Google Scholar]
- 16.Meyskens FL, Van Chau H, Tohidian N, Buckmeier JA. Luminol-enhanced chemiluminescence response of human melanocytes and melanoma cells to hydrogen peroxide stress. Pigment Cell Res. 1997;10:184–189. doi: 10.1111/j.1600-0749.1997.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 17.Meyskens FL, Buckmeier JA, McNulty SE, Tohidian NE. Activation of nuclear factor-kappa B in human metastatic melanoma cells and the effect of oxidative stress. Clin Cancer Res. 1999;5:1197–1202. [PubMed] [Google Scholar]
- 18.McNulty SE, Tohidian NB, Meyskens FL., Jr RelA, p50, and inhibitor of kappa B alpha are elevated in human metastatic melanoma cells and respond aberrantly to ultraviolet light B. Pigment Cell Res. 2001;14:456–465. doi: 10.1034/j.1600-0749.2001.140606.x. [DOI] [PubMed] [Google Scholar]
- 19.McNulty SE, del Rosario R, Cen D, Meyskens FL, Jr, Yang S. Comparative expression of NFkappa B proteins in melanocytes of normal skin vs. benign intradermal naevus and human metastatic melanoma biopsies. Pigment Cell Res. 2004;17:173–180. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, McNulty SE, Meyskens FL., Jr During human melanoma progression AP-1 binding pairs are altered with loss of c-Jun in vitro. Pigment Cell Res. 2004;17:74–83. doi: 10.1046/j.1600-0749.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 21.Fruehauf JP, Zonis S, Al-Bassam M, Kyshtoobayeva A, Dasgupta G, Milovanovic T, Parker R, Buzaid AC. Melanin content and down regulation of glutathione-S-transferase contribute to the action of L-buthionine-S-sulfoximine on human melanoma. Chem Biol Interact. 1998;111–112:277–305. doi: 10.1016/s0009-2797(97)00167-1. [DOI] [PubMed] [Google Scholar]
- 22.Picardo M, Maresca V, Eibenschutz L, DeBernado C, Rinaldi R, Grammatico P. Correlation between antioxidants and phototypes in melanocytes cultures. A possible link of physiologic and pathologic relevance. J Invest Dermatol. 1999;113:424–425. doi: 10.1046/j.1523-1747.1999.00714.x. [DOI] [PubMed] [Google Scholar]
- 23.Pavel S, van Nieuwpoort F, van der Meulen H, Out C, Pizinger K, Cetkovska P, Smit NP, Koerten HK. Disturbed melanin synthesis and chronic oxidative stress in dysplastic naevi. Eur J Cancer. 2004;9:1423–1430. doi: 10.1016/j.ejca.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Hill HZ, Hill GJ. Eumelanin causes DNA strand breaks and kills cells. Pigment Cell Res. 1987;1:163–170. doi: 10.1111/j.1600-0749.1987.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 25.Hubbard-Smith K, Hill HZ, Hill GJ. Melanin both causes and prevents oxidative base damage in DNA: Quantification by anti-thymine glycol antibody. Radiat Res. 1992;130:160–165. [PubMed] [Google Scholar]
- 26.Husain SS, Hadi M. DNA breakage by L-DOPA and Cu(II): Breakage by melanin and bacteriophage inactivation. Mut Res Fund Mol M. 1998;397:161–168. doi: 10.1016/s0027-5107(97)00206-6. [DOI] [PubMed] [Google Scholar]
- 27.Hong L, Liu Y, Simon JD. Binding of metal ions to melanin and their effects on the aerobic reactivity. Photochem Photobiol. 2004;80:477–481. doi: 10.1562/0031-8655(2004)080<0477:BOMITM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Hussein MR, Haemel AK, Sudilovsky O, Wood GS. Genomic instability in radial growth phase melanoma cell lines after ultraviolet irradiation. J Clin Pathol. 2005;58:389–396. doi: 10.1136/jcp.2004.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyskens FL, Farmer PJ, Yang S, Anton-Culver H. New perspectives on melanoma pathogenesis and chemoprevention. Rec Res Cancer Res. 2007;174:191–195. doi: 10.1007/978-3-540-37696-5_16. [DOI] [PubMed] [Google Scholar]
- 30.Berwick M, Wiggins C. The current epidemiology of cutaneous malignant melanoma. Front Biosci. 2006;11:1244–1254. doi: 10.2741/1877. [DOI] [PubMed] [Google Scholar]
- 31.Placzek M, Przybilla B, Kerkmann U, Gaube S, Gilbertz K-P. Effect of ultraviolet (UV) A, UVB or ionizing radiation on the cell cycle of human melanoma cells. Br J Dermatol. 2007;156:843–847. doi: 10.1111/j.1365-2133.2007.07795.x. [DOI] [PubMed] [Google Scholar]
- 32.Berwick M. Pathways to the development of melanoma: A complex issue. J Invest Dermatol. 2006;126:1932–1933. doi: 10.1038/sj.jid.5700419. [DOI] [PubMed] [Google Scholar]
- 33.Pavel S, Smit NP, van der Meulen H, Kolb RM, de Groot AJ, van der Velden PA, Grids NA, Bergman W. Homozygous germline mutation of CDKN2A/p16 and glucose-6-phosphate dehydrogenase deficiency in a multiple melanoma case. Melanoma Res. 2003;13:171–178. doi: 10.1097/00008390-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Meyskens FL, Berwick M. UV or not UV: Metals? The answer. Cancer Epidemiol Biomarkers Prev. 2008 doi: 10.1158/1055-9965.EPI-07-0653. in press. [DOI] [PubMed] [Google Scholar]
- 35.Bedrick AE, Ramaswamy G, Tchertkoff V. Histochemical determination of copper, zinc, and iron in some benign and malignant tissues. Am J Clin Pathol. 1986;86:637–640. doi: 10.1093/ajcp/86.5.637. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Simon JD. Isolation and biophysical studies of natural eumelanins: Applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res. 2003;16:606–618. doi: 10.1046/j.1600-0749.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 37.Leonard S, Harris G, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Borovansky J, Riley PA. The effect of divalent cations on Cloudman melanoma cells. Eur J Cancer Clin Oncol. 1983;19:91–99. doi: 10.1016/0277-5379(83)90403-0. [DOI] [PubMed] [Google Scholar]
- 39.Borovansky J, Blasko M, Siracky J, Schothorst AA, Smit NP, Pavel S. Cytotoxic interactions of Zn^sup 2+^ in vitro: Melanoma cells are more susceptible than melanocytes. Melanoma Res. 1997;7:449–453. doi: 10.1097/00008390-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Holly EA, Aston DA, Ahn DK, Smith AH. Intraocular melanoma linked to occupations and chemical exposure. Epidemiology. 1996;7:55–61. doi: 10.1097/00001648-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Meo SA, Al-Khlaiwi T. Health hazards of welding fumes. Saudi Med J. 2003;24:1176–1182. [PubMed] [Google Scholar]
- 42.Tenkate TD. Occupational exposure to ultraviolet radiation: A health risk assessment. Rev Environ Health. 1999;14:187–209. doi: 10.1515/reveh.1999.14.4.187. [DOI] [PubMed] [Google Scholar]
- 43.Carrasco-Pozo C, Alvarez-Lueje A, Olea-Azar C, López-Alarcón C, Speisky H. In vitro interaction between homocysteine and copper ions: potential redox implications. Exp Biol Med. 2006;231:1569–1575. doi: 10.1177/153537020623100918. [DOI] [PubMed] [Google Scholar]
- 44.Cen D, Gonzalez RI, Buckmeier JA, Kahlon RS, Tohidian NB, Meyskens FL., Jr Disulfiram induces apoptosis in human melanoma cells: A redox-related process. Mol Cancer Ther. 2002;1:197–204. [PubMed] [Google Scholar]
- 45.Cen D, Brayton D, Shahandeh B, Meyskens FL, Farmer PJ. Disulfiram causes intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47:6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 46.Samaraweera P, Donatien PD, Azi S, Kobayashi T, Hearing VJ, Panther JJ, Orlon SJ. Identification and characterization of a melanocyte-specific novel 65-kDa peripheral membrane protein. Eur J Biochem. 1999;266:924–934. doi: 10.1046/j.1432-1327.1999.00930.x. [DOI] [PubMed] [Google Scholar]
- 47.Marks MS, Seabra MC. The melanosome: Membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2(10):738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- 48.Raposo G, Marks MS. The dark side of lysosome-related organelles: Specialization of the endocytic pathway for melanosome biogenesis. Traffic. 2002;3:237–248. doi: 10.1034/j.1600-0854.2002.030401.x. [DOI] [PubMed] [Google Scholar]
- 49.Boissy RE, Huizing M, Gahl WA. Biogenesis of melanosomes. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System. 2. Blackwell Publishing; Maiden, MA: 2006. pp. 155–170. [Google Scholar]
- 50.Friedmann PS, Gilchrest BA. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol. 1987;133:88–94. doi: 10.1002/jcp.1041330111. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Malek Z, Swoope V, Samara D, Babcock G, Dawes S, Nordlund J. Analysis of the UV-induced melanogenesis and growth arrest of human melanocytes. Pigment Cell Res. 1994;7:326–332. doi: 10.1111/j.1600-0749.1994.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 52.Iyengar B. Expression of proliferating cell nuclear antigen (PCNA): Proliferative phase functions and malignant transformation of melanocytes. Melanoma Res. 1994;4:293–295. [PubMed] [Google Scholar]
- 53.Herlyn M, Herlyn D, Elder DE, Bondi E, LaRossa D, Hamilton R, Sears HF, Balaban G, Guerry D. Phenotypic characteristics of cells derived from precursors of human melanoma. Cancer Res. 1983;43:5502–5508. [PubMed] [Google Scholar]
- 54.Korytowski W, Sarna T. Bleaching of melanin pigments. Role of copper ions and hydrogen peroxide in autooxidation and photooxidation of synthetic dopa-melanin. J Biol Chem. 1990;265:12410–12416. [PubMed] [Google Scholar]
- 55.Borovanský J, Eileder M. Melanosome degradation: Fact or fiction. Pigment Cell Res. 2003;16:280–286. doi: 10.1034/j.1600-0749.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 56.Meyskens FL, Jr, McNulty SE, Buckmeier JA, Tohidian NB, Spillane TJ, Kahlon RS, Gonzalez RI. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic Biol Med. 2001;31:799–808. doi: 10.1016/s0891-5849(01)00650-5. [DOI] [PubMed] [Google Scholar]
- 57.Korytowski W, Pilas B, Sarna T, Kalyanaraman B. Photoinduced generation of hydrogen peroxide and hydroxyl radicals in melanins. Photochem Photobiol. 1987;45:185–190. doi: 10.1111/j.1751-1097.1987.tb05362.x. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz S, Thomas PD, Allen TM, Poznansky MJ, Jimbow K. Dual role of melanins and melanin precursors as photoprotective and phototoxic agents: Inhibition of ultraviolet radiation-induced lipid peroxidation. Photochem Photobiol. 1995;61:650–655. doi: 10.1111/j.1751-1097.1995.tb09883.x. [DOI] [PubMed] [Google Scholar]
- 59.Joshi R, Kumar S, Unnikrishnan M, Mukherjee T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: Mechanistic aspects and antioxidant activity. Free Radic Res. 2005;39:1163–1172. doi: 10.1080/10715760500177880. [DOI] [PubMed] [Google Scholar]
- 60.Nordlund JJ. The pigmentary system and inflammation. Pigment Cell Res. 1992;5:362–365. doi: 10.1111/j.1600-0749.1992.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 61.Doss LL, Memula N. The radio-responsiveness of melanoma. J Radiat Oncol Biol Phys. 1982;8:1131–1134. doi: 10.1016/0360-3016(82)90060-8. [DOI] [PubMed] [Google Scholar]
- 62.Egorov SY, Dontsov AE, Krasnovsky AA, Jr, Osrivsky MA. Quenching of singlet molecular oxygen by screening pigments-Melanin and ommochromes. Biofizika. 1987;32:685–686. [PubMed] [Google Scholar]
- 63.Sarna T, Pilas B, Land EJ, Truscott TG. Interaction of radicals from water radiolysis with melanin. Biochim Biophys Acta. 1986;88:162–167. doi: 10.1016/0304-4165(86)90147-9. [DOI] [PubMed] [Google Scholar]
- 64.Korytowski W, Kalyanaraman B, Menon IA, Saraa T, Sealy RC. Reaction of Superoxide anions with melanins: Electron spin resonance and spin trapping studies. Biochim Biophys Acta. 1986;882:145–153. doi: 10.1016/0304-4165(86)90149-2. [DOI] [PubMed] [Google Scholar]
- 65.Ben-Shachar D, Youdim MB. Iron, melanin and dopamine interaction: Relevance to Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:139–150. doi: 10.1016/0278-5846(93)90038-t. [DOI] [PubMed] [Google Scholar]
- 66.Gidanian S, Farmer PJ. Redox behavior of melanins: Direct electrochemistry of dihydroxyindole melanin and its Cu and Zn adducts. J Inorg Biochem. 2002;89:54–60. doi: 10.1016/s0162-0134(01)00405-6. [DOI] [PubMed] [Google Scholar]
- 67.Hintz P, Kalyanaraman B. Metal-ion induced activation of molecular oxygen in pigmented polymers. Biochim Biophys Acta. 1986;883:41–45. doi: 10.1016/0304-4165(86)90132-7. [DOI] [PubMed] [Google Scholar]
- 68.Luanda J, Henschke GI, Mogammed N. The Tanzanian human albino skin. Natural history Cancer. 1985;15:1823–1828. doi: 10.1002/1097-0142(19850415)55:8<1823::aid-cncr2820550830>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 69.Yoniba A, Mabogunje OA. Skin cancer in African albinos. Acta Oncol. 1993;32:621–622. doi: 10.3109/02841869309092440. [DOI] [PubMed] [Google Scholar]
- 70.Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci USA. 2006;103:4111–4113. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alaluf S, Atkins D, Barren K, Blount M, Carter N, Heath M. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002;15:119–126. doi: 10.1034/j.1600-0749.2002.1o072.x. [DOI] [PubMed] [Google Scholar]
- 72.Kuchino Y, Mori F, Kasai H, Inoue H, Iwai S, Miura K, Ohtsuka E, Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;327:77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- 73.Thong HY, Jee SH, Sun CC, Boissy RE. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br J Dermatol. 2003;149:498–505. doi: 10.1046/j.1365-2133.2003.05473.x. [DOI] [PubMed] [Google Scholar]