Abstract
Objective
Compared to children born near term, those born extremely preterm are at much higher risk for Attention Deficit Hyperactivity Disorder (ADHD). Little information is available about differences in neuropsychological outcomes among extremely preterm children with and without ADHD. The primary aim of our analyses was to evaluate the neuropsychological correlates of ADHD symptoms in extremely low gestational age newborns (ELGANs).
Method
We obtained Child Symptom Inventory-4 (CSI-4) reports from parents (n = 871) and teachers (n = 634) of 10-year old children born before the 28th week of gestation. The children completed standardized assessments of neurocognitive and academic functioning.
Results
In the total sample, children who screened positive for ADHD symptoms were at increased risk for neurocognitive limitations compared to ELGANs who did not meet for ADHD classification. These associations were weaker when the sample was limited to those with IQ ≥ 70 or those with IQ ≥ 85. Even the children with IQ ≥ 70 and those with IQ ≥ 85 who screened positive for ADHD symptoms were more likely than their peers to have deficits on the DAS-II assessment of working memory and the NEPSY-II Auditory Response subtest. The risks for impaired academic performance (Z ≤ −1) on components of the WIAT-III (Word Reading, Pseudoword Decoding, and Spelling) were 2-to-3 times higher in this group than among ELGANs not classified as having ADHD symptoms.
Conclusion
Among children born extremely preterm, those with ADHD symptoms are more likely than others to have global neurocognitive impairment. Even when IQ is within normal limits, ADHD symptoms are associated with deficits in executive functioning skills including working memory, inhibition, and cognitive flexibility. These findings highlight a group at risk for executive functioning deficits and related academic difficulties, even in the absence of intellectual disability.
Keywords: Extreme Prematurity, Extremely Low Gestational Age Newborns, Attention Deficit Hyperactivity Disorder, ADHD, Neuropsychological outcomes
INTRODUCTION
Compared to children born at term, those born before the 28th week gestation are at greater risk for a range of developmental problems including neurosensory disorders, global intellectual disability, and difficulties with aspects of broader cognition, academic achievement, and behavior (2–4). Even when controlling for IQ or vocabulary, children born extremely prematurely exhibit deficits in several domains of neuropsychological functioning, including attention, executive function, visual-spatial and perceptual analysis abilities, visual-motor skills, and memory, as well as in academic achievement (6–9).
These high-risk children are more likely than others to have attention and social problems at early school age (ages 5 and 6 years) (10–13) and are more likely to be diagnosed with Attention Deficit Hyperactivity Disorder (ADHD) in early childhood (13–16). With some exceptions, (13,16) most studies indicate that the inattentive subtype of ADHD is more common than the hyperactive/impulsive subtype among children born very preterm (17–20). Despite the uniform findings of higher rates of ADHD in extremely preterm children compared to the general population, little is known about the neurocognitive limitations that accompany ADHD in these high-risk children.
ADHD is associated with poor academic outcomes and comorbid disorders/diseases/conditions. For example, 45% of children who have ADHD also have a learning disability (21–23) and are more likely than others to repeat a grade, have lower academic achievement, and higher rates of dropping out of high school (24, 25). They are also more likely to need more academic support and special education services (24, 25).
About one half of children with ADHD exhibit deficits in a range of executive abilities, most prominently in sustained attention, response inhibition, working memory, and planning (26–31). Children with ADHD and working memory deficits have poorer academic outcomes including lower scores on measures of reading and math achievement as well as increased risk for grade retention and special services (32). While the specificity of executive functioning weaknesses in ADHD remains a topic of debate, the occurrence of deficits in these core problem-solving skills arguably serve as a defining feature of ADHD (26).
The ELGAN Study, which enrolled infants born before the 28th week of gestation and assessed their behavioral and neurocognitive characteristics at age 10 years, provided an opportunity to investigate the neurocognitive correlates of ADHD symptoms in a high risk sample of extremely low gestational age children. The primary aim of our analyses was to evaluate the association of ADHD symptoms in extremely low gestational age newborns (ELGANs) with the following outcomes: global cognitive ability, executive functions, language abilities, visual-spatial skills, and academic achievement. These associations are evaluated in the context of maternal, newborn, and educational variables.
METHODS
Participants
The ELGAN study is a multi-center prospective, observational study of the risk of structural and functional neurologic disorders in extremely preterm infants (33). A total of 1506 infants born before the 28th week of gestation were enrolled during the years 2002–2004 and 1200 survived to 2 years, when 1102 returned for a developmental assessment (34).
At age 10 years, 889 (92%) of 966 children who were actively recruited for follow-up (because of the availability of data on inflammation-related proteins in blood samples from their first postnatal month), returned for an age-appropriate assessment of cognition, executive function, behaviors, and achievement. The 871 for whom we have CSI-4 report from a parent constitute the sample for this report (Supplemental Digital Content 1). 634 also had a teacher-completed CSI-4.
The institutional review boards of all participating institutions approved enrollment and consent procedures for this follow up study.
Demographic and pregnancy variables
After delivery, a trained research nurse interviewed each mother in her native language using a structured data collection form and following procedures defined in a manual. The mother provided information regarding her own characteristics and exposures as well as the sequence of events leading to preterm delivery.
Shortly after the mother’s discharge, the research nurse reviewed the maternal chart using a second structured data collection form. The medical record was relied on for events following admission. The clinical circumstances that led to preterm delivery were operationally defined using both data from the maternal interview and data abstracted from the medical record (35). Each mother/infant pair was assigned to the category that described the primary reason for the preterm delivery.
Newborn variables
The gestational age estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), LMP without fetal ultrasound (7%), and gestational age recorded in the log of the neonatal intensive care unit (1%).
The birth weight Z-score is the number of standard deviations the infant’s birth weight is above or below the median weight of infants at the same gestational age in referent samples not delivered for preeclampsia or fetal indications (36,37).
Procedures for the assessments at age 10 years
All families who participated in the previous follow up were contacted by mail and then by phone to invite them to participate in the 10-year follow up. Lost to follow-up families were searched for on state vaccination registries, and other openly available websites. Facebook was also used where approved by the local institution’s IRB.
Families willing to participate were scheduled for one visit during which all of the measures reported here were administered in fixed order in 3 to 4 hours, including breaks. The assessments were selected to provide the most comprehensive information about neurocognitive and academic function in one testing session. While the child was tested, the parent or caregiver completed questionnaires regarding the child’s medical and neurological status and behavior.
Behavioral Measures
Child Symptom Inventory-4
The parent or caregiver completed the Child Symptom Inventory, Fourth Edition Parent Checklist (CSI-4) (38,39). The child’s current teacher was also asked to complete the CSI-4 Teacher Checklist. Both include the same 18 items specific for ADHD symptoms (9 for the inattentive domain and 9 for the hyperactive/impulsive domain) that are each rated on a scale from 0 (never) to 3 (very often). Classifications regarding significant ADHD symptoms were made based on norm-based cut-off scores provided in the CSI-4 manual. Though severity of impairment was not directly assessed, research suggests that classifications based on CSI-4 norm-based cut-off scores do not significantly differ from physician provided DSM-IV and ICD-10 based diagnoses (40).
Defining classification based on symptoms identified by two informants satisfied one of the DSM-5 criteria for ADHD. Importantly, these participants were not considered to meet diagnostic criteria, but rather research classification of significant ADHD symptom presentation. In order to parallel clinical decision-making, we considered three possible contexts in which ADHD symptoms were present. Two of the contexts were taken from the parent and teacher ADHD symptoms as reported on the CSI-4. A third context was based on the parent’s indication at interview of the child having been diagnosed previously by a clinician to have ADHD. Physician assessment of ADHD precedes treatment with stimulants, and the physician’s diagnosis of ADHD is typically based equally or more on parent and teacher report than their own observations (41) so we regarded this as pertinent to our research classification. Participants were included in the ADHD symptom group if they meet criteria in any two of the three contexts.
Neurocognitive measures
General cognitive ability
General cognitive ability (or IQ) was assessed with the School-Age Differential Ability Scales, Second Edition (DAS-II) Verbal and Nonverbal Reasoning scales (42).
Maternal cognitive ability
Maternal cognitive ability was assessed with the Kaufman Brief Intelligence Test-Second Edition (KBIT-2).
Language ability
Expressive and receptive language skills were evaluated with the Oral and Written Language Scales (OWLS), which assess semantic, morphological, syntactic, and pragmatic production and comprehension of elaborated sentences (43).
Attention and executive function
We assessed attention and executive functioning with both the DAS-II (42) and the NEPSY-II (A Developmental NEuroPSYchological Assessment, Second Edition) (44). The DAS Recall of Digits Backward and Recall of Sequential Order measured verbal working memory, while the NEPSY-II Auditory Attention and Response Set measured auditory attention, set switching and inhibition, the Inhibition and Switching scores from the NEPSY-II Inhibition subtest measured simple inhibition and inhibition in the context of set shifting, respectively, and the NEPSY-II Animal Sorting measured visual concept formation and set shifting. Speed of processing was assessed with NEPSY-II Inhibition Naming, which provides a baseline measure of processing speed and has no inhibitory component.
Latent profile analysis (LPA) was utilized to categorize extremely preterm children in the ELGAN cohort based on the severity of their limited performance on a number of cognitive measures (DAS-II: Verbal Reasoning, Nonverbal Reasoning, & Working Memory Clusters; NEPSY-II: Animal Sorting, Auditory Attention, Response Set, Inhibition: Inhibition, & Inhibition: Switching subtests). With LPA, we identified four subgroups corresponding to functioning on measures of IQ and executive functioning: normal (34% of cohort), low-normal (41%), moderately impaired (17%), and severely impaired (8%) (Unpublished). Children in the moderately and severely impaired groups exhibited more global deficits across both cognitive and executive functioning measures, whereas the low-normal group exhibited more significant deficits in inhibition (Unpublished).
Visual perception and fine motor function
Visual perception was assessed with NEPSY-II Arrows and Geometric Puzzles, while fine-motor function was measured with NEPSY-II Visuomotor Precision.
Academic Function
The Wechsler Individual Achievement Test-III (WIAT-III) provides standard scores in word recognition and decoding, spelling, and numeric operations (45). Scores were generated based on age-based normative data.
Data Analyses
We evaluated the null hypothesis that ELGANs who had significant ADHD symptoms are not at increased risk of neurocognitive impairment, including executive dysfunction, or academic delays. We began our analyses by exploring the distribution of Z-scores of each assessment separately in those with and without significant ADHD symptoms. To allow for the differences in age at the time of the assessment, and to facilitate a comparison of our findings to those reported for children presumably born very near term, we used Z-scores based on distributions of values reported for the historical normative samples that are described by the authors of the assessments we used (42–45). We then created logistic regression models of the risk of a score one or more standard deviations below the normative mean of each assessment. These models, which included potential confounders (including sex, birth weight Z-score < −1, and mother’s eligibility for government-provided medical-care insurance), allowed us to calculate odds ratios (and 95% confidence intervals) of each 10-year characteristic associated with any ADHD symptom classification.
RESULTS
Sample Characteristics (Supplemental Digital Content 2)
Teacher-completed CSI-4 forms were available for 634 of the 871 children for whom we had a parent-completed CSI-4 form. The children whose teacher did not provide a CSI-4 report differed minimally from the children whose teacher did return a completed form. Similarly, the socio-demographic characteristics of their mothers also differed minimally. Differences between the two groups were examined for statistical significance using 2-sided Fisher’s exact test. Across maternal characteristics, perinatal characteristics, and newborn characteristics, differences were seen at the p<0.05 level in race and gestational age.
Table 1 describes the sample characteristics in relation to combinations of observer reports of ADHD symptoms providing a context in which to view our results. Sample characteristics based on combinations of information sources do not differ appreciably and thus our results do not depend on the operational definition we used for ADHD.
Table 1.
Sample characteristics. Percent of children identified as having ADHD symptoms (according to the reporters identified at the top of the column) whom also had the characteristic listed on the left.
| Characteristic | Any ADHD based on | Non-ADHD by All 3 | ||||
|---|---|---|---|---|---|---|
| Parent + Teacher | Parent + Physician | Teacher + Physician | Any 2 or All 3 | |||
| Maternal | ||||||
| Racial identity | White | 8 | 12 | 9 | 17 | 82 |
| Black | 11 | 13 | 15 | 21 | 76 | |
| Other | 10 | 10 | 9 | 13 | 79 | |
| Hispanic | Yes | 7 | 12 | 7 | 12 | 84 |
| No | 9 | 13 | 11 | 18 | 80 | |
| Age, years | < 21 | 11 | 20 | 16 | 27 | 68 |
| 21–35 | 9 | 12 | 11 | 17 | 82 | |
| > 35 | 7 | 8 | 6 | 12 | 85 | |
| Education, years | ≤ 12 | 12 | 17 | 15 | 23 | 75 |
| > 12, < 16 | 9 | 11 | 11 | 16 | 83 | |
| ≥ 16 | 7 | 10 | 5 | 13 | 86 | |
| Single marital Status | Yes | 12 | 16 | 16 | 22 | 74 |
| No | 7 | 10 | 7 | 14 | 85 | |
| Public insurance | Yes | 13 | 19 | 17 | 26 | 72 |
| No | 7 | 9 | 7 | 13 | 85 | |
| Newborn | ||||||
| Sex | Male | 13 | 15 | 14 | 22 | 75 |
| Female | 5 | 10 | 6 | 13 | 86 | |
| Gestational age, weeks | 23–24 | 11 | 19 | 14 | 25 | 74 |
| 25–26 | 10 | 12 | 11 | 17 | 80 | |
| 27 | 7 | 9 | 8 | 13 | 85 | |
| Birth weight, Grams | ≤ 750 | 11 | 15 | 13 | 20 | 78 |
| 751–1000 | 7 | 10 | 8 | 14 | 83 | |
| > 1000 | 10 | 14 | 10 | 19 | 80 | |
| Birth weight Z-score | < –2 | 4 | 12 | 8 | 14 | 87 |
| ≥ −2, < −1 | 10 | 13 | 8 | 17 | 81 | |
| ≥ −1 | 9 | 13 | 10 | 18 | 80 | |
| Education | ||||||
| IEP | Yes | 14 | 19 | 17 | 27 | 71 |
| No | 3 | 5 | 3 | 7 | 92 | |
| Repeated Grade | Yes | 14 | 22 | 17 | 29 | 68 |
| No | 8 | 10 | 9 | 15 | 84 | |
| Remedial Class | Yes | 17 | 17 | 21 | 26 | 68 |
| No | 7 | 11 | 8 | 15 | 84 | |
| Current ADHD medication | Yes | 22 | 53 | 38 | 65 | 32 |
| No | 6 | 4 | 5 | 8 | 91 | |
| N ADHD (N total) | 57 (634) | 109 (871) | 66 (637) | 152 (874) | 511 (634) | |
Intelligence, Language, and Academic Achievement Characteristics (Figure 1; Supplemental Digital Content 3a)
Figure 1.
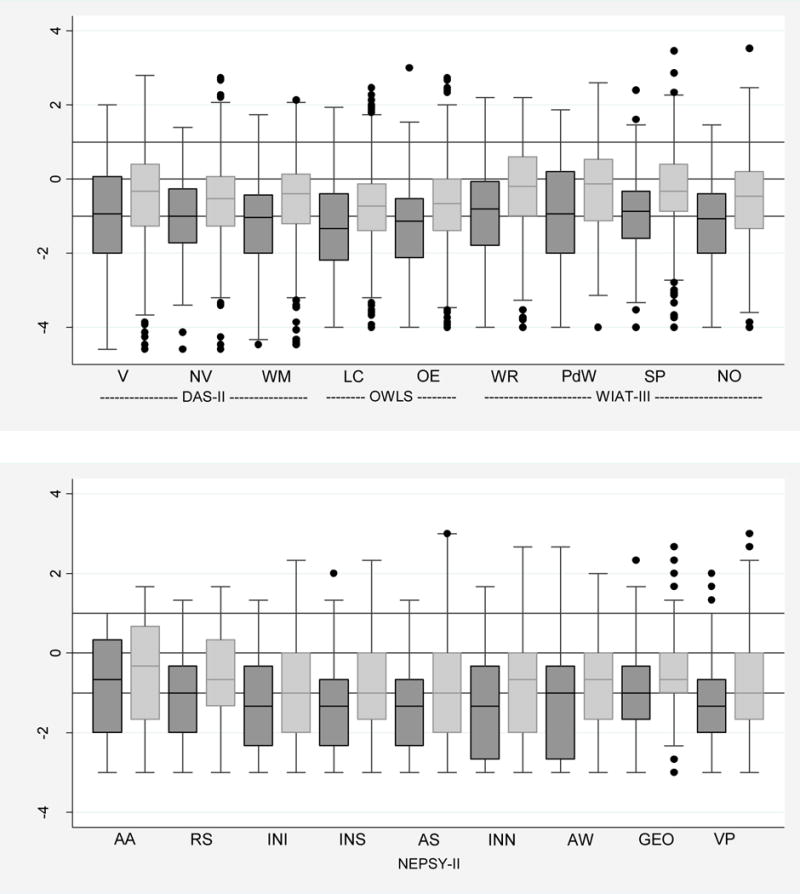
Box-and-whisker plots of each neurocognitive subtest by ADHD (defined as meeting criteria by 2 of the following: CSI-4 report by parent, CSI-4 report by teacher, or clinical diagnosis). All subtest Z-scores are adjusted to population norms. Key: dark gray is ADHD and light gray is Non-ADHD. The central line in the box indicates the median (50th centile), while the top of the box indicates the 75th centile and the bottom of the box indicates the 25th centile. V=Verbal, NV=Nonverbal reasoning, WM=Working memory, LC=Listening comprehension, OE=Oral expression, WR=Word reading, PwD=Pseudoword decoding, Sp=Spelling, NO=Numerical operations, AA=Auditory attention, RS=Auditory response set, INI=Inhibition inhibition, INS=Inhibition switching, AS=Animal sorting, INN=Inhibition naming, AW=Arrows, GEO=Geometric puzzles, VP=Visuomotor precision.
The median Z-scores on all tests for children classified as having significant ADHD symptoms were approximately −1.0 and were consistently .25 to .75 standard deviations below the scores for children not classified as having significant ADHD symptoms.
Children with a classification of ADHD symptoms were much more likely than those without ADHD symptoms to have low IQ scores. For example, a total IQ Z-score ≤ −2 was found in 23% of children with ADHD symptoms but only 12% of those without ADHD symptoms, and a verbal IQ Z-score ≤ −2 was found in 27% of children with ADHD symptoms but only 14% of those without ADHD symptoms.
Similarly, 26% of children with a classification of ADHD symptoms had an OWLS Listening Comprehension Z-score of ≤ −2 compared to 16% of their peers, while 30% of children with a classification of ADHD symptoms had an OWLS Oral Expression Z-score of ≤ −2 compared to 16% of the children who did not meet criteria for ADHD symptom classification. This almost doubling of the frequency of low scores associated with ADHD symptom classification was also seen for each of the four WIAT-III components.
Executive Function, Processing Speed, Visual Perception, Fine-Motor Characteristics (Figure 1; Supplemental Digital Content 3b)
The DAS-II Working Memory subtest and five of the NEPSY-II subtest scores (e.g., Auditory Attention, Auditory Response Set, Inhibition and Switching from the Inhibition subtest, and Animal Sorting) assess components of executive function. Children with a classification of ADHD symptoms had a doubling of the frequency of Z-scores of ≤ −2 on the DAS-II Working Memory assessments, while the multiples of increased frequencies of Z-scores of ≤ −2 were more modest on the NEPSY-II items (1.6 for Auditory Response Set and Inhibition Switching, and 1.4 for NEPSY-II Auditory Attention, Inhibition, and Animal Sorting). Even with the assessment of fine-motor function (NEPSY-II Visuomotor Precision subtest) the multiple was 1.8, while the multiple dropped to 1.5 for the assessments of visual perception (NEPSY-II Arrows and Geometric Puzzles).
Forest plots of odds ratios and 95% confidence intervals of a Z-score ≤ −1 on each DAS-II, OWLS, WIAT-III, and NEPSY-II neurocognitive assessment associated with ADHD symptoms (Figure 2)
Figure 2.
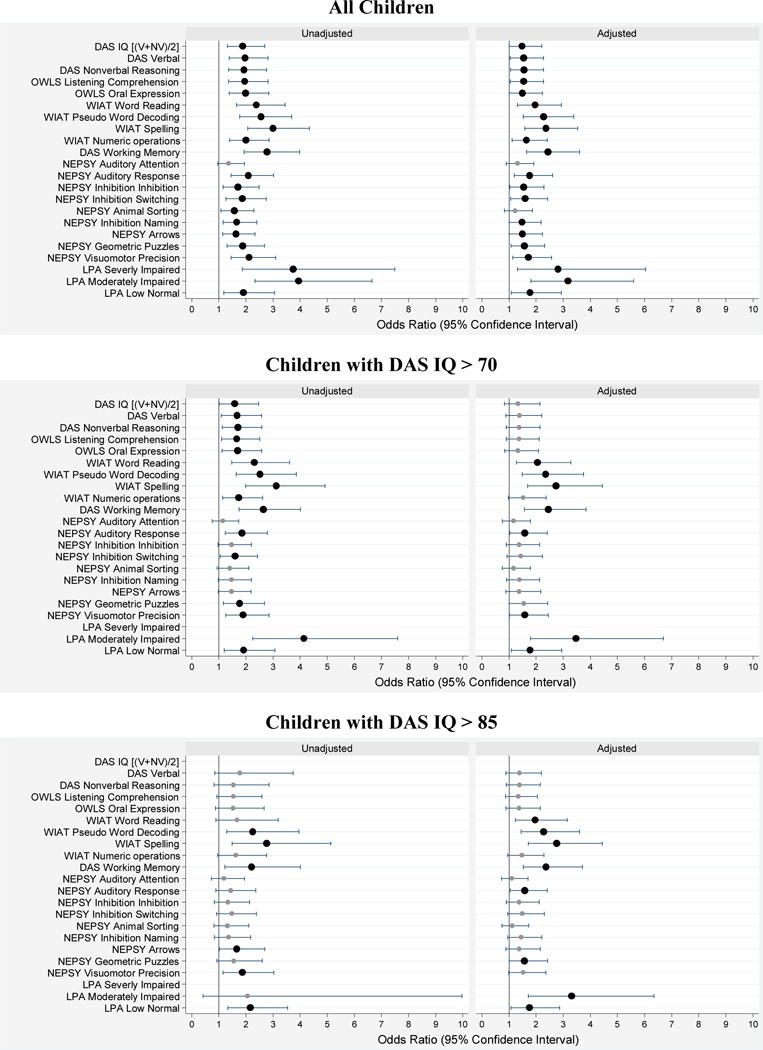
Forest plots of odds ratios (ORs) and 95% confidence intervals of a Z-score ≤ –1 on each DAS-II, OWLS, WIAT-III, and NEPSY-II neurocognitive assessment at age 10 associated with ADHD (defined as meeting criteria by 2 of the following: CSI-4 report by parent, CSI-4 report by teacher, or clinical diagnosis). Odds ratios in the left panel are unadjusted, while those in the right panel are adjusted for mother’s identification as black, mother’s age < 21 years, mother eligibility for public insurance, male sex, gestational age 23–24 weeks and birth weight Z-score < –2. The top set of panels is for all children, while the middle set is for children with an IQ > 70, and the bottom set is for children with an IQ > 85. Large dark circles indicate ORs significantly different from 1.0 (p < 0.05) while smaller gray circles indicate non-significant ORs. The ends of the horizontal lines indicate the bounds of the 95% confidence interval.
In these forest plots, the large dark circles and the smaller lighter circles mark are the point estimates of the odds ratios and the vertical ends of the horizontal lines that run through the circles identify the lower and upper ends of the 95% confidence intervals (46). The vertical line marks the null, an odds ratio of 1.0. When the lower end of a confidence interval does not include 1.0, the odds ratio, marked with a large dark circle, is statistically significantly different from 1.0 at p < 0.05.
After adjusting for three maternal characteristics (identification as black, age < 21 years, and eligibility for government-provided medical care insurance), and three newborn characteristics (male sex, gestational age 23–24 weeks, and birth weight Z-score < −2), an ADHD symptom classification was significantly associated with Z-scores ≤ −1 on all DAS-II, OWLS, and WIAT-III subtests, on all but two NEPSY-II subtests, and for LPA-based level of executive function impairment.
To address the possibility that IQ, and not ADHD symptoms, is responsible for these findings, we re-analyzed our data after excluding the 68 children with potential intellectual disabilities (i.e., IQ < 70) as well as after excluding the data of children with IQ < 85 in order to focus on children whose cognitive abilities are broadly average to above average. After eliminating the children with IQ < 70, the ADHD-associated adjusted risk of a Z-score ≤ −1 was essentially unchanged for WIAT-III Word Reading, Pseudoword Decoding, and Spelling, DAS-II Working Memory, and NEPSY-II Auditory Response and Visuomotor Precision, and for LPA-based level of executive function impairment. For all other subtests, there was no longer a significant association of a Z-score ≤ −1 with ADHD symptom classification, when the sample was restricted to children with IQ > 70. Similarly, after eliminating the children with IQ < 85, the ADHD-associated adjusted risk of a Z-score ≤ −1 was essentially unchanged for WIAT-III Word Reading, Pseudoword Decoding, and Spelling, DAS-II Working Memory, and NEPSY-II Auditory Response, and for LPA-based level of executive function impairment. Interestingly, NEPSY-II Visuomotor Precision was no longer associated with ADHD symptoms, though NEPSY-II Geometric Puzzles indicated some visual spatial analysis challenges in this group.
DISCUSSION
Three of our main findings are worthy of comment. First, relative to normative expectations, the distribution of IQ scores was shifted downward across the entire ELGAN sample, and this shift was much more prominent for ELGANs with ADHD symptom classification than for their peers. Second, children who meet classification for ADHD symptoms are at increased risk for many neurocognitive limitations, especially in regard to executive function and visual-motor skills. These limitations are evident even in the subsample restricted to children whose IQ ≥ 70 as well as in the subsample of children whose IQ ≥ 85, although less severely than in the entire sample. Thus, we show that low IQ in ELGANs can account for a sizable proportion of ADHD-associated limitations, but not all. Third, ELGANs with ADHD symptoms are at an increased risk for poor performance on assessments of school achievement in contrast with ELGANs without ADHD symptoms, with difficulties observed across reading, spelling, and math.
Our finding of a downward shift of the IQ distribution in such a low gestational age sample is a replicates previous findings from very preterm and/or low birth weight cohorts (47), but in an extremely preterm cohort. To minimize the potential contribution of low IQ to our understanding of impaired neuropsychological function, we restricted some analyses to children whose IQs were ≥ 70 as well as to children whose IQs were ≥ 85. Even then, children classified as having ADHD symptoms were more likely than their peers to have specific deficits on the DAS-II working memory and NEPSY-II Auditory Response. In those with IQs ≥ 70 specific deficits in NEPSY-II Visuomotor Precision were identified, however, these deficits were not seen in the group with IQs ≥ 85 though deficits on NEPSY-II Geometric Puzzles were identified in this group. Across both groups, ADHD symptoms were associated with LPA-defined moderate impairment and low normal function again supporting our findings that both IQ and executive functioning are areas of concern in ELGANs with significant ADHD symptoms. ELGANs in the LPA-defined Low Normal group have significant challenges in aspects of executive functioning (specifically inhibition) as compared to performance on measures of verbal and nonverbal IQ (Unpublished). Overall, these results are consistent with previous studies that have identified significant deficits in attention, working memory, executive function, and visuomotor and visual spatial skills, beyond the generalized cognitive weaknesses seen in children born extremely preterm (9, 48–51). Moreover, the deficits we found on the DAS-II working memory and NEPSY-II Auditory Response tasks, which require sustained and dual attention, working memory, inhibitory control, and cognitive flexibility, were present even after adjusting for key sociodemographic and medical variables.
In the general population, (26, 27, 28, 29) and in children born preterm or at low birth weight (52, 53,13), those with ADHD exhibit deficits in a range of executive abilities. In a meta-analysis, groups with ADHD exhibited significant impairment across executive functioning tasks compared to those without ADHD, with the strongest effect sizes identified on measures of response inhibition, vigilance, working memory, and planning (54). While there remains some debate regarding the specificity of executive function weaknesses in ADHD, it is clear that the two are associated (31). Among children born preterm who do not necessarily meet criteria for ADHD, frequently reported executive dysfunction includes challenges with working memory, visual attention, and set shifting (29, 53). When examining neuropsychological correlates, relationships between behavioral ratings of ADHD symptoms and poorer performances on measures of rapid naming, set shifting, and focused and sustained attention have been identified in a cohort of children with extreme prematurity (29). Similarly, performance on neuropsychological measures of attention and executive functioning is correlated with parent and teacher report of ADHD symptoms in a more recent extreme prematurity cohort (53). Given the significant heterogeneity in executive dysfunction found among individuals with ADHD (31), the finding that cognitively intact ELGANs (IQ>70 and IQ>85) with ADHD symptoms are at significantly increased risk for deficits in working memory, inhibition, and cognitive flexibility strongly suggests that, within this population, there is a higher co-occurrence of these deficits than in the typical ADHD profile. While ADHD symptoms and executive function are associated, specific domains of executive dysfunction that are associated with ADHD are less clear, and may vary by age, executive functions assessed, and population evaluated. Nonetheless, these results help support previous findings of deficits in working memory and set-shifting in ELGANs who have ADHD symptoms, and add to the literature by identifying an association with inhibition as well.
In ELGANs, delays in early motor development at 2.5 years predict inattention at age 11 years (55). Our results suggest that motor delays are not simply a predictor of inattentive symptoms, but that children with ADHD symptoms are more likely than others to have persistent difficulties with visuomotor coordination.
Finally, we found that children who had an ADHD symptom classification are at significantly increased risk for poor academic outcomes, even in the subsample of children whose IQ was > 70 and >85. These findings highlight the cumulative impact of attention and executive function deficits on learning and academic development among children born very (56) or extremely preterm (57). Improved academic function might require a multifaceted intervention in this population
This study has several limitations. First, we did not receive teacher ratings for 237 of the 871 children for whom we did receive parent ratings. The missing data limited the number of children who could have reports from two observers (usually parent and teacher, although occasionally teacher and physician). We are uncertain about the potential bias. Second, we did not utilize a standardized or conventional diagnostic instrument to assess ADHD presentation (59). Although the CSI-4 appears attractive in light of its orientation to DSM-5, there are limits to this measure as is the case with many parent or teacher report questionnaires assessing ADHD symptoms. This limited our ability to assess the psychometric properties and reliability of the CSI-4 ratings. The CSI-4 was used principally to assess ADHD symptom presentation, which did not provide a scale of functional impairment as is required for diagnostic decision making. We did not obtain information about the severity of each participants ADHD symptoms. The validity of the CSI-4 for diagnosis of ADHD has not been adequately assessed (60, 61). Despite these weaknesses, a recent study of ADHD diagnoses in a pediatric population in central and eastern Europe and Asia indicated that ADHD classifications based on CSI-4 norm-based cut-off scores did not significantly differ from physician provided DSM-IV and ICD-10 based diagnoses (40). Third, although the CSI-4 does generate ADHD subtyping information we were not able to take advantage of this because of the high rate of disagreement about subtype even when the reporters agreed on the presence of ADHD. We did utilize physician diagnosis in defining our ADHD symptom group. We do not know to what extent the physician’s diagnosis of ADHD is based on personal observation and therefore provides independent information, or provides redundant information because it was based on parent and teacher reports. Because some of the physicians’ diagnoses may have been made years earlier, the physicians have, in essence, provided “life-time” diagnoses, whereas both teacher and parent provided information about current symptoms. Although we were able to document the working memory, inhibition, and switching limitations of children identified to have ADHD symptoms, we did not assess the effect of executive dysfunction on activities of daily life. It will be helpful for future assessments of the neuropsychological correlates of ADHD symptoms to more directly assess executive dysfunction within the context of daily life, as reported by parents and teachers, through the inclusion of questionnaires to directly assess day-to-day executive functioning (e.g., the Behavior Rating Inventory of Executive Function-2). Our comparison group is based on data from a national-based normative sample rather than full-term sibling paired controls. We acknowledge that we cannot separate the impact of ADHD symptoms on test performance from the very dysfunctions we assessed. Finally, it will be important for future research to examine possible mitigating factors on ADHD behaviors and neuropsychological functioning in this population such as medication response or receipt of early intervention, behavioral, or academic services.
Our study has several strengths. First, we included a large number of children, making it unlikely that we have missed important associations due to lack of statistical power, or claimed associations that might reflect the instability of small numbers of participants. Second, we selected infants based on gestational age, not birth weight, in order to minimize confounding due to factors related to fetal growth restriction (17). Third, we collected all of our data prospectively. Fourth, attrition was modest, limiting the resultant bias.
Conclusions
Among children born extremely preterm, those who were classified as having ADHD symptoms had lower IQs and lower scores on assessments of executive function indicators than those not considered to have ADHD symptoms. These children are at substantial risk for academic underachievement even when intellectual abilities are within normal limits. This places them at particular risk in the classroom, because these neurocognitive deficits likely will interfere with learning, accessing information, and perhaps behavior. Our findings add to the growing literature on neurocognitive outcomes of extreme prematurity and highlight the need for early identification of and intervention to limit adverse consequences.
Supplementary Material
Acknowledgments
This study was supported by The National Institute of Neurological Disorders and Stroke (5U01NS040069-05, and 2R01NS040069 - 06A2), and the National Institute of Child Health and Human Development (5P30HD018655-34). The authors also gratefully acknowledge the contributions of their subjects, and their subjects’ families, as well as those of their colleagues.
Source Funding: Scott J. Hunter, Ph.D. receives royalties from Cambridge University Press and is funded through grants (PAR-12-068 Fogarty HIV Research Training Program for Low and Middle Income Country Institutions (D43); NIH-NIDDK R03 DK103096-01; NIH-NICHD R01 HD074757).
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
Contributor Information
Megan N. Scott, Department of Psychiatry and Behavioral Neuroscience, The University of Chicago Medicine, Chicago IL, USA
Scott J. Hunter, Department of Psychiatry and Behavioral Neuroscience, The University of Chicago Medicine, Chicago, IL
Robert M. Joseph, Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA
T. Michael O’Shea, Division of Neonatology, Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill NC
Stephen R Hooper, Department of Allied Health Sciences, University of North Carolina School of Medicine, Chapel Hill NC
Elizabeth N. Allred, Department of Neurology, Boston Children’s Hospital and Harvard Medical School, Boston MA
Alan Leviton, Department of Neurology Research, Boston Children’s Hospital and Harvard Medical School, Boston MA, USA
Karl Kuban, Division of Pediatric Neurology, Boston Medical Center and Boston University School of Medicine, Boston, MA, USA
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. 2014;19:90–96. doi: 10.1016/j.siny.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Moreira RS, Magalhães LC, Alves CR. Effect of preterm birth on motor development, behavior, and school performance of school-age children: a systematic review. J Pediatr. 2014;90:119–34. doi: 10.1016/j.jped.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Johnson S. Cognitive and behavioural outcomes following very preterm birth. Semin Fetal Neonatal Med. 2007;12:363–373. doi: 10.1016/j.siny.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Orchinik LJ, Taylor HG, Espy KA, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc. 2011;17:1067–1079. doi: 10.1017/S135561771100107X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor HG, Klien N, Anselmo MG, et al. Learning problems in kindergarten students with extremely preterm birth. Arch Pediatr Adolesc Med. 2011;165:819–825. doi: 10.1001/archpediatrics.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2010;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 8.Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev. 2007;83:247–254. doi: 10.1016/j.earlhumdev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Joseph RM, O’Shea TM, Allred EN, et al. ELGAN Study Investigators Neurocognitive and Academic Outcomes at Age 10 Years of Extremely Preterm Newborns. Pediatrics. 2016;137:pii: e20154343. doi: 10.1542/peds.2015-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sykes DH, Hoy EA, Hill JM, et al. Behavioural adjustment in school of very low birthweight children. J Child Psychol Psychiatry. 1997;38:315–325. doi: 10.1111/j.1469-7610.1997.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 11.Clark CAC, Woodward LJ, Horwood LJ, et al. Development of emotional and behavioral regulation in children born extremely preterm and very preterm: Biological and social influences. Child Dev. 2008;79:1444–1462. doi: 10.1111/j.1467-8624.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 12.Spittle AJ, Treyvaud K, Doyle LW, et al. Early Emergence of behavior and social-emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry. 2009;48:9099–18. doi: 10.1097/CHI.0b013e3181af8235. [DOI] [PubMed] [Google Scholar]
- 13.Scott MN, Taylor HG, Fristad MA, et al. Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. J Dev Behav Pediatr. 2012;33(3):202–13. doi: 10.1097/DBP.0b013e3182475287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26:427–40. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Delobel-Ayoub M, Kaminski M, Marret S, et al. Behavioral outcome at 3 years of age in very preterm infants: the EPIPAGE study. Pediatrics. 2006;117:1996–2005. doi: 10.1542/peds.2005-2310. [DOI] [PubMed] [Google Scholar]
- 16.Reijneveld SA, de Kleine MJK, van Baar AL, et al. Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Arch Dis Child Fetal Neonatal Ed. 2006;91:F423–28. doi: 10.1136/adc.2006.093674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hack M, Taylor HG, Schluchter M, et al. Behavioral outcomes of extremely low birth weight children at age 8 years. J Dev Behav Pediatr. 2009;30:122–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson S, Hollis C, Kochhar P, et al. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49(5):453–63 e1. [PubMed] [Google Scholar]
- 19.Johnson S, Marlow N. Growing up after extremely preterm birth: lifespan mental health outcomes. Semin Fetal Neonatal Med. 2014;19(2):97–104. doi: 10.1016/j.siny.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PJ, De Luca CR, Hutchinson E, et al. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol. 2011;36(1):57–73. doi: 10.1080/87565641.2011.540538. [DOI] [PubMed] [Google Scholar]
- 21.Seidman LJ, Biederman J, Valera EM, et al. Neuropsychological functioning in girls with attention-deficit/hyperactivity disorder with and without learning disabilities. Neuropsychology. 2006;20:166–177. doi: 10.1037/0894-4105.20.2.166. [DOI] [PubMed] [Google Scholar]
- 22.Seidman LJ. Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev. 2006;26:466–485. doi: 10.1016/j.cpr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.DuPaul GJ, Gormley MJ, Laracy SD. Comorbidity of LD and ADHD: implications of DSM-5 for assessment and treatment. J Learn Disabil. 2013;46(1):43–51. doi: 10.1177/0022219412464351. [DOI] [PubMed] [Google Scholar]
- 24.Biederman J, Faraone SV, Mick E, et al. Clinical correlates of ADHD in females: findings from a large group of girls ascertained from pediatric and psychiatric referral sources. J Am Acad Child Adolesc Psychiatry. 1999;38(8):966–975. doi: 10.1097/00004583-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265–273. doi: 10.1097/DBP.0b013e31811ff87d. [DOI] [PubMed] [Google Scholar]
- 26.Nigg JT, Blaskey LG, Stawicki JA, et al. Evaluating the endophenotype model of ADHD neuropsychological deficit: Results for parents and siblings of children with ADHD combined and inattentive subtypes. J Abnorm Psychol. 2004;113:614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- 27.Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 28.Willcutt EG, Doyle AE, Nigg JT, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Taylor HG, Hack M, Klein NK. Attention deficits in children with <750 gm birth weight. Child Neuropsychol. 1998;4:21–34. doi: 10.1076/0929-7049(200003)6:1;1-B;FT049. [DOI] [PubMed] [Google Scholar]
- 30.Shum D, Neulinger K, O’Callahan M, et al. Attentional problems in children born very preterm or with extremely low birth weight at 7–9 years. Arch Clin Neuropsych. 2008;23:103–112. doi: 10.1016/j.acn.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Nigg JT, Willcutt EG, Doyle, et al. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Fried R, Chan J, Feinberg L, et al. Clinical correlates of working memory deficits in youth with and without ADHD: a controlled study. J Clin Exp Neuropsyc. 2016;38(5):487–496. doi: 10.1080/13803395.2015.1127896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helderman JB, O’Shea TM, Goldstein DJ, et al. Antenatal antecedents of low scores on the Bayley Scales of Infant Development at 24 months among children born before the 28th post-menstrual week. The ELGAN Study. Pediatrics. 2012;129(3):494–502. [Google Scholar]
- 35.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–9. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yudkin PL, Aboualfa M, Eyre JA, et al. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 37.Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birthweight infants. Pediatr Res. 1999;46(5):566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Sprafkin J, Gadow KD, Salisbury H, et al. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Clin Child Adolesc. 2002;31(4):513–24. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- 39.Gadow KD, Sprafkin J. Child Symptom Inventory–4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- 40.Martenyi F, Treuer T, Gau SS, et al. Attention-deficit/hyperactivity disorder diagnosis, co-morbidities, treatment patterns, and quality of life in a pediatric population in central and eastern europe and asia. J Child Adolesc Psychopharmacol. 2009;19(4):363–376. doi: 10.1089/cap.2008.0148. [DOI] [PubMed] [Google Scholar]
- 41.Power TJ, Mautone JA, Manz PH, et al. Managing attention-deficit/hyperactivity disorder in primary care: a systematic analysis of roles and challenges. Pediatrics. 2008;121(1):e65–72. doi: 10.1542/peds.2007-0383. [DOI] [PubMed] [Google Scholar]
- 42.Elliott CD. Differential Ability Scales. 2nd. San Antonio, TX: Pearson; 2007. [Google Scholar]
- 43.Carrow-Woolfolk E. Oral and Written Language Scales: Written Expression Scale Manual. Circle Pines, MN: American Guidance Service; 1996. [Google Scholar]
- 44.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. New York: The Psychological Corporation; 1998. [Google Scholar]
- 45.Wechsler D. The Wechsler Individual Achievement Test-III. Oxford, UK: Pearson Assessment; 2009. [Google Scholar]
- 46.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. Brit Med J. 2001;322(7300):1479–1480. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hack M, Taylor G, Klein N, et al. School-age outcomes in children with birth weights under 750g. N Engl J Med. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 48.Taylor HG, Minich N, Bangert B, et al. Long-term neuropsychological outcomes of very low birth weight: Associations with early risks for periventricular brain insults. J Int Neuropsychol Soc. 2004;10:987–1004. doi: 10.1017/s1355617704107078. [DOI] [PubMed] [Google Scholar]
- 49.Taylor HG, Klein N, Drotar D, et al. Consequences of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27:459–469. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Orchinik LJ, Taylor HG, Espy KA, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc. 2011;17:1067–1079. doi: 10.1017/S135561771100107X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulseng S, Jennekens-Schinkel A, Naess P, et al. Very-low-birthweight and term small-for-gestational-age adolescents: Attention revisted. Acta Paediatr. 2006;95:224–230. doi: 10.1080/08035250500421568. [DOI] [PubMed] [Google Scholar]
- 52.Nedeau L, Boivin M, Tessier R, et al. Mediators of behavioral problems in 7-year-old children born after 24 to 28 weeks of gestation. J Dev Behav Pediatr. 2001;22:1–10. doi: 10.1097/00004703-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Shum D, Neulinger K, O’Callaghan M, et al. Attentional problems in children born with very preterm or with extremely low birth weight at 7–9 years. Arch Clin Neuropsych. 2008;23:103–112. doi: 10.1016/j.acn.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Edwards J, Berube M, Erlandson K, et al. Developmental coordination disorder in school-aged children born very preterm and/or at very low birth weight: A systematic review. J Dev Behav Pediatr. 2011;32:678–687. doi: 10.1097/DBP.0b013e31822a396a. [DOI] [PubMed] [Google Scholar]
- 56.Johnson S, Kochhar P, Hennessy E, et al. Antecedents of attention-deficit/hyperactivity disorder symptoms in children born extremely preterm. J Dev Behav Pediatr. 2016;37:285–297. doi: 10.1097/DBP.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaekal J, Wolke D, Bartmann P. Poor attention rather than hyperactivity/impulsivity predicts academic achievement in very preterm and full-term adolescents. Psychol Med. 2013;43:183–196. doi: 10.1017/S0033291712001031. [DOI] [PubMed] [Google Scholar]
- 58.Aarnoudse-Moens CSH, Weisglas-Kuperus N, Duivenvoorden J, et al. Executive function and IQ predict mathematical and attention problems in very preterm children. PLoS ONE. 2013;8:e55994. doi: 10.1371/journal.pone.0055994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tripp G, Schaughency EA, Clarke B. Parent and teacher rating scales in the evaluation of attention-deficit hyperactivity disorder: contribution to diagnosis and differential diagnosis in clinically referred children. J Dev Behav Pediatr. 2006;27:209–18. doi: 10.1097/00004703-200606000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Sprafkin J, Gadow KD, Salisbury H, et al. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Clin Child Adolesc. 2002;31:513–524. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- 61.Ghanizadeh A. Psychometric analysis of the new ADHD DSM-V derived symptoms. BMC Psychiatry. 2012;12:21. doi: 10.1186/1471-244X-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


