Abstract
In 1923, Dr. Warburg had observed that tumors acidified the Ringer solution when 13 mM glucose was added, which was identified as being due to lactate. When glucose is the only source of nutrient, it can serve for both biosynthesis and energy production. However, a series of studies revealed that the cancer cell consumes glucose for biosynthesis through fermentation, not for energy supply, under physiological conditions. Recently, a new observation was made that there is a metabolic symbiosis in which glycolytic and oxidative tumor cells mutually regulate their energy metabolism. Hypoxic cancer cells use glucose for glycolytic metabolism and release lactate which is used by oxygenated cancer cells. This study challenged the Warburg effect, because Warburg claimed that fermentation by irreversible damaging of mitochondria is a fundamental cause of cancer. However, recent studies revealed that mitochondria in cancer cell show active function of oxidative phosphorylation although TCA cycle is stalled. It was also shown that blocking cytosolic NADH production by aldehyde dehydrogenase inhibition, combined with oxidative phosphorylation inhibition, resulted in up to 80% decrease of ATP production, which resulted in a significant regression of tumor growth in the NSCLC model. This suggests a new theory that NADH production in the cytosol plays a key role of ATP production through the mitochondrial electron transport chain in cancer cells, while NADH production is mostly occupied inside mitochondria in normal cells.
Keywords: Cancer energy metabolism, Warburg effect, TCA cycle, Malate-aspartate shuttle, Electron transport chain, Oxidative phosphorylation
INTRODUCTION
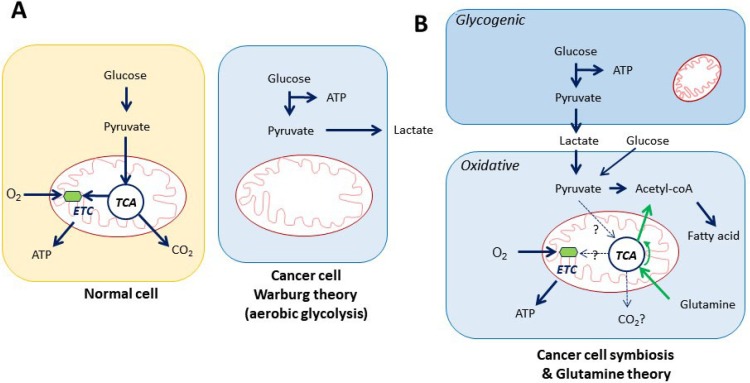
Biomolecules cannot be produced without an energy supply. Growth signaling, driver gene activation, and mTOR activation requires ATP for phosphorylation, and translation machineries including DNA/RNA synthesis enzymes also requires ATP. Therefore, cancer cells need to have huge supply of ATP. What is the major ATP source that cancer cells require for biomolecule synthesis? We have at least three theories of cancer energy metabolism. First, the Classical Warburg effect explains that cancer cells ferments glucose as an ATP source via glycolysis (Fig. 1A). Warburg thought that glucose is the source of cancer growth through fermentation, instead of oxidative respiration (Warburg, 1956a). Until now, it was widely accepted that glucose is the main source of cancer ATP. However, this only happens when glucose is the only nutrient supply under hypoxia. The second theory of cancer metabolism posits that cancer cells elaborate survival through symbiosis: one cancer cell produces lactate with ATP production by consuming glucose (Warburg effect), and the neighbor cancer cell consumes the secreted lactate to produce ATP through the TCA cycle and oxidative phosphorylation (Fig. 1B) (Sonveaux et al., 2008; Faubert et al., 2017). Some cancer cells use lactate as a substrate for TCA intermediates through monocarboxylate transporters (MCT1/4) and also for ATP production (Faubert et al., 2017). Lactate can be converted to pyruvate by LDH (lactate dehydrogenase) and further to acetyl-CoA through ATP-citrate lyase for fatty acid synthesis (Zaidi et al., 2012). Therefore, the symbiosis theory may contribute more importantly to biosynthesis instead of ATP production (Fig. 1B). The third theory is that glutamine is converted to glutamate by glutamine synthetase 1, which is further metabolized to α-ketoglutarate by glutamate dehydrogenase and consumed for ATP synthesis through TCA cycle under oxidative condition (Fig. 1B) (Reitzer et al., 1979; McKeehan, 1982). In the glutaminolysis, flux analysis revealed that glutamine was significantly used to replenish TCA cycle intermediates for anaplerosis and fatty acid synthesis which are considered as biosynthetic precursors (DeBerardinis et al., 2007, 2008) (Fig. 1B). Interestingly the latter two hypotheses of cancer energy metabolism are opposite to the Warburg effect that cancer cell consuming oxygen for operation of oxidative phosphorylation and TCA cycle as normal cell does. This implies that glutamine and lactate can be a part of ATP supply. However, the glucose dependent TCA cycle is not likely an operative mechanism, because cancer cells under hypoxia induce pyruvate dehydrogenase kinase (PDK) that inactivates pyruvate dehydrogenase, which causes depletion of TCA cycle intermediates (Kim et al., 2006). The major ATP source in cancer cell remains in question.
Fig. 1.
Cancer energy metabolism theories. (A) Classical Warburg effect. Cancer cell adopts oxygenated glycolysis. (B) Cancer cell symbiosis. The fate of lactate remains to be clarified in the energy metabolism. TCA: tri-carboxylic acid cycle (Kreb’s cycle), ETC: electron transport chain.
MISUNDERSTANDING THE CANCER ENERGY METABOLISM
All cancer cells produce lactate from glucose regardless of thousands of mutation combinations. We need to look carefully this finding that has been discovered by Dr. Warburg. The classical Warburg effect implies that there must be common metabolic pathways for all type of cancer cells to survive. It is a good news for us to find cancer-specific vulnerabilities. It is often explained that cancer glycolysis is responsible for ATP production and biosynthesis (Warburg, 1956a, 1956b). However, although glucose may be used for both biosynthesis and ATP production, cancer cell cannot survive only with glycolytic production of ATP. By simple calculation, glycolysis does not add up sufficient energy supply in cancer cells. It generates 2 molar ATPs, 2 molar NADH, and 2 molar pyruvates from 1 molar glucose. However, only 1 molar ATP is left after consumption of 2 molar NADH for 2 molar lactate production and 1 molar ATP for pentose pathway. It is revealed that glucose greatly contributes towards building biomolecules in cancer anabolic metabolism instead of ATP production (DeBerardinis et al., 2008). Cancer cells depends on glycolysis for biosynthesis in cancer. In glycolysis, glucose-6-phosphate goes into pentose phosphate pathway (Patra and Hay, 2014) to produce RNA, DNA, ATP, NADH, FADH2, and coenzyme A using pentose ribose-5-phosphate as well as NADPH. 3-phosphoglycerate in glycolysis also goes to serine synthesis pathway (Yang and Vousden, 2016) that converts to glycine, which goes into one carbon pathway for producing nucleotides and proteins. Therefore, cancer glycolysis is designated for anabolic metabolism instead of energy production (DeBerardinis et al., 2008).
People often confuse the TCA cycle with oxidative phosphorylation because Dr. Warburg mentioned that mitochondria in the cancer cell is destroyed. However, mitochondrial membrane potential in cancer cells are more active compared to normal cells, although it does produce lactate avoiding TCA cycle. The TCA cycle is a charger of electron carrier NAD+ to NADH by converting glucose to CO2 by a series of enzymes in the normal mitochondria. Oxidative phosphorylation occurs at electron transport chain in the mitochondrial membrane using NADH from the TCA cycle in the normal cell. One key Achilles’ heel dilemma is, when we admit active oxidative phosphorylation in the cancer cell, we need to admit that cancer cells use oxygenated respiration.
Misunderstanding the mitochondrial function in the cancer cell has led to the wrong interpretation of cancer energy metabolism. First, the cancer cell environment provides hypoxic condition due to fast growth of tumors and failure of proper blood supply. Hypoxia stops the glucose driven TCA cycle by HIF (Hypoxia Inducible Factor) induction. HIF triggers induction of pyruvate PDK inactivating pyruvate dehydrogenase to rewire flow of pyruvate to LDH (Kim et al., 2006). Therefore, the hypoxia is a critical switch of cancer cell metabolism that metabolic flow adopts fermentation. Second, mitochondrial membrane potential activity is 50% active under 1% oxygen (Chandel et al., 1997), which implies that cancer cell mitochondrial membrane potential is functionally active. When mitochondrial membrane potential is active, oxidative phosphorylation requires oxygen to produce ATP. This suggests that Warburg’s mitochondrial malfunction theory in cancer cell is invalid. We also have observed increased mitochondrial membrane potential in cancer cells (Kang et al., 2016b). The electron transport chain (ETC) can produce sufficient ATP using NADH and FADH2 produced from malate dehydration or FAO (fatty acid oxidation) in mitochondria. FAO is carried out in energy-demanding heart and skeletal muscle tissues as well as in cancer cells (Carracedo et al., 2013). A report showed that fatty acid receptor (CD36) is important for tumor metastasis model (Pascual et al., 2017).
A NEW PROPOSAL FOR CANCER ENERGY METABOLISM
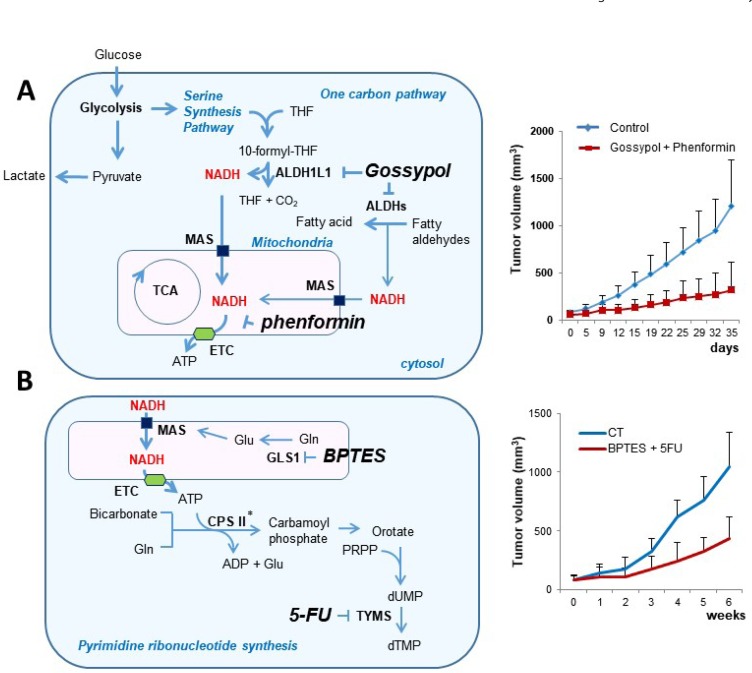
Recently, we have reported two independent studies about NSCLC metabolism, which describe the increase of cytosolic NADH production as an electron source of the cancer cell. The cytosolic NADH is transported into mitochondria through the MAS, which results in ATP production through the ETC. The first study was based upon omics data analysis using NSCLC related metabolic enzyme profiling, which suggests close association of ALDH in NSCLC (Kang et al., 2016a). By mechanism analysis of ALDH contribution, we found that ALDH contributes vast amount of ATP production following cytosolic NADH production. It has been demonstrated that MAS is required for transporting cytosolic NADH into mitochondria for oxidative phosphorylation (Fig. 2A) (Kang et al., 2016b). Therefore, ALDH inhibition using gossypol, combined with mitochondrial complex I inhibition using phenformin, resulted in up to 80% depletion of ATP production in cancer cells, accompanied by significant growth regression of NSCLC tumors in the animal xenograft model, while normal cells do not have a loss of ATP production (Kang et al., 2016b).
Fig. 2.
Two experiments related with cancer energy metabolism. (A) ALDH1L1 in one-carbon pathway produces abundant cytosolic NADH which generates ATP following to transport to mitochondria via MAS in NSCLC. By combined inhibition of ALDH and mitochondrial complex I, xenograft cancer model showed significant regression of tumor growth (Kang et al., 2016b). (B) GLS1 supplies abundant glutamate for MAS to produce ATP that is needed for pyrimidine synthesis in NSCLC. By combined inhibition of GLS1 and TYMS, xenograft cancer model showed significant regression of tumor growth (Lee et al., 2016a). THF: tetrahydrofolate, GLS1: glutaminase 1, TYMS: thymidylate synthetase, ALDH: aldehyde dehydrogenase, CPSII: carbamoylphosphate synthase II, *: ATP sensitive enzyme, MAS: malate aspartate shuttle, ETC: electron transfer complex, BPTES: bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide.
The second study was based on drug screening by random combination with conventional drugs and metabolic enzyme inhibitors. We found that GLS1 inhibition is very effective to reduce growth of NSCLC (Lee et al., 2016b). Furthermore, GLS1 inhibition with 5-FU resulted in almost total growth regression of NSCLC tumor growth in animal xenograft model (Fig. 2B) (Lee et al., 2016a). By mechanism analysis, we found that GLS1 contributes to the induction of MAS via glutamate supply. MAS activation provides ATP production, which induces carbamoyl phosphate synthetase II for pyrimidine synthesis. Therefore, inhibition of GLS1 using BPTES combined with 5-FU inhibiting thymidylate synthase causes depletion of ATP and decrease of pyrimidine synthesis, which resulted in significant tumor growth regression (Lee et al., 2016a).
This is a very provocative challenge in cancer metabolism, because Dr. Warburg proposed that cancer cells cannot use oxygen as much as normal cell due to mitochondrial malfunction, which leads to an increase of lactate production instead of CO2 production. His idea was established without the concepts of the TCA cycle and oxidative phosphorylation, because those were discovered later following his discovery. O2 consumes in oxidative phosphorylation via electron transfer chain and CO2 is produced from the TCA cycle by carbohydrate dehydration. Cancer cells do not use as much oxygen as normal cells to produce lactate when glucose is the only nutrient supply. However, under glucose-limited conditions, cancer cells may use fatty acids as an energy source through fatty acid oxidation (Carracedo et al., 2013). In that case, the cancer cells operate oxidative phosphorylation consuming oxygen. ATP production was depleted over 80% in NSCLC cells by ALDH inhibition combined with phenformin treatment, while the ATP level was not changed in normal cells (Kang et al., 2016b). By down regulation of ATP production through ALDH inhibition using gossypol together with mitochondrial complex I inhibition using phenformin, tumor growth was almost totally retarded in NSCLC xenograft model (Kang et al., 2016b). This is quite a convincing result that the regulation of cancer energy metabolism may have great benefits in killing cancer cells selectively without any harm to normal tissue.
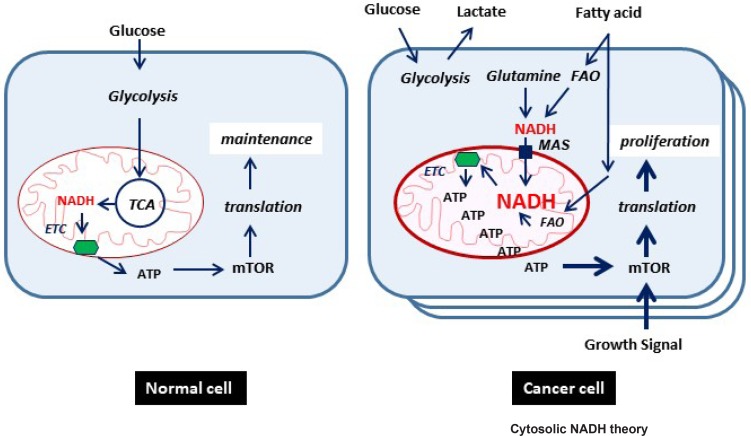
In our proposed model (Fig. 3), cytosolic NADH as an electron donor is the key player in ATP production in cancer cells. One of major cytosolic NADH production enzymes is ALDH that catalyzes aldehyde to carboxylic acid and NADH. Major substrates of ALDH is fatty aldehyde, acetaldehyde, retinaldehyde, and 10-formyl-tetrahydrofolate in one-carbon pathway. In the cancer condition, ROS radicals are abundant that are usually involved in the peroxidation process. Fatty acid turns into fatty aldehyde under the peroxidation condition, which can be further catalyzed to fatty acid with NADH production by ALDH. Although it remains to be elucidated that peroxidation of fatty acid is increased in cancer cells, knockdown of ALDH expression or treatment with ALDH inhibitor significantly decreased cytosolic NADH and mitochondrial ATP production in cancer cells (Kang et al., 2016b). Cytosolic NADH transports into mitochondria through MAS, which is constituted with malate-α-ketoglutarate antiporter, glutamate-aspartate antiporter, MDH1/2 and GOT1/2. Alternatively, it has been demonstrated that GOT or MDH knockdown or inhibition mimics decrease of NADH/ATP induced by ALDH inhibition, which can be rescued also by malate treatment. Glutamate as a core component of MAS is supplied from glutamine by GLS1. Therefore GLS1 knockdown significantly reduced NADH and ATP production in cancer cells (Lee et al., 2016a). Previously, it was also mentioned that tumor cells significantly depend on cytosolic NADH for ATP production through MAS (Greenhouse and Lehninger, 1977). Although an abundant growth signal drives mega-translation in cancer cells, it happens only when abundant ATP production is supplied to the cancer cells (Fig. 3).
Fig. 3.
A new proposed model of cancer energy metabolism. Major source of electron may be cytosolic NADH produced by metabolic enzymes such as ALDH. ETC: electron transport chain, FAO: fatty acid oxidation, MAS: malate-aspartate shuttle, TCA: tri-carboxylic acid cycle.
POSSIBLE THERAPEUTIC APPROACH BY REGULATION OF CANCER ENERGY METABOLISM
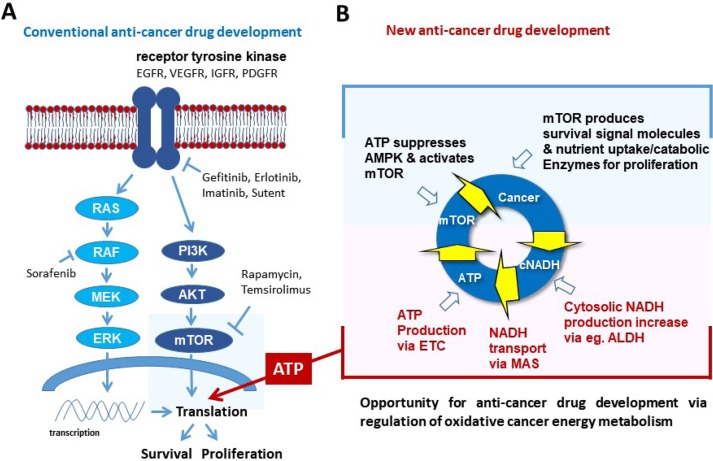
Fast growth of tumor needs efficient ATP supply as well as abundant biomolecules. All recent targeted drugs ultimately aim at cancer translation (anabolism) suppression through inhibiting signaling molecules (Fig. 4A). But blocking signal pathways always induce signal rewiring to promote translation. Although mTOR was a conceptually perfect target for blocking cancer translation, cancer cells evade blocking a biomolecule supply through recycling cell compartments via autophagy (Wu et al., 2013).
Fig. 4.
Anti-cancer therapeutic approach. (A) Targeting cancer translation by targeting signaling molecules such as receptor tyrosine kinases and mTOR. (B) Targeting cancer energy metabolism by regulating energy supplies such as cytosolic NADH producing enzymes, ETC and MAS. ETC: electron transport chain, MAS: malate-aspartate shuttle.
Continuous growth signaling cannot reach the next level when ATP is not properly supplied in cancer cells, because all kinases requires ATP as a substrate. Therefore, cancer growth should be turned down when the major ATP supplier is blocked. As an alternate approach, targeting ATP supply was attempted by blocking glycolysis and/or inhibiting mitochondrial complex I. Blocking glycolysis using hexokinase inhibitor 2-deoxyglucose, combined with blocking oxidative phosphorylation using mitochondrial complex I inhibitor metformin (Cheong et al., 2011), resulted in delayed tumor growth. However, hexokinase inhibitor such as 2-DG and 3-bromopyruvate have been discontinued in clinical trials. We need to find effective targets responsible for energy supply, as well as develop effective inhibitors against them.
Recently we have demonstrated that ALDH inhibition using gossypol combined with mitochondrial complex I inhibition using phenformin resulted in up to 80% ATP depletion in NSCLC, which induced significant tumor regression in the cancer xenograft model (Kang et al., 2016b). This warrants that a key molecule regulating cancer energy metabolism can be a therapeutic target. We found three major spots including mitochondrial complex I, malate-aspartate shuttle, and cytosolic NADH producing enzymes such as glutamate dehydrogenase, alcohol dehydrogenase, LDH and ALDH (Fig. 4B).
CONCLUSIONS
The three major cancer metabolic theories, the “Classic Warburg Effect”, the more recent “Cancer Cell Symbiosis” and “Glutaminolysis,” are based on biosynthesis as promotion of cancer cell proliferation. I proposes a new theory that NADH production in the cytosol using carbohydrate, fatty acid, glutamine and NADH transportation to mitochondria via MAS plays a key role of ATP production through the mitochondrial electron transport complex in the cancer cell (Fig. 3). Further studies of these hot-spots may lead us to answer the question of how we can cure cancer.
Acknowledgments
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korean government (MSIP) (No. 2017R1A2B2003428). I thank Kee-Hwan Kim for English editing.
REFERENCES
- Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Budinger GR, Choe SH, Schumacker PT. Cellular respiration during hypoxia. Role of cytochrome oxidase as the oxygen sensor in hepatocytes. J Biol Chem. 1997;272:18808–18816. doi: 10.1074/jbc.272.30.18808. [DOI] [PubMed] [Google Scholar]
- Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, Wa Cheng K, Hall H, Zhang D, Lu Y, Ravoori M, Kundra V, Ajani J, Lee JS, Ki Hong W, Mills GB. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011;10:2350–2362. doi: 10.1158/1535-7163.MCT-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, Li H, Huet G, Yuan Q, Wigal T, Butt Y, Ni M, Torrealba J, Oliver D, Lenkinski RE, Malloy CR, Wachsmann JW, Young JD, Kernstine K, DeBerardinis RJ. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371.e9. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse WV, Lehninger AL. Magnitude of malate-aspartate reduced nicotinamide adenine dinucleotide shuttle activity in intact respiring tumor cells. Cancer Res. 1977;37:4173–4181. [PubMed] [Google Scholar]
- Kang JH, Lee SH, Hong D, Lee JS, Ahn HS, Ahn JH, Seong TW, Lee CH, Jang H, Hong KM, Lee C, Lee JH, Kim SY. Aldehyde dehydrogenase is used by cancer cells for energy metabolism. Exp Mol Med. 2016a;48:e272. doi: 10.1038/emm.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Lee SH, Lee JS, Nam B, Seong TW, Son J, Jang H, Hong KM, Lee C, Kim SY. Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion. Oncotarget. 2016b;7:49397–49410. doi: 10.18632/oncotarget.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kang JH, Lee SH, Hong D, Son J, Hong KM, Song J, Kim SY. Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC. Cell Death Dis. 2016a;7:e2511. doi: 10.1038/cddis.2016.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kang JH, Lee SH, Lee CH, Son J, Kim SY. Glutaminase 1 inhibition reduces thymidine synthesis in NSCLC. Biochem Biophys Res Commun. 2016b;477:374–382. doi: 10.1016/j.bbrc.2016.06.095. [DOI] [PubMed] [Google Scholar]
- McKeehan WL. Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep. 1982;6:635–650. doi: 10.1016/0309-1651(82)90125-4. [DOI] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescos C, Di Croce L, Benitah SA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956a;124:269–270. [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956b;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wu L, Feng Z, Cui S, Hou K, Tang L, Zhou J, Cai G, Xie Y, Hong Q, Fu B, Chen X. Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway, resulting in reduced podocyte injury. PLoS ONE. 2013;8:e63799. doi: 10.1371/journal.pone.0063799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72:3709–3714. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]