Abstract
Rapidly proliferating cancer cells require energy and cellular building blocks for their growth and ability to maintain redox balance. Many studies have focused on understanding how cancer cells adapt their nutrient metabolism to meet the high demand of anabolism required for proliferation and maintaining redox balance. Glutamine, the most abundant amino acid in plasma, is a well-known nutrient used by cancer cells to increase proliferation as well as survival under metabolic stress conditions. In this review, we provide an overview of the role of glutamine metabolism in cancer cell survival and growth and highlight the mechanisms by which glutamine metabolism affects cancer cell signaling. Furthermore, we summarize the potential therapeutic approaches of targeting glutamine metabolism for the treatment of numerous types of cancer.
Keywords: Cancer, Glutamine, Anaplerosis, Redox homeostasis
INTRODUCTION
Since the discovery that cancer cells can reprogram glucose metabolism towards aerobic glycolysis instead of oxidative phosphorylation by Warburg in the 1920s, there have been significant advancements in understating cancer metabolism (Vander Heiden et al., 2009). Metabolic reprogramming is a hallmark of cancer cells, whereby numerous changes in cellular bioenergetics occur, causing the cells to adapt to a variety of stress conditions (Yoshida, 2015). Cancer cells can orchestrate metabolic reprogramming by altering the uptake and catabolism of nutrients, enabling them to maintain proliferative capacity, conferring resistance to oxidative stress, and promoting the evasion of immune-mediated destruction (Hanahan and Weinberg, 2011). In early studies on cancer metabolism, dysregulated glucose metabolism, also called the “Warburg effect”, received much attention as a hallmark of cancer, since the glycolytic pathway produces ATP and metabolic intermediates for cancer cell proliferation. However, glucose only supplies a carbon source for biosynthesis; it cannot supply the amino acids and glutathione that rapidly proliferating cancer cells require for the synthesis of nucleic acids.
Several studies have shown that glutamine is a major nutrient involved in multiple aspects of cancer metabolism (Hensley et al., 2013). Glutamine is the most abundant amino acid in the blood and muscle (5) and is largely utilized for energy generation and as a precursor for the biomass required for rapid cancer cell proliferation (Windmueller and Spaeth, 1974). In addition to providing a carbon source, glutamine metabolism also acts as a source of nitrogen for the synthesis of nucleic acids and other amino acids and also participates in the regulation of cellular redox homeostasis through a variety of mechanisms (Altman et al., 2016). Therefore, most cancer cells are dependent on glutamine and cannot survive in the absence of exogenous glutamine, which has been termed “glutamine addiction” (Eagle, 1955). In light of the importance of glutamine in cancer cell biology, a comprehensive understanding of glutamine metabolism is important for developing effective therapeutic strategies.
In this review, we summarize the diverse aspects of glutamine metabolism including its role in biosynthetic fluxes, the modulation of signal transduction pathways, and the mitigation of oxidative stress. Finally, we discuss potential cancer therapy targeting approaches based on glutamine metabolism.
ROLE OF GLUTAMINE IN CELLULAR GROWTH AND REDOX HOMEOSTASIS
Glutamine addiction in cancer cells
Enhanced glutamine uptake is mediated by several transporters, including the well-investigated SLC1A5 (also called ASCT2) (Bhutia et al., 2015). Many cancer cells, including non-small cell lung cancer (NSCLC), breast cancer, and brain tumor cells have a high dependency on glutamine for their growth and survival and exhibit upregulated SLC1A5 expression (Mohamed et al., 2014; Marquez et al., 2017). Following the entry of glutamine into the cell via its transporter, the first step of its catabolism occurs through the activation of glutaminase (GLS), which catalyzes the conversion of glutamine to glutamate. Two different isoforms of glutaminase are expressed in mammals, kidney-type glutaminase (GLS1) and liver-type glutaminase (GLS2) (Mates et al., 2013). GLS1 is overexpressed in many cancer types and converts glutamine to glutamate, which is then converted to α-KG and channeled into the TCA cycle. In some human cancer tissues, increased levels of GLS1 are associated with a higher disease stage and poor prognosis (Yu et al., 2015). On the other hand, the role of GLS2 in cancer is still not completely understood and GLS2 function appears to be context-specific. Some studies have shown that the overexpression of GLS2 reduces the growth of cancer cells, suggesting that GLS2 works as a tumor suppressor and a putative transcription factor (Hu et al., 2010). By contrast, other studies have reported that GLS2 in some neuroblastomas is upregulated and contributes to cell survival (Qing et al., 2012). The relationship between GLS2 and tumorigenesis is discussed below. In rapidly proliferating cancer cells, glutamine is avidly taken up through a transporter and metabolized by a catalyzing enzyme. Thus, it can provide precursors for energy production and macromolecule biosynthesis, in addition to its ability to regulate cellular signaling.
Glutamine as a source of nitrogen
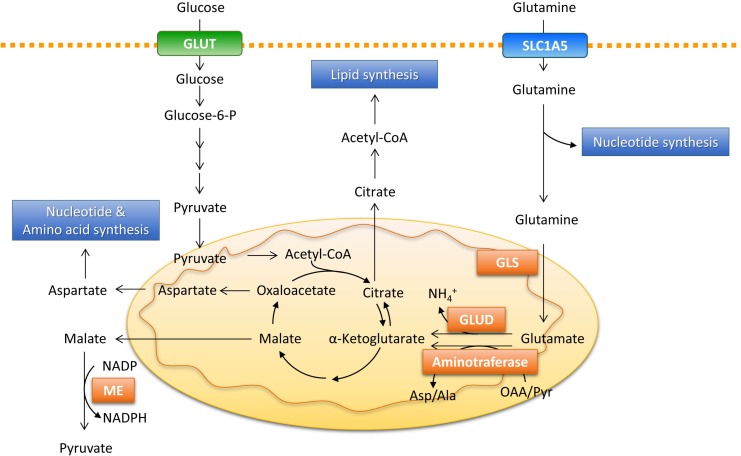
One of the most important metabolic fates of glutamine is supplying amino and amide nitrogen for biosynthetic pathways, including the production of nonessential amino acids and nucleotides (Fig. 1). Glutamate is converted to α-ketoglutarate by either glutamate dehydrogenase (GLUD), which releases ammonium, or by aminotransferases, which transfers amino nitrogen from glutamate to produce another amino acid (alanine and aspartate) and α-ketoglutarate, without producing ammonia (Yang et al., 2017). The aminotransferases glutamate-pyruvate transaminase (GPT), glutamate-oxalate transaminase (GOT), and phosphoserine aminotransferase 1 (PSAT1) catalyze the reversible transfer of amino nitrogen between glutamate to alanine, aspartate, and phosphoserine, respectively (Yang et al., 2017). Aspartate contributes to the generation of asparagine through incorporation into urea or is used in nucleotide synthesis. In addition to aspartate, alanine is also used in protein synthesis, but can also be released outside of the cancer cell, carrying some of the excess carbon from glycolysis (DeBerardinis and Cheng, 2010). Phosphoserine is subsequently converted to glycine by serine hydroxymethyltransferase, as part of one-carbon metabolism. This integrates cellular nutrients by cycling carbon units from serine inputs to generate diverse outputs, including the biosynthesis of NADPH and nucleotides (Locasale, 2013). A tracer study showed that at least 50% of nonessential amino acids required for protein synthesis arise from glutamine in cancer cells, highlighting the role of glutamine during rapid cellular proliferation (Alberghina and Gaglio, 2014). Glutamine-derived amide nitrogen also contributes to de novo synthesis of purines and pyrimidines, which are required for rapidly proliferating cancer cells (DeBerardinis and Cheng, 2010). Glutamine-derived amide nitrogen units are added to the growing purine and pyrimidine rings, which can explain the observation that K-RAS-transformed cells exhibit a delayed transit through S phase under low glutamine conditions, due to a reduced supply of DNA building blocks (Gaglio et al., 2009). All of these findings suggest that glutamine serves as a major nitrogen source for amino acid and nucleotide biosynthesis required for cancer cell growth.
Fig. 1.
Glutamine provides a nitrogen and carbon source in biosynthetic pathways. Glutamine enters the cells via the SLC1A5 transporter and contributes to nucleotide biosynthesis directly or is converted to glutamate by GLS. Glutamate is converted to α-ketoglutarate by either GLUD or aminotransferases. Malate from the TCA cycle can be exported to the cytoplasm and converted to pyruvate and generate NAPDH by ME. Oxaloacetate can be converted to aspartate, which supports amino acid and nucleotide synthesis. Glutamine-derived α-ketoglutarate can provide an alternative carbon source for the formation of acetyl-CoA required for lipid synthesis via reductive carboxylation. Glucose-6-P: glucose-6-phosphate, GLS: glutaminase, GLUD: glutamate dehydrogenase, ME: malic enzyme.
Glutamine-derived anaplerosis
Cancer cells require large amounts of lipids as well as nucleotides and amino acids during cell division. Most of the carbon required for fatty acid synthesis in non-proliferating cells comes from glucose, which is converted to acetyl CoA that condensed with oxaloacetate to produce citrate (Vander Heiden et al., 2009). During the rapid proliferation of cancer cells, citrate is continuously exported from the mitochondria to the cytosol for lipid biosynthesis. To accommodate this, the replenishment of metabolic intermediates in the TCA cycle, also called anaplerosis, is required. The flux experiments revealed that glutamine, via anaplerotic entry to TCA cycle, replenishes the biosynthetic precursors required for fatty acid synthesis (DeBerardinis et al., 2007, 2008). In glioblastoma cells, glutamine-derived oxaloacetate accounts for a high fraction of citrate synthesis, whereas glucose supplies a major carbon source for acetyl-CoA, suggesting that anaplerosis is central to glutamine metabolism (DeBerardinis et al., 2007). In addition to citrate synthesis, glutamine provides an alternative carbon source for the formation of acetyl-CoA that is required for lipid synthesis via reductive carboxylation under conditions of hypoxia or mitochondrial dysfunction (Metallo et al., 2011; Jiang et al., 2016). Tracer experiments demonstrated that 10–25% of lipogenic acetyl-CoA is derived from glutamine via reductive carboxylation in various types of cancer cells (Metallo et al., 2011). Glutamine-derived malate is also converted to pyruvate by malic enzyme, which can be metabolized to oxaloacetate or acetyl-CoA for reentry into the TCA cycle (Le et al., 2012). Thus, anaplerosis for energy production and fatty acid synthesis in cancer cells is highly dependent on glutamine metabolism, especially under conditions of metabolic stress or oncogenic activation.
Effects of glutamine metabolism on redox homeostasis
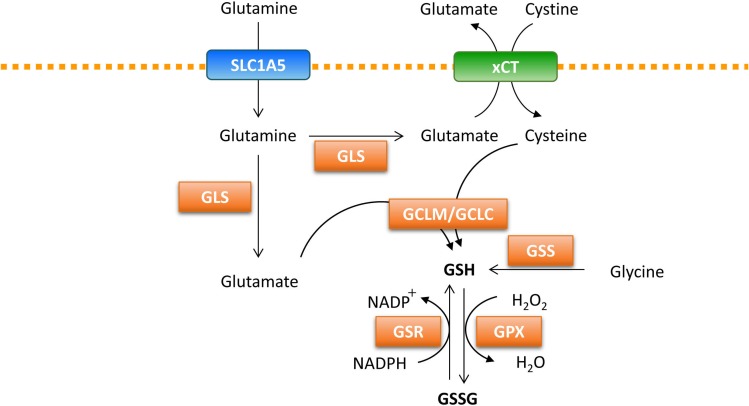
Cancer cells are inevitably exposed to more reactive oxygen species (ROS), generated by the mitochondrial electron transport chain during disease progression. When ROS is present in excess, it can damage DNA and other cellular components (Gorrini et al., 2013). Cancer cells have several protective mechanisms to avoid death upon excessive ROS exposure. One of the well-known mechanisms is the ability of cancer cells to increase antioxidant defense substrates that can lower ROS levels. Glutathione (GSH), a tripeptide of glutamate, glycine, and cysteine, is an abundant endogenous antioxidant molecule that can promote cancer cell survival and redox homeostasis (Fig. 2). Glutamine-derived glutamate and cysteine from the cysteine/glutamate transporter are required for de novo GSH synthesis through the activity of the glutamate-cysteine ligase modifier subunit (GCLM) and the glutamate-cysteine ligase catalytic subunit (GCLC) (Lee et al., 2006). Next, glycine is added during the second step of de novo GSH synthesis by glutathione synthetase (GSS) (Lushchak, 2012). GSH acts directly to eliminate hydrogen peroxide through the action of glutathione peroxidase (Lubos et al., 2011). GSH can be regenerated from its oxidized form (GSSG), along with the conversion of NADPH to NADP+ (Gorrini et al., 2013). The reducing agent NADPH is generated via several mechanisms, including the conversion of malate to pyruvate by malic enzyme as well as through the pentose phosphate pathway and serine/glycine metabolism (Boroughs and DeBerardinis, 2015). Among these, glutamine availability contributes to the production of NADPH by malic enzyme and also participates in the maintenance of redox homeostasis (Son et al., 2013). In addition, the IDH1-dependent reductive carboxylation of glutamine generates NADPH, which suppresses mitochondrial ROS in anchorage-independent growth conditions (Jiang et al., 2016). The cytosolic reductive carboxylation of glutamine, followed by the import of isocitrate/citrate into the mitochondria can suppress mitochondrial ROS via the generation of NADPH in mitochondria, consequently enabling cells to adapt in anchorage-independent conditions (Jiang et al., 2016).
Fig. 2.
Glutamine regulates reactive oxidative stress. Glutamine contributes to the generation of GSH, a tripeptide of glutamate, glycine, and cysteine. Glutamate reacts with cysteine to produce GSH via GLCL/GCLC. Glycine is added during the second step of de novo GSH synthesis via GSS. GSH directly eliminates ROS through the action of GPX. NADPH is required for the regeneration of the reduced form of GSH by GSR. GSH: reduced glutathione, GLCL: glutamate-cysteine ligase catalytic subunit, GCLM: glutamate-cysteine ligase modifier subunit, GSS: glutathione synthetase, GPX: glutathione peroxidase, GSR: glutathione reductase, GSSG: oxidized glutathione.
ROLE OF GLUTAMINE ON MODULATION OF SIGNAL TRANSDUCTION
mTORC1 activation
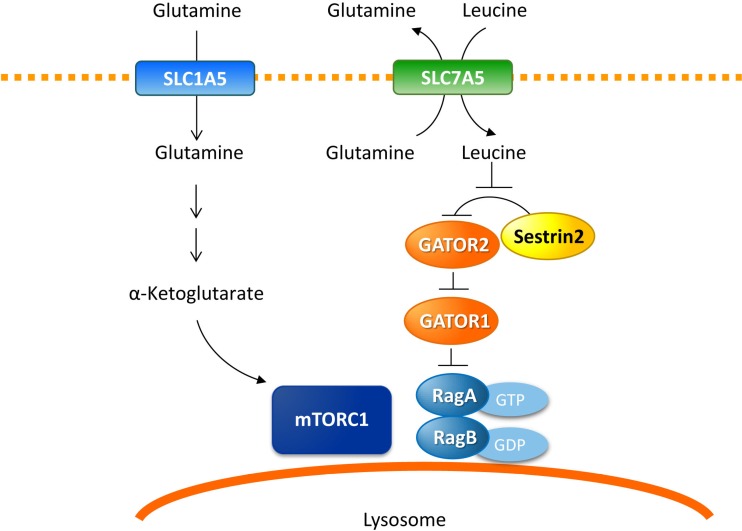
Glutamine coordinates intracellular signaling to promote cancer growth in addition to acting as an important substrate for carbon and nitrogen production. Glutamine regulates mechanistic target of rapamycin complex (mTORC1) activity through several mechanisms (Fig. 3). mTORC1 regulates cell growth and proliferation, which are tightly controlled by multiple signals, including growth factor stimulation, amino acid withdrawal, and hypoxia (Bhaskar and Hay, 2007). Most tumor cells exhibit upregulated mTORC1 activity, which favors tumorigenesis by driving the translation of oncogenic factors, inhibiting autophagy, and enhancing lipid biosynthesis (Zoncu et al., 2011). Glutamine activates mTORC1 through the simultaneous efflux of essential amino acids, including leucine, into cells via the bidirectional transporter, SLC7A5/SLC3A2 (van Geldermalsen et al., 2016). Imported leucine binds to Sestrin2 and disrupts the Sestrin2-GATOR2 interaction, an inhibitor of mTORC1, leading to the translocation of mTORC1 to lysosomes where Rheb-GTPase enhances mTORC1 activity (Saxton et al., 2016; Wolfson et al., 2016). Glutamine catabolism results in the production of α-KG and stimulates the lysosomal localization of mTORC1 (Sancak et al., 2008; Duran et al., 2012). Inhibition of glutaminolysis prevents RagB activation and lysosomal translocation of mTORC1, whereas a cell-permeable α-KG analog stimulates the activation of mTORC1, indicating that mTORC1 activation occurs downstream of glutaminolysis. In our recent study, glutamine deprivation increased Sestrin2 expression in lung and liver cancer cell lines and created a positive feedback loop between Sestrin2 and mTORC2, which is required for the suppression of mTORC1 activity under glutamine-deprived conditions (Byun et al., 2017). Thus, by regulating mTORC1, glutamine metabolism participates in modulating multiple cellular signaling pathways.
Fig. 3.
Glutamine regulates mTORC1 activation. Glutamine activates mTORC1 through the simultaneous efflux of leucine into cells by the bidirectional transporter, SLC7A5. Imported leucine binds to Sestrin2 and disrupts the Sestrin2-GATOR2 interaction, resulting in the recruitment of mTORC1 to lysosomes. Glutamine-derived α-ketoglutarate can directly stimulate lysosomal localization and activation of mTORC1. mTORC1: mechanistic target of the rapamycin complex 1.
Glutamine and autophagy
Autophagy is the tightly regulated process by which cells sequester intracellular components in autophagosomes and deliver them to lysosomes, where they are broken down and recycled to provide new building blocks for cell growth in response to extra- or intracellular signals (He and Klionsky, 2009). The role of autophagy in cancer development and progression is context-dependent: while autophagy can suppress the initiation of tumors by removing aberrant proteins and damaged organelles, it also can promote cancer growth by providing substrates for cellular growth and survival in established cancers (White, 2012). Glutamine stimulates mTORC1 activity and in turn, impairs autophagy initiation through the negative regulation of ULK1 by several mechanisms (Hosokawa et al., 2009; Jung et al., 2009; Nazio et al., 2013). In addition, since ROS is an inducer of autophagy, glutamine may also repress autophagy through the elimination of ROS by glutathione and NADPH (Dewaele et al., 2010). By contrast, ammonia, generated in catalytic reactions by GLS1 and GLUD, also can act as a signaling molecule, supporting basal autophagy activity in both transformed and non-transformed human cells (Eng et al., 2010; Cheong et al., 2012). As autophagy provides cellular substrates required for tumor growth, the production of ammonia can reduce cellular stress, and consequently protect cancer cells from death.
ONCOGENE AND TUMOR SUPPRESSORS
KRAS
The oncogenic KRAS mutation is one of the most frequent mutations found in numerous tumor types (Fernandez-Medarde and Santos, 2011). Accumulating evidence supports a molecular link between oncogenic signals, such as KRAS, and glutamine metabolism. Transformed cells harboring high levels of KRAS can orchestrate pleiotropic metabolic reprogramming, including increased glycolytic flux, utilization of glutamine, autophagy, and macropinocytosis (Bryant et al., 2014). Flux experiments showed that glutamine supports tumor growth by supplying the increased levels of carbon and nitrogen required for biomass synthesis in KRAS-driven cancer cells (Gaglio et al., 2011). More recently, Son et al. (2013) demonstrated that oncogenic KRAS altered glutamine metabolism to make it dependent on transaminases, which in turn, supports redox balance due to the conversion of cytosolic aspartate into oxaloacetate, malate, and then pyruvate, simultaneously generating NADPH. Moreover, inhibition of glutamine metabolism increases the sensitivity of pancreatic ductal adenocarcinoma (PDAC) cells to radiotherapy by increasing oxidative stress (Li et al., 2015). Thus, cells harboring the oncogenic KRAS are dependent on glutamine for growth and survival, as glutamine provides carbon for biosynthetic pathways and supports the maintenance of redox homeostasis. These findings suggest that targeting KRAS-regulated glutamine metabolism may potentiate the effects of ROS-generating treatments, such as chemotherapy and radiation in PDAC patients.
MYC
Similar to KRAS, MYC-overexpressing cancer cells are often addicted to glutamine, such that glutamine deprivation results in MYC-dependent apoptosis (Yuneva et al., 2007). MYC coordinates the gene expression that regulates glutamine metabolism at the transcriptional and post transcriptional levels (Gao et al., 2009). Interestingly, Gao et al. (2009) reported that GLS mRNA levels do not correlate with changes in MYC levels in the human B cell-derived P493-6 cell line or in prostate cancer cells, suggesting that MYC regulates GLS levels post-transcriptionally. In agreement with this notion, MYC transcriptionally represses miR-23a/b, leading to higher expression of mitochondrial glutaminase (Gao et al., 2009). In addition, MYC appears to selectively bind to the promoter regions of glutamine transporters ASCT2 [SLC1A5] and SN2 [SNAT5], and indirectly induces GLUD (Wise et al., 2008; Yuneva et al., 2012). Le et al. (2012) also found that MYC-inducible human Burkitt lymphoma cells have the ability to survive and even proliferate under hypoxic and glucose-deficient conditions by utilizing the glutamine-driven TCA cycle as an alternative source for energy generation. More recently, MYC-induced reprogramming of glutamine was verified in studies on latent infection of Kaposi’s sarcoma-associated herpesvirus and optimal progeny virion generation (Sanchez et al., 2015; Thai et al., 2015).
p53
p53 is a well-known tumor suppressor that participates in many cellular functions including cell cycle arrest, apoptosis, senescence, and differentiation (Daye and Wellen, 2012). One of the metabolic tumor suppressor functions of p53 is the direct induction of GLS2 expression, which displays an opposing function to GLS1 in tumorigenesis (Hu et al., 2010; Suzuki et al., 2010). Accumulating evidence suggests that GLS1 and GLS2 have opposite functions in tumorigenesis, although both are involved in the same pathway of catalyzing the conversion of glutamine to glutamate. GLS2 levels in some types of cancers are lower than those in distant and adjacent non-tumor tissues and GLS2 overexpression in cancer cells induces G2/M phase cell cycle arrest (Zhang et al., 2013). A previous report has demonstrated that GLS2 is downregulated in glioblastoma cells through DNA hypermethylation, which occurs independently of p53 (Szeliga et al., 2016). It is possible that the transcription of GLS2 may be controlled by other members of the p53 family, such as p63 and p73 (Giacobbe et al., 2013; Velletri et al., 2013). More recently, Kuo et al. (2016) demonstrated that GLS2 inversely correlated with advanced-stage, vascular invasion, early recurrence and poor prognosis in HCC patients. One possible mechanism that could explain the different roles of GLS2 and GLS1 in tumorigenesis is the nonenzymatic action of GLS2. Several studies have demonstrated that GLS2 suppresses HCC metastasis through the inhibition of snail expression or small GTPase Rac activity, neither of which are related to the glutaminolysis function of GLS2 (Kuo et al., 2016; Zhang et al., 2016). Given that GLS2 is associated with increased glutathione levels, similarly to GLS1, it is likely that it can also play a role in regulating the oxidative stress-resistant properties of cancer cells. Indeed, some studies have reported that tumor tissues from radio-resistant patients exhibit significantly higher GLS2 levels than those from radio-sensitive patients and that apoptosis in response to radiation is increased in GLS2-knockdown cancer cells (Xiang et al., 2013). GLS1 and GLS2 have different structural and kinetic properties and are subject to different regulatory mechanisms (Curthoys and Watford, 1995). Therefore, further study is required to explore the context-dependent divergent effects of GLS2 on tumorigenesis and its regulatory mechanisms in response to external stimuli.
CLINICAL OPPORTUNITIES
Pharmacological strategies to inhibit glutamine metabolism in cancer cells
As previously reviewed, glutamine metabolism exhibits pleiotropic effects on cancer cell signaling and proliferation and therapeutic suppression of glutamine metabolism is considered to be an attractive anticancer strategy (Table 1). Benzylserine and L-γ-glutamyl-p-nitroanilide (GPNA), the inhibitor of the glutamine transporter SLC1A5, have been shown to be effective agents in the treatment of glutamine-dependent cancers (Hassanein et al., 2015). However, unless targeted to a precise pathway in tumor cells, these drugs induce toxicity in healthy cells that require glutamine for other pathways. Small molecule inhibitors, such as bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES), CB-839, and compound 968, represent a new class of metabolism-targeted drugs that inhibit GLS isoforms not commonly expressed in normal cells (Chen and Cui, 2015; Xiang et al., 2015). BPTES, a specific GLS1 inhibitor, suppresses tumor growth in vitro and in vivo in various cancer cell types. However, BPTES is not a good candidate for GLS inhibition because of its poor solubility and bioavailability (Chen and Cui, 2015). CB839, currently undergoing a phase one clinical trial, is a selective and more potent GLS1 inhibitor than BPTES. CB839 exhibits a significant antitumor effect in triple-negative breast cancer cells and in leukemia cells that require glutamine for their growth (Gross et al., 2014; Jacque et al., 2015). In contrast to BPTES and CD-839, 968 is a specific inhibitor of GAC, a shorter isoform of the kidney-type glutaminase (Erickson and Cerione, 2010). The effects of 968 were also demonstrated in a variety of cancers including brain, pancreatic, and breast cancer cells that are highly resistant to conventional chemotherapy (Katt et al., 2015). Although newly discovered GLS inhibitors such as CB839 and 968 have a higher efficacy and a lower toxicity, the potential side effects of inhibiting glutamine metabolism should be considered (Masson et al., 2006; Bunpo et al., 2008). Epigallocatechin gallate (EGCG) and R162, an inhibitor of GLUD, as well as aminooxyacetic acid (AOA), a transaminase inhibitor, attenuated tumor growth in preclinical studies by disturbing the anaplerotic use of glutamine in the TCA cycle (Korangath et al., 2015).
Table 1.
Pharmacological strategies to inhibit glutamine metabolism in cancer cells
| Class | Drug | Status | Ongoing clinical trials | |
|---|---|---|---|---|
|
| ||||
| Cancer type | NCT number | |||
| SLC1A5 inhibitor | GPNA | Preclinical tool | - | - |
| γ-FBP | ||||
| Benzylserine | ||||
| GLS inhibitors | BPTES | Preclinical tool | - | - |
| CB-839 | Phase I clinical | Hematologic tumors | NCT02071888 | |
| Solid tumors (TNBC, NSCLC, RCC, Mesothelioma…) |
NCT02071862 NCT02771626 |
|||
| 968 | Preclinical tool | |||
| GLUD inhibitor | EGCG | Preclinical study | Colorectal Cancer (not yet open for participants recruitment) | NCT02891538 |
| R162 | Preclinical tool | - | - | |
| Aminotransferase inhibitors | AOA | Clinically used to treat tinnitus | - | - |
GPNA: Benzylserine and L-γ-gluatamyl-p-nitroanilide, γ-FBP: γ-folate binding protein, GLS: glutaminase, BPTES: bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide, TNBC: Triple-negative Breast Cancer, NSCLC: Non Small Cell Lung Cancer, RCC: Renal Cell Carcinoma, GLUD: glutamate dehydrogenase, EGCG: pigallocatechin gallate, AOA: aminooxyacetate.
Key strategies for circumventing therapeutic resistance in cancer
Cancer cells gain their stemness and chemoresistant properties through the upregulation of compensatory pathways when conventional therapy induces metabolic stress (Kim, 2015). As glutamine metabolism contributes to cancer cell proliferation and to the development of adaptation to metabolic stress, the inhibition of glutamine metabolism may be a promising adjuvant strategy to suppress the development of resistance to conventional cancer treatments (Hernandez-Davies et al., 2015; Baenke et al., 2016). For example, glioblastoma treatment resistance to mTOR inhibitors is likely the result of a compensatory glutamine metabolism mechanism, suggesting that a combined inhibition of GLS1 and mTOR could potentially overcome this type, of resistance (Tanaka et al., 2015). In addition, our recent study demonstrated increased glutamine metabolism and reduced glutamine carboxylation in sorafenib-resistant liver cancer cells, where metabolic reprogramming overcame sorafenib resistance (Kim et al., 2017). Given that highly invasive and metastatic cancer cells are more dependent on glutamine compared to less invasive cells (Yang et al., 2014), combination therapy involving the inhibition of glutamine metabolism could constitute a novel approach to preventing metastasis. Indeed, dual inhibition with BPTES and 5-fluorouracil elicits cell death synergistically through cell cycle arrest, which results in remarkable antitumor effect in a preclinical xenograft model of NSCLC (Lee et al., 2016). Collectively, targeting compensatory glutamine metabolism pathways may be a key strategy for circumventing therapeutic resistance in various cancers.
Challenges for clinical use
Despite the significant advances in the understanding of glutamine metabolism in cancer cells, there are still obstacles to overcome in the clinical application of inhibitors of glutamine metabolism pathways. Notably, in the Kras-driven lung cancer mouse model, glutamine was not the preferred carbon source for the TCA cycle in a study that utilized isotope-labeled glucose (Davidson et al., 2016). This in vivo result is in contrast with in vitro observations. Moreover, the study using human glioblastoma orthotopic tumors which have metabolic similarities to primary human GBMs showed the accumulated glutamine in tumor tissues is synthesized by de novo from glucose-derived glutamate and minimal glutaminolysis (Marin-Valencia et al., 2012). Moreover, high expression of glutamine synthase in cancer cells can promote glutamine-independent growth and resistance to therapies that restrict glutamine metabolism (Hernandez-Davies et al., 2015; Baenke et al., 2016). As tumors contain numerous cell types that work together to support tumor growth, the metabolic crosstalk between cancer cells and neighboring cells is crucial for the understanding of tumorigenesis. A recent study demonstrated that cancer–associated fibroblasts upregulated the glutamine anabolic pathway to support cancer cell growth. Thus, disrupting metabolic crosstalk between cancer cells and stromal cells by co-targeting stromal glutamine synthetase and cancer cell glutaminase could represent a promising approach to counteract tumor growth (Yang et al., 2016). Further investigations are needed to understand how glutamine bioavailability is regulated in the tumor microenvironment and to guide the selection of successful metabolic therapies in the clinic.
CONCLUSION AND FUTURE PERSPECTIVES
During rapid proliferation, cancer cells must optimize metabolic adaptability by balancing nutrient utilization for the synthesis of building blocks, generation of ATP, and maintenance of redox homeostasis. Glutamine metabolism acts as a central player in the regulation of uncontrolled tumor growth by modulating bioenergetic and redox homeostasis and serving as a precursor for biomass synthesis. Intrinsic oncogenic alterations as well as the surrounding tumor microenvironment regulate metabolic reprogramming, resulting in cancer cells that are “addicted” to glutamine metabolism. Although targeting glutamine metabolism pathways represents a promising strategy for the clinical design of therapeutic agents, developing an effective drug has been challenging. Nevertheless, a comprehensive understanding of glutamine metabolism is of the utmost importance, because it provides valuable insights into the pathways that could be targeted for the development of novel therapeutic strategies for the treatment of advanced or drug resistant cancers.
Acknowledgments
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2015).
REFERENCES
- Alberghina L, Gaglio D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014;5:e1561. doi: 10.1038/cddis.2014.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:773. doi: 10.1038/nrc.2016.131. [DOI] [PubMed] [Google Scholar]
- Baenke F, Chaneton B, Smith M, Van Den Broek N, Hogan K, Tang H, Viros A, Martin M, Galbraith L, Girotti MR, Dhomen N, Gottlieb E, Marais R. Resistance to BRAF inhibitors induces glutamine dependency in melanoma cells. Mol Oncol. 2016;10:73–84. doi: 10.1016/j.molonc.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino Acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
- Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39:91–100. doi: 10.1016/j.tibs.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunpo P, Murray B, Cundiff J, Brizius E, Aldrich CJ, Anthony TG. Alanyl-glutamine consumption modifies the suppressive effect of L-asparaginase on lymphocyte populations in mice. J Nutr. 2008;138:338–343. doi: 10.1093/jn/138.2.338. [DOI] [PubMed] [Google Scholar]
- Byun JK, Choi YK, Kim JH, Jeong JY, Jeon HJ, Kim MK, Hwang I, Lee SY, Lee YM, Lee IK, Park KG. A positive feedback loop between sestrin2 and mTORC2 is required for the survival of glutamine-depleted lung cancer cells. Cell Rep. 2017;20:586–599. doi: 10.1016/j.celrep.2017.06.066. [DOI] [PubMed] [Google Scholar]
- Chen L, Cui H. Targeting glutamine induces apoptosis: a cancer therapy approach. Int J Mol Sci. 2015;16:22830–22855. doi: 10.3390/ijms160922830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H, Lindsten T, Thompson CB. Autophagy and ammonia. Autophagy. 2012;8:122–123. doi: 10.4161/auto.8.1.18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, Bauer MR, Jha AK, O’Brien JP, Pierce KA, Gui DY, Sullivan LB, Wasylenko TM, Subbaraj L, Chin CR, Stephanopolous G, Mott BT, Jacks T, Clish CB, Vander Heiden MG. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 2016;23:517–528. doi: 10.1016/j.cmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol. 2012;23:362–369. doi: 10.1016/j.semcdb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6:838–854. doi: 10.4161/auto.6.7.12113. [DOI] [PubMed] [Google Scholar]
- Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates RagmTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. doi: 10.1126/scisignal.2000911. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA. Glutaminase: a hot spot for regulation of cancer cell metabolism? Oncotarget. 2010;1:734–740. doi: 10.18632/oncotarget.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C, Alberghina L, Stephanopoulos G, Chiaradonna F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011;7:523. doi: 10.1038/msb.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio D, Soldati C, Vanoni M, Alberghina L, Chiaradonna F. Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS ONE. 2009;4:e4715. doi: 10.1371/journal.pone.0004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe A, Bongiorno-Borbone L, Bernassola F, Terrinoni A, Markert EK, Levine AJ, Feng Z, Agostini M, Zolla L, Agro AF, Notterman DA, Melino G, Peschiaroli A. p63 regulates glutaminase 2 expression. Cell Cycle. 2013;12:1395–1405. doi: 10.4161/cc.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J, Mackinnon AL, Parlati F, Rodriguez ML, Shwonek PJ, Sjogren EB, Stanton TF, Wang T, Yang J, Zhao F, Bennett MK. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hassanein M, Qian J, Hoeksema MD, Wang J, Jacobovitz M, Ji X, Harris FT, Harris BK, Boyd KL, Chen H, Eisenberg R, Massion PP. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int J Cancer. 2015;137:1587–1597. doi: 10.1002/ijc.29535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Davies JE, Tran TQ, Reid MA, Rosales KR, Lowman XH, Pan M, Moriceau G, Yang Y, Wu J, Lo RS, Kong M. Vemurafenib resistance reprograms melanoma cells towards glutamine dependence. J Transl Med. 2015;13:210. doi: 10.1186/s12967-015-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–979. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]
- Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA. 2010;107:7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacque N, Ronchetti AM, Larrue C, Meunier G, Birsen R, Willems L, Saland E, Decroocq J, Maciel TT, Lambert M, Poulain L, Hospital MA, Sujobert P, Joseph L, Chapuis N, Lacombe C, Moura IC, Demo S, Sarry JE, Recher C, Mayeux P, Tamburini J, Bouscary D. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood. 2015;126:1346–1356. doi: 10.1182/blood-2015-01-621870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Shestov AA, Swain P, Yang C, Parker SJ, Wang QA, Terada LS, Adams ND, McCabe MT, Pietrak B, Schmidt S, Metallo CM, Dranka BP, Schwartz B, DeBerardinis RJ. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016;532:255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katt WP, Antonyak MA, Cerione RA. Simultaneously targeting tissue transglutaminase and kidney type glutaminase sensitizes cancer cells to acid toxicity and offers new opportunities for therapeutic intervention. Mol Pharm. 2015;12:46–55. doi: 10.1021/mp500405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Choi YK, Park SY, Jang SY, Lee JY, Ham HJ, Kim BG, Jeon HJ, Kim JH, Kim JG, Lee IK, Park KG. PPARδ reprograms glutamine metabolism in sorafenib-resistant HCC. Mol Cancer Res. 2017;15:1230–1242. doi: 10.1158/1541-7786.MCR-17-0061. [DOI] [PubMed] [Google Scholar]
- Kim SY. Cancer metabolism: targeting cancer universality. Arch Pharm Res. 2015;38:299–301. doi: 10.1007/s12272-015-0551-5. [DOI] [PubMed] [Google Scholar]
- Korangath P, Teo WW, Sadik H, Han L, Mori N, Huijts CM, Wildes F, Bharti S, Zhang Z, Santa-Maria CA, Tsai H, Dang CV, Stearns V, Bhujwalla ZM, Sukumar S. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clin Cancer Res. 2015;21:3263–3273. doi: 10.1158/1078-0432.CCR-14-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TC, Chen CK, Hua KT, Yu P, Lee WJ, Chen MW, Jeng YM, Chien MH, Kuo KT, Hsiao M, Kuo ML. Glutaminase 2 stabilizes Dicer to repress Snail and metastasis in hepatocellular carcinoma cells. Cancer Lett. 2016;383:282–294. doi: 10.1016/j.canlet.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, Zimmerman LJ, Liebler DC, Slebos RJ, Lorkiewicz PK, Higashi RM, Fan TW, Dang CV. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Kang J, Stipanuk MH. Differential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation. Biochem J. 2006;393:181–190. doi: 10.1042/BJ20051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kang JH, Lee SH, Hong D, Son J, Hong KM, Song J, Kim SY. Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC. Cell Death Dis. 2016;7:e2511. doi: 10.1038/cddis.2016.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Fu Z, Chen R, Zhao X, Zhou Y, Zeng B, Yu M, Zhou Q, Lin Q, Gao W, Ye H, Zhou J, Li Z, Liu Y, Chen R. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget. 2015;6:31151–31163. doi: 10.18632/oncotarget.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Mates JM, Pascual JM, Maher EA, Malloy CR, Deberardinis RJ, Bachoo RM. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez J, Alonso FJ, Mates JM, Segura JA, Martin-Rufian M, Campos-Sandoval JA. Glutamine addiction in gliomas. Neurochem Res. 2017;42:1735–1746. doi: 10.1007/s11064-017-2212-1. [DOI] [PubMed] [Google Scholar]
- Masson J, Darmon M, Conjard A, Chuhma N, Ropert N, Thoby-Brisson M, Foutz AS, Parrot S, Miller GM, Jorisch R, Polan J, Hamon M, Hen R, Rayport S. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26:4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates JM, Segura JA, Martin-Rufian M, Campos-Sandoval JA, Alonso FJ, Marquez J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr Mol Med. 2013;13:514–534. doi: 10.2174/1566524011313040005. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A, Deng X, Khuri FR, Owonikoko TK. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer. 2014;15:7–15. doi: 10.1016/j.cllc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, Cecconi F. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, Hogarty MD, Simon MC. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez EL, Carroll PA, Thalhofer AB, Lagunoff M. Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival. PLoS Pathog. 2015;11:e1005052. doi: 10.1371/journal.ppat.1005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016;351:53–58. doi: 10.1126/science.aad2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, Kimmelman AC. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, Sugano S, Sato E, Nagao T, Yokote K, Tatsuno I, Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeliga M, Bogacinska-Karas M, Kuzmicz K, Rola R, Albrecht J. Downregulation of GLS2 in glioblastoma cells is related to DNA hypermethylation but not to the p53 status. Mol Carcinog. 2016;55:1309–1316. doi: 10.1002/mc.22372. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Sasayama T, Irino Y, Takata K, Nagashima H, Satoh N, Kyotani K, Mizowaki T, Imahori T, Ejima Y, Masui K, Gini B, Yang H, Hosoda K, Sasaki R, Mischel PS, Kohmura E. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. J Clin Invest. 2015;125:1591–1602. doi: 10.1172/JCI78239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai M, Thaker SK, Feng J, Du Y, Hu H, Ting Wu T, Graeber TG, Braas D, Christofk HR. MYC-induced reprogramming of glutamine catabolism supports optimal virus replication. Nat Commun. 2015;6:8873. doi: 10.1038/ncomms9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N, Harvey K, Beith JM, Selinger CI, O’Toole SA, Rasko JE, Holst J. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201–3208. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velletri T, Romeo F, Tucci P, Peschiaroli A, Annicchiarico-Petruzzelli M, Niklison-Chirou MV, Amelio I, Knight RA, Mak TW, Melino G, Agostini M. GLS2 is transcriptionally regulated by p73 and contributes to neuronal differentiation. Cell Cycle. 2013;12:3564–3573. doi: 10.4161/cc.26771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974;249:5070–5079. [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Xie G, Liu C, Zhou J, Chen J, Yu S, Li J, Pang X, Shi H, Liang H. Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim Biophys Acta. 2013;1833:2996–3005. doi: 10.1016/j.bbamcr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, Hsieh AL, Gouw AM, Thomas AG, Gao P, Sun L, Song L, Yan B, Slusher BS, Zhuo J, Ooi LL, Lee CG, Mancuso A, McCallion AS, Le A, Milone MC, Rayport S, Felsher DW, Dang CV. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015;125:2293–2306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, Baddour J, Marini JC, Ni J, Nakahara R, Wahlig S, Chiba L, Kim SH, Morse J, Pradeep S, Nagaraja AS, Haemmerle M, Kyunghee N, Derichsweiler M, Plackemeier T, Mercado-Uribe I, Lopez-Berestein G, Moss T, Ram PT, Liu J, Lu X, Mok SC, Sood AK, Nagrath D. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 2016;24:685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Moss T, Mangala LS, Marini J, Zhao H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, Roopaimoole R, Rodriguez-Aguayo C, Mercado-Uribe I, Lopez-Berestein G, Liu J, Tsukamoto T, Sood AK, Ram PT, Nagrath D. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol. 2014;10:728. doi: 10.1002/msb.20134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Venneti S, Nagrath D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu Rev Biomed Eng. 2017;19:163–194. doi: 10.1146/annurev-bioeng-071516-044546. [DOI] [PubMed] [Google Scholar]
- Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Shi X, Meng G, Chen J, Yan C, Jiang Y, Wei J, Ding Y. Kidney-type glutaminase (GLS1) is a biomarker for pathologic diagnosis and prognosis of hepatocellular carcinoma. Oncotarget. 2015;6:7619–7631. doi: 10.18632/oncotarget.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Mates JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu J, Zhao Y, Yue X, Zhu Y, Wang X, Wu H, Blanco F, Li S, Bhanot G, Haffty BG, Hu W, Feng Z. Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis. Elife. 2016;5:e10727. doi: 10.7554/eLife.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang C, Chen M, Cao J, Zhong Y, Chen L, Shen HM, Xia D. Epigenetic silencing of glutaminase 2 in human liver and colon cancers. BMC Cancer. 2013;13:601. doi: 10.1186/1471-2407-13-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]