Abstract
To investigate alleviative effect of selenium (Se) on lead (Pb)-induced apoptosis in chicken nervous tissues, 7-day-old chickens were randomly divided into four groups. The control group was fed a standard diet and drinking water. In the Pb and Se/Pb groups, (CH3OO)2Pb was dissolved in drinking water. In the Se and Se/Pb groups, Na2SeO3 was put into the standard diet. Embryonic neurocytes were divided into the control, Se (containing Na2SeO3), Pb (containing (CH3COO)2Pb), and Se/Pb (containing Na2SeO3 and (CH3COO)2Pb) groups. The following contents were performed: Morphologic observation for 90 days in brain tissues and for 12, 24, 36, and 48 hours in embryonic neurocytes; and antioxidant indexes, messenger RNA (mRNA) expression of twenty-five selenoproteins, and mRNA and protein expression of five apoptosis-related genes for 30, 60, and 90 days in brain tissues and for 12, 24, 36, and 48 hours in embryonic neurocytes. The results indicated that Se alleviated Pb-caused morphological changes; the decrease of superoxide dismutase, glutathione peroxidase (GPx), GPx1, GPx2, GPx3, GPx4, thioredoxin reductases (Txnrd)1, Txnrd2, Txnrd3, iodothyronine deiodinases (Dio)1, Dio2, Dio3, selenoprotein (Sel)T, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, selenoprotein (Sep)n1, Sepp1, Sepx1, Sepw1, 15-kDa selenoprotein, and selenophosphate synthetases 2, and B-cell lymphoma-2 (Bcl-2); the increase of malondialdehyde, p53, Bcl-2 associated X protein, cytochrome c, and Caspase-3. Pb had time-dependent effects on GPx4, SelM, and malondialdehyde in the brain tissues; and on SelU in the embryonic neurocytes. Our data demonstrated that Se alleviated Pb-induced apoptosis in the chicken nervous tissues via mitochondrial pathway.
Keywords: lead, selenium, chicken nervous tissue, apoptosis, mitochondrial pathway
INTRODUCTION
Lead (Pb) is a toxic heavy metal and can cause bird and human poisoning through contaminated soil and vegetation. Pb caused topsoil and vegetation pollution near Pb battery manufacture factory in Ibadan, Nigeria [1]. Pb poisoning was diagnosed in 8% of birds in mallards and coots from two hunting activity areas in Poland [2]. Children and adolescents had neurocognitive deficit in a local ceramic glazing cottage industry in Ecuadorian villages with Pb exposure [3]. Occupational exposure to Pb caused cognitive deficit in Pittsburgh battery workers [4]. Pb caused behavioral changes of wild birds in Belgium hunting areas [5]. Excess Pb caused human and animal neurotoxicity, including behavioral deficit in herring gull brains [6], hippocampus damage and apoptosis in rat adrenal medulla pheochromocytoma cells (PC 12) [7], oxidative stress and apoptosis in human neuroblastoma cells [8], and apoptosis in rat brains [9]. Cadmium (Cd) disrupted mitochondrial membrane integrity and triggered release of mitochondrial proteins cytochrome c (Cyt c) into cytosol, then Cyt c activated Caspase-3, finally apoptosis occurred in PC 12 [10]. Pb induced apoptosis via mitochondrial pathway in rat proximal tubular cells [11]. B-cell lymphoma-2 (Bcl-2), p53, Bcl-2 associated X protein (Bax), Cyt c, and Caspase-3 involved in Pb-induced apoptosis in rat brains [9]. Therefore, we wanted to study neurotoxicity via inducing apoptosis in birds.
Selenium (Se) is an essential trace element in organisms [12]. Se alleviated Pb-caused the decrease of neural cell adhesion molecules in Wistar rat blood and hippocampus [13], and Cd-induced oxidative stress and apoptosis in mouse kidneys [14]. Se exerts its biological activity through incorporating into selenoproteins in form of selenocysteine [15]. Glutathione peroxidases (GPx)1, GPx2, GPx3, GPx4, thioredoxin reductases (Txnrd)1, Txnrd2, Txnrd3, iodothyronine deiodinases (Dio)1, Dio2, Dio3, selenoprotein (Sel)T, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, selenoprotein (Sep)n1, Sepp1, Sepx1, Sepw1, 15-kDa selenoprotein (Sep15), and selenophosphate synthetases 2 (SPS2) are selenoproteins in animals and humans [16]. Se can alleviate Cd-caused the decrease of GPx4 expression in rat testes [17] and the decrease of SelT, SelK, and SelS messenger RNA (mRNA) expression in chicken splenic lymphocytes [18]. SelT, SelK, and SelS may be related to protection of Se against Cd toxicity in chicken splenic lymphocytes [18]. Se is a cofactor of GPx, which is an antioxidant enzyme [18]. SelP and GPx participated in protection of human astrocytes from tert-butylhydroperoxide-induced oxidative damage [19]. Antioxidant of Se may be a mechanism of protective effect against Pb toxicity [20]. It was found that Se can antagonize Cd-induced apoptosis via mitochondrial pathway in chicken splenic lymphocytes [21] and in mouse kidneys [14].
Studies about alleviative effect of Se on Pb poisoning in chickens were focused on in vivo, such as cartilages about mRNA expression of GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepp1, Sepx1, Sep15, and SPS2 [22]; kidneys about mitochondrial dynamics and protein expression of Bcl-2, p53, Bax, Cyt c, and Caspase-3 [23]; bursa of Fabricius about oxidative stress indicators and cytokines mRNA expression [24]; and testes about mRNA and protein expression of inflammatory factors and heat shock proteins [25], mRNA expression of heat shock proteins and GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Selpn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2 [26]. However, little is known about alleviative effect of Se on Pb-caused apoptosis in bird nervous tissues in vivo and in vitro via mitochondrial pathway. Therefore, in this study, we wanted to establish chicken and chicken embryonic neurocytes models of alleviative effect of Se on Pb poisoning in vivo and in vitro; observe morphological changes; examine superoxide dismutase (SOD) and GPx activities, malondialdehyde (MDA) content, mRNA expression of twenty-five selenoproteins (GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2), and mRNA and protein expression of five apoptosis-related genes (Bcl-2, p53, Bax, Cyt c, and Caspase-3); and investigate alleviative effect of Se on Pb-induced apoptosis in chicken nervous tissues via mitochondrial pathway.
RESULTS
Cell viability
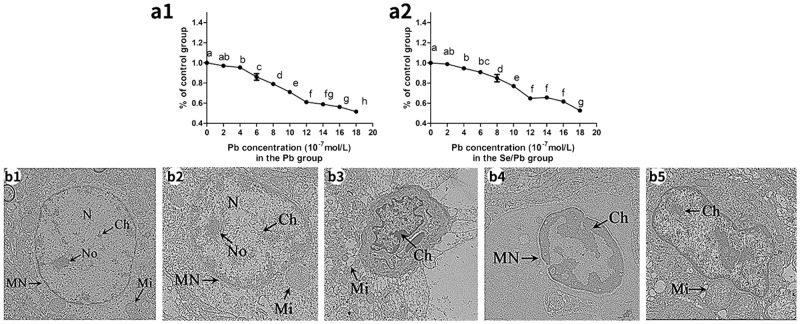
As shown in Figure 1, 50% inhibitory concentration (IC50) of chicken embryonic neurocytes for 48 hours in the Pb (Figure 1a1) and Se/Pb groups (Figure 1a2) were measured. Cell viabilities in the Pb group were 97.07%, 95.46%, 86.06%, 79.00%, 71.03%, 61.03%, 58.91%, 56.24%, and 56.24%, respectively, with Pb concentrations of 2, 4, 6, 8, 10, 12, 14, 16, and 18 × 10−7 mol/L. Cell viabilities in the Se/Pb group were 98.82%, 94.54%, 90.80%, 84.86%, 77.02%, 64.81%, 65.66%, 61.66%, and 52.66%, respectively, with Se concentration of 10 × 10−8 mol/L and Pb concentrations of 2, 4, 6, 8, 10, 12, 14, 16, and 18 × 10−7 mol/L. Cell viabilities in the Pb and Se/Pb groups with 18 × 10−7 mol/L Pb were 56.24% and 52.66%, respectively. IC50 of Pb at 48 hours was 18 × 10−7 mol/L. Cell viabilities of chicken embryonic neurocytes in the Pb and Se/Pb groups with Pb concentration of 10 × 10−7 mol/L were 71.03% and 77.02%, respectively. Pb concentration of 10 × 10−7 mol/L was equivalent to 5/9 of IC50. Therefore, Se concentration of 10 × 10−8 mol/L and Pb concentration of 10 × 10−7 mol/L for 48 hours were selected in the experiment.
Figure 1. Cell viabilities of the chicken embryonic neurocytes for 48 hours and the ultrastructure of the chicken brain tissues on the 90th day.
Different lowercase letters indicate significant differences (P < 0.05). (a1): Cell viability in the Pb group; (a2): cell viability in the Se/Pb group; (b1): the control group; (b2): the Se group; (b3): the Pb group; (b4): the Pb group; (b5): the Se/Pb group. Magnification: b1 × 30,000; b2 × 25,000; b3 × 15,000; b4 × 25,000; b5 × 20,000.
Apoptosis observation in chicken brain tissues
Ultrastructure observation of chicken brain tissues on the 90th day was shown in Figure 1. In the control (Figure 1b1) and Se (Figure 1b2) groups, cells were normal with complete nucleus (N) and nucleolus (No), intact mitochondria (Mi), and normal chromatin (Ch). In the Pb group, nucleus became shrunk (Figure 1b3), swollen mitochondria appeared (Figure 1b3), chromatin margination was clearly present (Figure 1b3 and 1b4), abnormal nuclear shape occurred (Figure 1b3 and 1b4), nucleolus disappeared (Figure 1b4), and nuclear membrane fusion appeared (Figure 1b4). In the Se/Pb group (Figure 1b5), degrees of abnormal nuclear shape, chromatin margination, and swollen mitochondria were lower than those in the Pb group.
Apoptosis observation in chicken embryonic neurocytes
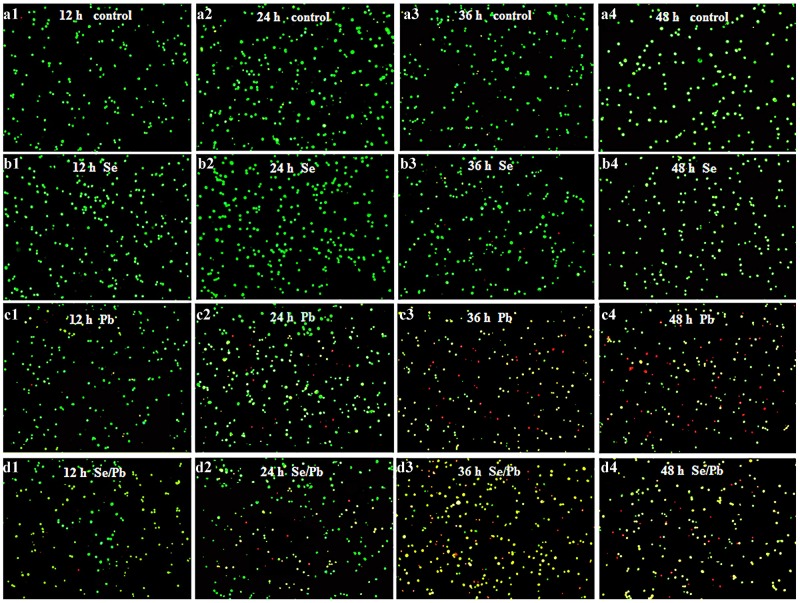
In this experiment, apoptosis observation of chicken embryonic neurocytes was performed. As shown in Figure 2, in the control (Figure 2a1-2a4) and Se (Figure 2b1-2b4) groups at all the time points, cells were round and green with the same size. In the Pb group for 12 (Figure 2c1), 24 (Figure 2c2), 36 (Figure 2c3), and 48 hours (Figure 2c4), cells showed irregular shape. Chromatin condensation occurred and there were yellow and red cells. Degree of irregular shape and numbers of yellow and red cells increased with the increase of treatment time. In the Se/Pb group for 12 (Figure 2d1), 24 (Figure 2d2), 36 (Figure 2d3), and 48 hours (Figure 2d4), irregular degree of cells, the degree of chromatin condensation, and the numbers of yellow and red cells increased with the increase of treatment time. Irregular degree of cells and the degree of chromatin condensation in the Se/Pb group were lower than those in the Pb group at all the same time points. Numbers of yellow and red cells in the Se/Pb group were less than those in the Pb group at all the same time points.
Figure 2. Apoptosis observation in the chicken embryonic neurocytes.
(a): The control groups; (b): the Se groups; (c): the Pb groups; (d): the Se/Pb groups; (1): 12 hours; (2): 24 hours; (3): 36 hours: (4): 48 hours.
Relative mRNA expression of twenty-five selenoproteins in chicken brain tissues and embryonic neurocytes
Relative mRNA expression of twenty-five selenoproteins (GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2) in chicken brain tissues and embryonic neurocytes was shown in Supplementary Tables 1 and 2, respectively. Twenty-five selenoproteins in the Pb group were significantly lower (P < 0.05) than those in the control, Se, and Se/Pb groups at all the time points in the brain tissues and embryonic neurocytes except GPx1, Txnrd3, Dio3, SelH, and SelI for 30 days; Txnrd3, SelT, and SelO for 60 days; GPx1, GPx3, Dio2, Dio3, and SelS for 90 days; GPx1, GPx2, GPx3, Txnrd1, Dio1, Dio2, and SelO for 12 hours; GPx1, GPx4, Txnrd1, Dio1, Dio2, SelS, SelH, SelM, SelU, SelO, Sepn1, Sepp1, Sepx1, Sepw1, and SPS2 for 24 hours; GPx1, GPx2, GPx4, Txnrd2, Txnrd3, Dio1, Dio2, SelS, SelH, SelU, SelI, SelO, and Sepn1 for 36 hours; and GPx1, GPx2, Txnrd3, Dio1, SelS, SelH, SelM, SelU, SelO, Sepn1, Sepp1, Sepw1, and SPS2 for 48 hours. The twenty-five selenoproteins in the Se/Pb group were significantly lower (P < 0.05) than those in the control and Se groups at all the time points in the brain tissues and embryonic neurocytes.
In the brain tissues for the Pb group, GPx1, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio3, SelH, SelI, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, and Sep15 for 60 and 90 days were significantly lower (P < 0.05) than those for 30 days. GPx2, SelT, and SelO for 90 days were significantly lower (P < 0.05) than those for 30 and 60 days. GPx4 and SelM decreased significantly (P < 0.05) with the increase of treatment time. In the embryonic neurocytes for the Pb group, Txnrd1 decreased significantly (P < 0.05) with the increase of treatment time for 12, 24, and 36 hours. Dio1, Dio3, and SelK decreased significantly (P < 0.05) with the increase of treatment time for 24, 36, and 48 hours. Dio2, SelS, and SelM for 36 and 48 hours were significantly lower (P < 0.05) than those for 12 and 24 hours. SelU decreased significantly (P < 0.05) with the increase of treatment time. Sepp1 for 24, 36, and 48 hours was significantly lower (P < 0.05) than that for 12 hours.
SOD and GPx activities, and MDA content in chicken brain tissues and embryonic neurocytes
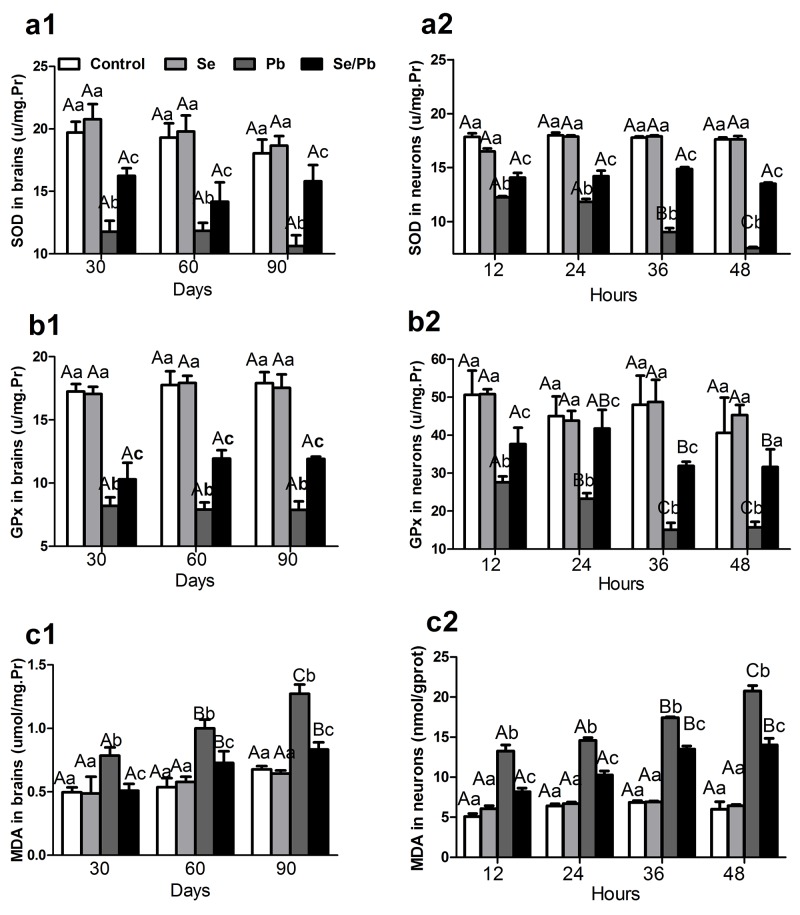
Oxidative stress in chicken brain tissues and embryonic neurocytes was evaluated by determining SOD and GPx activities, and MDA content. As shown in Figure 3, SOD (Figure 3a1 and 3a2) and GPx (Figure 3b1 and 3b2) activities in the Pb group decreased significantly (P < 0.05) compared with those in the control, Se, and Se/Pb groups at all the time points in the brain tissues and embryonic neurocytes. SOD and GPx activities in the Se/Pb group decreased significantly (P < 0.05) compared with those in the control and Se groups at all the time points in the brain tissues and embryonic neurocytes except GPx activity in the embryonic neurocytes for 24 hours. MDA (Figure 3c1 and 3c2) content in the Pb group increased significantly (P < 0.05) compared with that in the control, Se, and Se/Pb groups at all the time points in the brain tissues and embryonic neurocytes. MDA content in the Se/Pb group increased significantly (P < 0.05) compared with that in the control and Se groups at all the time points.
Figure 3. SOD and GPx activities, MDA content in the chicken brain tissues and embryonic neurocytes.
(a): SOD activities; (b): GPx activities; (c): MDA content (1): brain tissues; (2) embryonic neurocytes. Different lowercase letters indicate that there were significant differences (P < 0.05) among all groups at the same time point. Different uppercase letters indicate that there were significant differences (P < 0.05) among different time points in the same group.
In the embryonic neurocytes for the Pb group, SOD activity for 36 and 48 hours decreased significantly (P < 0.05) compared with that for 12 and 24 hours. SOD activity for 48 hours decreased significantly (P < 0.05) compared with that for 36 hours. GPx activity for 24 hours decreased significantly (P < 0.05) compared with that for 12 hours. GPx activity for 36 and 48 hours decreased significantly (P < 0.05) compared with that for 24 hours. MDA content increased significantly (P < 0.05) with the increase of treatment time. MDA content for 36 and 48 hours increased significantly (P < 0.05) compared with that for 12 and 24 hours. MDA content for 48 hours increased significantly (P < 0.05) compared with that for 36 hours.
Relative mRNA and protein expression of five apoptosis-related genes in chicken brain tissues and embryonic neurocytes
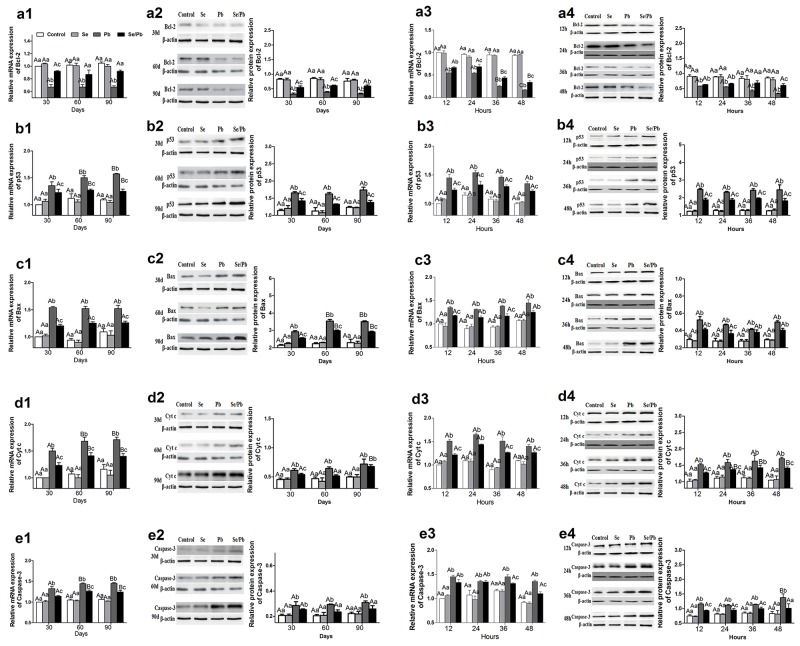
To investigate toxic effect of excess Pb on apoptosis and alleviative effect of Se on Pb-induced apoptosis, mRNA and protein expression of five apoptosis-related genes (Bcl-2, p53, Bax, Cyt c, and Caspase-3) was examined in chicken brain tissues and embryonic neurocytes. Bcl-2 (Figure 4a1-4a4) in the Pb group was significantly lower (P < 0.05) than that in all the other groups at all the time points in the brain tissues and embryonic neurocytes except mRNA expression for 12 hours; and protein expression for 90 days, 12 hours, and 24 hours in the Se/Pb group. Bcl-2 in the Se/Pb group was significantly lower (P < 0.05) than that in the control and Se groups at all the time points except mRNA expression for 60 and 90 days; and protein expression for 36 hours. p53 (Figure 4b1-4b4), Bax (Figure 4c1-4c4), Cyt c (Figure 4d1-4d4), and Caspase-3 (Figure 4e1-4e4) in the Pb group were significantly higher (P < 0.05) than those in all the other groups at all the time points except Bax mRNA expression for 24 and 48 hours; Bax protein expression for 12, 36, and 48 hours; Cyt c mRNA expression for 24 hours, Cyt c protein expression for 90 days and for 36 hours; Caspase-3 mRNA expression for 12 and 24 hours; Caspase-3 protein expression for 30 days in the Se/Pb group. p53, Bax, Cyt c, and Caspase-3 in the Se/Pb group were significantly higher (P < 0.05) than those in the control and Se groups at all the time points except Cyt c protein expression for 60 days; Caspase-3 protein expression for 60 and 90 days, and for 48 hours.
Figure 4. Relative mRNA and protein expression of five apoptosis-related genes in the chicken brain tissues and embryonic neurocytes.
(a): Bcl-2 expression; (b): p53 expression; (c): Bax expression; (d): Cyt c expression; (e): Caspase-3 expression (1): mRNA expression in brain tissues; (2): protein expression in brain tissues; (3): mRNA expression in embryonic neurocytes; (4): protein expression in embryonic neurocytes. Different lowercase letters indicate that there were significant differences (P < 0.05) among all groups at the same time point. Different uppercase letters indicate that there were significant differences (P < 0.05) among different time points in the same group.
In the brain tissues for the Pb group, mRNA expression of p53 and Cyt c and protein expression of Bax for 60 and 90 days were significantly higher (P < 0.05) than those for 30 days. In the embryonic neurocytes for the Pb group, Bcl-2 mRNA expression decreased significantly (P < 0.05) with the increase of treatment time for 24, 36, and 48 hours. Caspase-3 protein expression for 48 hours was significantly higher (P < 0.05) than that for 12, 24, and 36 hours.
Multivariate correlation analysis
Pearson's r correlation coefficient was used for multivariate correlation analysis among all twenty-five selenoproteins and five apoptosis-related genes in chicken brain tissues and embryonic neurocytes, respectively. The results showed that there were significantly positive relationships at the 0.01 level among twenty-five selenoproteins; among four apoptosis-related genes (p53, Bax, Cyt c, and Caspase-3); and between twenty-five selenoproteins and Bcl-2 in the brain tissues and embryonic neurocytes. There were significantly negative relationships at the 0.01 level between twenty-five selenoproteins and four apoptosis-related genes (p53, Bax, Cyt c, and Caspase-3) in the brain tissues and embryonic neurocytes.
PCA
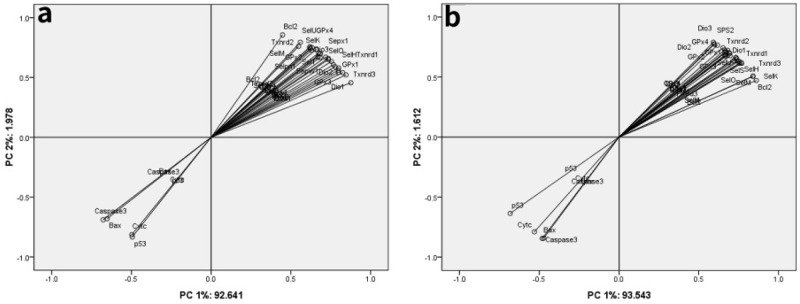
Twenty-five selenoproteins and five apoptosis-related genes were used for PCA in chicken brain tissues and embryonic neurocytes (Tables 1 and 2 and Figure 5). The results showed that all the parameters focused on the first two principal components. In the brain tissues, the first two principal components reflected 94.619% original data. Principal component (PC) 1 and PC 2 accounted for 92.641% and 1.978% of explained variances, respectively (Table 1). In the embryonic neurocytes, the first two principal components reflected 95.155% original data. PC 1 and PC 2 accounted for 93.543% and 1.612% of explained variances, respectively (Table 2). Twenty-five selenoproteins and Bcl-2 belonged to PC 1 in the brain tissues (Figure 5a) and embryonic neurocytes (Figure 5b). p53, Bax, Cyt c, and Caspase-3 belonged to PC 2 in the brain tissues (Figure 5a) and embryonic neurocytes (Figure 5b).
Table 1. Principle component analysis results of twenty-five selenoproteins and five apoptosis-related genes in the chicken brain tissues.
| Component | Initial eigenvalues | Extraction sums of squared | ||||
|---|---|---|---|---|---|---|
| Total | % of variance | Cumulative % | Total | % of variance | Cumulative % | |
| 1 | 27.792 | 92.641 | 92.641 | 27.792 | 92.641 | 92.641 |
| 2 | 0.593 | 1.978 | 94.619 | 0.593 | 1.978 | 94.619 |
| 3 | 0.401 | 1.336 | 95.956 | |||
| 4 | 0.196 | 0.653 | 96.608 | |||
| 5 | 0.166 | 0.553 | 97.161 | |||
| 6 | 0.130 | 0.434 | 97.595 | |||
| 7 | 0.111 | 0.371 | 97.966 | |||
| 8 | 0.099 | 0.329 | 98.295 | |||
| 9 | 0.085 | 0.283 | 98.578 | |||
| 10 | 0.071 | 0.238 | 98.815 | |||
| 11 | 0.065 | 0.216 | 99.031 | |||
| 12 | 0.052 | 0.175 | 99.206 | |||
| 13 | 0.043 | 0.143 | 99.349 | |||
| 14 | 0.037 | 0.125 | 99.474 | |||
| 15 | 0.034 | 0.112 | 99.586 | |||
| 16 | 0.026 | 0.088 | 99.674 | |||
| 17 | 0.025 | 0.085 | 99.759 | |||
| 18 | 0.019 | 0.062 | 99.821 | |||
| 19 | 0.014 | 0.046 | 99.867 | |||
| 20 | 0.010 | 0.034 | 99.901 | |||
| 21 | 0.007 | 0.024 | 99.926 | |||
| 22 | 0.006 | 0.021 | 99.946 | |||
| 23 | 0.006 | 0.019 | 99.965 | |||
| 24 | 0.004 | 0.013 | 99.978 | |||
| 25 | 0.002 | 0.007 | 99.986 | |||
| 26 | 0.002 | 0.006 | 99.992 | |||
| 27 | 0.001 | 0.004 | 99.996 | |||
| 28 | 0.001 | 0.002 | 99.998 | |||
| 29 | 0.000 | 0.001 | 99.999 | |||
| 30 | 0.000 | 0.001 | 100.000 | |||
Table 2. Principle component analysis results of twenty-five selenoproteins and five apoptosis-related genes in the chicken embryonic neurocytes.
| Component | Initial eigenvalues | Extraction sums of squared | ||||
|---|---|---|---|---|---|---|
| Total | % of variance | Cumulative % | Total | % of variance | Cumulative % | |
| 1 | 28.063 | 93.543 | 93.543 | 28.063 | 93.543 | 93.543 |
| 2 | 0.484 | 1.612 | 95.156 | 0.484 | 1.612 | 95.156 |
| 3 | 0.332 | 1.107 | 96.263 | |||
| 4 | 0.199 | 0.663 | 96.926 | |||
| 5 | 0.157 | 0.525 | 97.450 | |||
| 6 | 0.142 | 0.474 | 97.924 | |||
| 7 | 0.101 | 0.335 | 98.259 | |||
| 8 | 0.090 | 0.301 | 98.560 | |||
| 9 | 0.072 | 0.239 | 98.800 | |||
| 10 | 0.056 | 0.187 | 98.987 | |||
| 11 | 0.051 | 0.170 | 99.156 | |||
| 12 | 0.046 | 0.153 | 99.309 | |||
| 13 | 0.037 | 0.124 | 99.433 | |||
| 14 | 0.033 | 0.110 | 99.543 | |||
| 15 | 0.028 | 0.094 | 99.638 | |||
| 16 | 0.024 | 0.081 | 99.718 | |||
| 17 | 0.020 | 0.068 | 99.786 | |||
| 18 | 0.016 | 0.052 | 99.839 | |||
| 19 | 0.013 | 0.044 | 99.883 | |||
| 20 | 0.012 | 0.039 | 99.921 | |||
| 21 | 0.008 | 0.027 | 99.949 | |||
| 22 | 0.006 | 0.020 | 99.968 | |||
| 23 | 0.003 | 0.010 | 99.978 | |||
| 24 | 0.003 | 0.010 | 99.988 | |||
| 25 | 0.002 | 0.006 | 99.994 | |||
| 26 | 0.001 | 0.003 | 99.997 | |||
| 27 | 0.000 | 0.002 | 99.999 | |||
| 28 | 0.000 | 0.001 | 99.999 | |||
| 29 | 0.000 | 0.000 | 100.000 | |||
| 30 | 2.849E-5 | 9.498E-5 | 100.000 | |||
Figure 5. Principle component analysis among twenty-five selenoproteins and five apoptosis-related genes in the chicken brain tissues and embryonic neurocytes.
(a) brain tissues; (b) embryonic neurocytes.
DISCUSSION
Pb can induce neuronal apoptosis such as Pb-induced apoptosis in rat neuronal [9]. Se mainly performs its function through Se-containing proteins [15]. Se can protect against oxidative damage [27, 28] via selenoproteins [28]. Se had a protective effect for Cd-induced oxidative stress and apoptosis via mitochondria pathway in mouse kidneys [14]. Yao et al. found that Sepw1 served as an antioxidant [29] and protected embryonic myoblasts against apoptosis induced by hydrogen peroxide (H2O2) in chickens [30]. The decrease of GPx1, GPx2, GPx3, GPx4, Txnrd1, SelT, SelK, SelH, SelM, and SelI mRNA expression caused oxidative stress in swine peripheral blood neutrophils in vitro [31]. The decrease of SelW increased MDA content, and caused oxidative stress in chicken myoblasts and embryos [32]. Se can alleviate the decrease of selenoproteins, and oxidative stress caused by heavy metals in animals. Se alleviated Cd-caused the decrease of GPx4 mRNA expression, and oxidative stress in rat testes [17], and the decrease of SelT, SelK, and SelS mRNA expression in chicken splenic lymphocytes in vitro [18]. Se alleviated Pb-caused the decrease of GPx1, GPx2, GPx4, Txnrd2, Txnrd3, Dio1, Dio2, SelT, SelK, SelS, SelM, SelU, SelI, SelO, Sepn1, Sepx1, and Sep15 mRNA expression in chicken meniscus cartilages; the decrease of GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Dio2, Dio3, SelT, SelK, SelH, SelM, SelI, SelO, Selpb, Sepn1, Sepx1, Sep15, and SPS2 mRNA expression in chicken sword cartilages; and oxidative stress in chicken meniscus cartilages and sword cartilages [22]. Our results were consistent with the above studies. We found that Se alleviated Pb-caused the decrease of GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2 mRNA expression in the chicken brain tissues and embryonic neurocytes. Our results indicated that Se alleviated Pb-induced oxidative stress via increasing selenoproteins in the chicken nervous tissues in vivo and in vitro. Moreover, we also found that there was a time-dependent effect on Pb-caused the decrease of GPx4 and SelM mRNA expression in the brain tissues and the decrease of SelU mRNA expression in the embryonic neurocytes. In addition, multivariate correlation analysis showed that there were positive relationships among the twenty-five selenoproteins in the chicken brain tissues and embryonic neurocytes. Huang et al. [26] also found that there were positive relationships among twenty-five selenoproteins in chicken testes. PCA revealed that the twenty-five selenoproteins belonged to PC 1 in the chicken brain tissues and embryonic neurocytes. Results of multivariate correlation analysis and PCA were confirmed the reliability of our results. The mechanisms of strong positive relationships among selenoproteins need to be further studied.
Excess Pb can cause oxidative stress, which is one possible mechanism of negative effect of Pb on organisms [33]. Oxidative stress can disrupt prooxidant/antioxidant balance in mammalian cells [33]. Pb caused oxidative stress and induced apoptosis in rat proximal tubular cells [34]. SOD, GPx, and MDA are antioxidant indexes. Pb decreased SOD and GPx activities, increased MDA content, and caused oxidative stress in mouse kidneys [35]. Arsenic exposure induced neurotoxicity and oxidative stress by the decrease of GPx activity, the increase of MDA content, and the changes of histopathology in chicken brain tissues [36]. Se alleviated Cd-caused the decrease of SOD and GPx activities, the increase of MDA content, and oxidative stress in chicken kidneys [37]. In accordance with the above researches, our study indicated that Se alleviated Pb-caused the decrease of SOD and GPx activities, the increase of MDA content, and oxidative stress in the chicken brain tissues and embryonic neurocytes. In addition, in our experiment, there was a time-dependent effect on Pb-induced the increase of MDA content in the chicken brain tissues. Schlorff et al. [38] also found that ethanol increased MDA content in a time-dependent manner in rat plasmas.
Apoptosis is a physiological cell suicide program that is essential for regulation of development and maintenance of homeostasis [39]. Oxidative stress induced apoptosis in rat proximal tubular cells [40]. Bcl-2 is known as an anti-apoptotic protein which protects cells from apoptosis [41]. Bcl-2 can inhibit the release of Cyt c from mitochondria and protect apoptosis induced by oxidative stress [41]. Pro-apoptotic proteins such as Bax can promote apoptosis [42]. p53 accumulates during apoptosis process [43, 44]. p53 can promote neuronal apoptosis by increasing Bax transcription [45]. The induction of p53 leads to Cyt c release [46]. The release of Cyt c activates Caspase-3 [10]. The activation of Caspase-3 induces apoptosis [10]. Our results also demonstrated the above mechanism. We found that Pb decreased mRNA and protein expression of Bcl-2; increased mRNA and protein expression of p53, Bax, Cyt c, and Caspase-3; and induced apoptosis in the chicken brain tissues and embryonic neurocytes via mitochondrial pathway. In our results, morphological changes also demonstrated that Pb caused apoptosis in the chicken brain tissues and embryonic neurocytes. Other researches were similar with our research results. Liu et al. [11] found that Pb caused ultrastructural changes and apoptosis in rat proximal tubular cells. Zhang et al. [47] found that molybdenum and Cd changed ultrastructural structure and induced apoptosis in duck spleens. Nickel chloride decreased Bcl-2 mRNA expression, increased Bax, Cyt c, and Caspase-3 mRNA expression, and induced apoptosis via mitochondrial pathway in chicken bursa of Fabricius [48]. Sodium fluoride decreased Bcl-2 protein expression, increased Bax and Caspase-3 protein expression, and induced apoptosis in a time- and dose-dependent manner in mice splenic lymphocytes via mitochondria pathway [49]. Flora et al. [9] demonstrated that Pb decreased Bcl-2 protein expression, increased p53, Bax, Cyt c, and Caspase-3 protein expression, and induced apoptosis in rat brains. Pb caused the disruption of mitochondrial structure, the release of Cyt c, the activation of Caspase-3, and apoptosis in rat proximal tubular cells [11]. In addition, multivariate correlation analysis showed that there were positive relationships among four apoptosis-related genes (p53, Bax, Cyt c, and Caspase-3). There were negative relationships between Bcl-2 and four apoptosis-related genes (p53, Bax, Cyt c, and Caspase-3) in the chicken brain tissues and embryonic neurocytes. The PCA also revealed that Bcl-2 and four apoptosis-related genes (p53, Bax, Cyt c, and Caspase-3) belonged to different components in the chicken brain tissues and embryonic neurocytes. In our study, we also found that there were positive relationships between twenty-five selenoproteins and Bcl-2; and there were negative relationships between twenty-five selenoproteins and four apoptosis-related genes (p53, Bax, Cyt c, and Caspase-3) in the chicken brain tissues and embryonic neurocytes. The PCA also revealed that twenty-five selenoproteins and Bcl-2 belonged to PC 1. Twenty-five selenoproteins are closely related with Bcl-2. Other researches have also found similar mechanism. Yao et al. [30] reported that H2O2 caused the decrease of SelW and Bcl-2 protein expression in chicken myoblasts. Wang et al. [50] found that there were strong positive relationships between Bcl-2 and ten selenoproteins (GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, SelS, SelI, Selpb, and Sep15); there were strong negative relationships between Cyt c and eight selenoproteins (GPx4, Dio1, SelS, SelH, SelI, Selpb, Sepp1, and Sep15); and there were strong negative relationships between Caspase-3 and ten selenoproteins (GPx3, GPx4, Dio1, Txnrd1, Txnrd2, Txnrd3, SelS, SelI, Selpb, and SPS2) in chick embryonic vascular smooth muscle cells. The mechanisms of positive relationships between twenty-five selenoproteins (GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2) and Bcl-2, and negative relationships between twenty-five selenoproteins and the four apoptosis-related genes (p53, Bax, Cyt c, and Caspase-3) need to be further studied. Se can alleviate apoptosis induced by heavy metals via mitochondria pathway [14]. Se was found to prevent Cd-induced apoptosis mediated by oxidative stress via ameliorating mitochondrial dysfunction in porcine renal epithelial cells [51]. Wang et al. [14] found that Se alleviated Cd-caused the decrease of Bcl-2 protein expression, the increase of Bax protein expression, and apoptosis via mitochondria pathway in mouse kidneys. Se alleviated Cd-caused the decrease of Bcl-2 mRNA expression; the increase of p53, Bax, Cyt c, and Caspase-3 mRNA expression; and apoptosis via mitochondrial pathway in chicken splenic lymphocytes in vitro [21]. Consistent with these reports, our results indicated that Se alleviated Pb-caused the decrease of Bcl-2 mRNA and protein expression, the increase of p53, Bax, Cyt c, and Caspase-3 mRNA and protein expression, and apoptosis via mitochondrial pathway in the chicken brain tissues and embryonic neurocytes. Moreover, our morphological examination revealed that Se alleviated Pb-caused apoptosis in the chicken brain tissues and embryonic neurocytes.
In summary, our data indicated that Pb caused morphological changes; decreased SOD and GPx activities; increased MDA content; decreased GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2 mRNA expression, Bcl-2 mRNA and protein expression; and increased p53, Bax, Cyt c, and Caspase-3 mRNA and protein expression in the chicken brain tissues and embryonic neurocytes. Se alleviated Pb-caused all of the above changes in the chicken brain tissues and embryonic neurocytes. There were time-dependent manners on GPx4, SelM, and MDA in the chicken brain tissues; and on SelU in the chicken embryonic neurocytes. Consistent with researches in mammals, our data demonstrated that Se alleviated Pb-induced apoptosis in the chicken nervous tissues via mitochondrial pathway.
MATERIALS AND METHODS
Animal model and tissue samples
One hundred and eighty 1-day-old healthy Hyline male chickens were fed a standard commercial diet (containing 0.49 mg/kg Se) and drinking water. Chickens were randomly divided into four groups (forty-five chickens per group) at 8 days of age. The control group was fed the standard commercial diet and drinking water. The Se group was fed sodium selenite (Na2SeO3, analytical reagent grade, Tianjinzhiyuan Chemical Reagent Co. Ltd., Tianjin, China) added to the standard commercial diet at 1 mg/kg Se and drinking water. The Pb group was fed the standard commercial diet and lead acetate ((CH3COO)2Pb, analytical reagent grade, Tianjinzhiyuan Chemical Reagent Co. Ltd., Tianjin, China) added to drinking water at 350 mg/L Pb, according to median lethal dose (1739.3 mg/kg body weight) for Pb in chickens [52] and the need for the chicken experiments in toxicology [53]. The Se/Pb group was fed Na2SeO3 added to the standard commercial diet at 1 mg/kg Se and (CH3COO)2Pb added to drinking water at 350 mg/L Pb. Food and water were provided ad libitum. The chickens were fed in the Laboratory Animal Center, Animal Medical College, Northeast Agricultural University (Harbin, China). All procedures used in this experiment were approved by the Northeast Agricultural University's Institutional Animal Care and Use Committee under the approved protocol number SRM-06.
Fifteen chickens per group were randomly selected and euthanized on the 30th, 60th, and 90th days of the experiment, respectively. Next, brain tissues were quickly removed and rinsed with ice-cold sterile deionized water. Some samples were immediately frozen in liquid nitrogen and stored at -80 °C to determine mRNA expression of twenty-five selenoproteins (GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2); and mRNA and protein expression of five apoptosis-related genes (Bcl-2, p53, Bax, Cyt c, and Caspase-3). Some samples were immediately homogenized to detect SOD and GPx activities, and MDA content. The remaining samples were stored in 2.5% glutaraldehyde phosphate buffered saline (PBS, 0.1 M phosphate buffer with 0.85% sodium chloride, v/v, pH 7.2) to observe ultrastructure.
Chicken embryonic neurocytes
Fertilized eggs were hatched in artificial hatching incubator (38 °C, 50% humidity) for 6-9 days. Embryos were carefully removed from eggs under aseptic conditions. Brain tissues were obtained and put into a sterile Petri dish containing PBS and washed twice at room temperature. After meninges and vessels being removed, embryos were cut into small pieces (approximately 1-2 mm3) and transferred into a sterile Petri dish containing PBS with 0.125% trypsin (pH 7.2, Sigma-Aldrich, USA) at 37 °C in an atmosphere of 5% CO2 for 15 minutes. The Petri dish was brought out from the incubator and mixed complete Dulbecco's modified Eagle's medium (DMEM) culture solution (Gibco, USA) [50 mL of fetal bovine serum (FBS, Gibco, USA), 5 mL of penicillin/streptomycin (Sigma, USA), and 500 mL of DMEM] was put into the Petri dish. The Petri dish was rested for 2 minutes. Supernatant liquid was poured out. Neuronal cell suspension was washed twice in the same culture medium. The neuronal cell suspension was filtered with a sterile stainless steel mesh with 400 mm pore size. Cell density was adjusted to 5 × 106 cells/mL with the same culture medium. Cell viability was detected using trypan blue exclusion test and was above 95%. Na2SeO3 at 10−7 mol/L Se in the Se group, (CH3COO)2Pb at 10−6 mol/L Pb in the Pb group, and Na2SeO3 at 10−7 mol/L Se and (CH3COO)2Pb at 10−6 mol/L Pb in the Se/Pb group were immediately added to the culture medium, respectively. Embryonic neurocytes in the control, Se, Pb, and Se/Pb groups were cultured in poly-l-lysine (Sigma-Aldrich, USA)-coated (0.1%, 4 hours) cell culture plates for 12, 24, 36, and 48 hours. Supernatant was poured out. 1 mL of RNAiso Plus was added into the culture plates. The embryonic neurocytes were used to perform cell morphology, mRNA and protein expression of five apoptosis-related genes, mRNA expression of twenty-five selenoproteins, SOD and GPx activities, and MDA content.
Determination of IC50 of Pb in chicken embryonic neurocytes
The IC50 of chicken embryonic neurocytes in the Pb and Se/Pb groups for 48 hours was measured using cell counting kit-8 produced by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's instructions. In the Pb group, Pb concentration was 2, 4, 6, 8, 10, 12, 14, 16, and 18 × 10−7 mol/L, respectively. In the Se/Pb group, Pb concentration was 2, 4, 6, 8, 10, 12, 14, 16, and 18 × 10−7 mol/L, respectively; and Se concentration was 10 × 10−8 mol/L.
Ultrastructure
Brain tissue specimens were embedded in araldite (Sigma-Aldrich, USA). Ultrathin section was stained with Mg-uranyl acetate and lead citrate (Sigma-Aldrich, USA) for transmission electron microscope observation. Specimen handling procedure was performed following the method described in our previous study [54].
Cell morphology
Apoptosis observation of chicken embryonic neurocytes was performed using acridine orange (Amrisco, USA) and ethidium bromide (Sigma-Aldrich, USA) double staining. Cell staining process was performed following the method described in our previous study [55].
SOD and GPx activities, and MDA content
SOD and GPx activities, and MDA content were examined using SOD, GPx, and MDA detection kits according to the instructions of the reagent company (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Relative mRNA expression of five apoptosis-related genes and twenty-five selenoproteins
Primer sequences (Table 3) of β-actin; five apoptosis-related genes (Bcl-2, p53, Bax, Cyt c, and Caspase-3); and twenty-five selenoproteins (GPx1, GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, SelT, SelK, SelS, SelH, SelM, SelU, SelI, SelO, Selpb, Sepn1, Sepp1, Sepx1, Sepw1, Sep15, and SPS2) published in GenBank were synthesized by Invitrogen Biotechnology Co. Ltd., Shanghai, China. β-actin was used as an internal reference gene. Total RNA was extracted using RNAiso Plus reagent (Takara, Japan), and then RNA was reverse transcribed in 40 μL of reaction mixture according to the manufacturer's instructions (Invitrogen, USA).
Table 3. Primers used for quantitative real-time PCR.
| Gene | Serial number | Primer sequence | |
|---|---|---|---|
| GPx1 | NM_001277853.1 | F: 5’-ACGGCGCATCTTCCAAAG-3’ | R: 5’-TGTTCCCCCAACCATTTCTC-3’ |
| GPx2 | NM_001277854.1 | F: 5’-ATCGCCAAGTCCTTCTACGA-3’ | R: 5’-ACGTTCTCGATGAGGACCAC-3’ |
| GPx3 | NM_001163232.2 | F: 5’-CCTGCAGTACCTCGAACTGA-3’ | R: 5’-CTTCAGTGCAGGGAGGATCT-3’ |
| GPx4 | AF498316.2 | F: 5’-CTTCGTCTGCATCATCACCAA-3’ | R: 5’-TCGACGAGCTGAGTGTAATTCAC-3’ |
| Txnrd1 | NM_001030762.2 | F: 5’-TACGCCTCTGGGAAATTCGT-3’ | R: 5’-CTTGCAAGGCTTGTCCCAGTA-3’ |
| Txnrd2 | NM_001122691.1 | F: 5’-GCTCTTAAAGATGCCCAGCACTAC-3’ | R: 5’-GAACAGCTTGAGCCATCACAGA-3’ |
| Txnrd3 | NM_001122777.1 | F: 5’-CCTGGCAAAACGCTAGTTGTG-3’ | R: 5’-CGCACCATTACTGTGACATCTAGAC-3’ |
| Dio1 | NM_001097614.1 | F: 5’-GCGCTATACCACAGGCAGTA-3’ | R: 5’-GGTCTTGCAAATGTCACCAC-3’ |
| Dio2 | NM_204114.3 | F: 5’-ATTTGCTGATCACGCTTCAG-3’ | R: 5’-GCTCAGAAACAGCACCATGT-3’ |
| Dio3 | NM_001122648.1 | F: 5’-CTGTGCATTCGCAAGAAGAT-3’ | R: 5’-GCCGACTTGAAGAAGTCCAG-3’ |
| SelT | NM_001006557.3 | F: 5’-AGGAGTACATGCGGGTCATCA-3’ | R: 5’-GACAGACAGGAAGGATGCTATGTG-3’ |
| SelK | NM_001025441.2 | F: 5’-ATGACGACCACCCTCACGAT-3’ | R: 5’-CCAGCGTTAACCGGAATGAT-3 |
| SelS | NM_173120.2 | F: 5’-GCCTGCGTCGCCATCTATCTCA-3’ | R: 5’-TTCTGCCTTCGCTTCTGTTCTTCAA-3’ |
| SelH | NM_001277865.1 | F: 5’-CATCGAGCACTGCCGTAG-3’ | R: 5’-GACACCTCGAAGCTGTTCCT-3’ |
| SelM | NM_001277859.1 | F: 5’-AAGAAGGACCACCCAGACCT-3’ | R: 5’-GCTGTCCTGTCTCCCTCATC-3’ |
| SelU | NM_001193518.1 | F: 5’-GATGCTTTCAGGCTTCTTCC-3’ | R: 5’-CTGTCTTCCTGCTCCAATCA-3’ |
| SelI | NM_001031528.2 | F: 5’-TGCCAGCCTCTGAACTGGAT-3’ | R: 5’-TGCAAACCCAGACATCACCAT-3’ |
| SelO | NM_001115017.1 | F: 5’-CCAGCGTTAACCGGAATGAT-3’ | R: 5’-GCCTACAGAATGGATCCAACTGA-3’ |
| Selpb | XM_003641687.2 | F: 5’-AGGCCAACAGTACCATGGAG-3’ | R: 5’-GTGGTGAGGATGGAGATGGT-3’ |
| Sepn1 | NM_001114972.1 | F: 5’-CAGGATCCATGCTGAGTTCCA-3’ | R: 5’-GAGAGGACGATGTAACCCGTAAAC-3’ |
| Sepp1 | NM_001031609 | F: 5’-CCAAGTGGTCAGCATTCACATC-3’ | R: 5’-ATGACGACCACCCTCACGAT-3’ |
| Sepx1 | NM_001135558.2 | F: 5’-TGGCAAGTGTGGCAATGG-3’ | R: 5’-GAATTTGAGCGAGCTGCTGAAT-3’ |
| Sepw1 | NM_001166327.1 | F: 5’-TGGTGTGGGTCTGCTTTACG-3’ | R: 5’-CCAAAGCTGGAAGGTGCAA-3’ |
| Sep15 | NM_001012926.2 | F: 5’-ACTTGGCTTCTCCAGTAACTTGCT-3’ | R: 5’-GCCTACAGAATGGATCCAACTGA-3’ |
| SPS2 | BM489698.1 | F: 5’-CGTTGGGTATCGGAACTGAC-3’ | R: 5’-CGTCCACCAGAGGGTAGAAA-3’ |
| Bcl-2 | NM_205339.1 | F: 5’-ATCGTCGCCTTCTTCGAGTT-3’ | R: 5’-ATCCCATCCTCCGTTGTCCT-3’ |
| p53 | NM_205264.1 | F: 5’-GAGATGCTGAAGGAGATCAATGAG-3’ | R: 5’-GTGGTCAGTCCGAGCCTTTT-3’ |
| Bax | FJ977571.1 | F: 5’-GATGAAGCCACCCAGCAGTA-3’ | R: 5’-TGGATTCTCACAGTAGGAGGATG-3’ |
| Cyt c | K02303.1 | F: 5’-AGGCAAGCACAAGACTGGA-3’ | R: 5’-CTGACTATCACCAAGAACCACC-3’ |
| Caspase-3 | NM_204725.1 | F: 5’-CATCTGCATCCGTGCCTGA-3’ | R: 5’-CTCTCGGCTGTGGTGGTGAA-3’ |
| β-actin | L08165 | F: 5’-CCGCTCTATGAAGGCTACGC-3’ | R: 5’-CTC TCG GCT GTG GTGGTG AA-3’ |
Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR® Premix Ex TaqTM II (Takara, Japan) with the Applied Biosystems PRISM 7500 real-time PCR system according to the manufacturer's instructions (Applied Biosystems, Foster, USA). The experiment was repeated three times for each sample. Relative mRNA abundance for each gene was calculated according to the method of 2−ΔΔCt [56].
Relative protein expression of five apoptosis-related genes
Relative protein expression of five apoptosis-related genes (Bcl-2, p53, Bax, Cyt c, and Caspase-3) was measured using Western blot assay followed the method described in previous study (28). The first antibody (1:100), secondary antibody (1:1000), monoclonal β-actin antibody (1:1000), and goat antimouse IgG (1:1000) of Bcl-2, p53, Bax, Cyt c, and Caspase-3 were purchased from Santa Cruz Biotechnology, USA. The signal was detected using X-ray films (TianGen Biotech Co. Ltd., Beijing, China). The optical density of each band was measured using Image VCD gel imaging system (Beijing Sage Creation Science And Technology Co. Ltd., China).
Statistical analysis
All data were performed using SPSS for Windows (version 19; SPSS Inc., Chicago, IL, USA). Statistical comparisons for all data were performed using one-way or two-way analysis of variance (ANOVA) and verified by nonparametric Kruskal-Wallis ANOVA and Mann-Whitney U test. Data were expressed as the mean ± standard deviation (n = 5). Relative mRNA expression data of twenty-five selenoproteins and five apoptosis-related genes were used to perform multivariate correlation analysis and principal component analysis (PCA). Multivariate correlation analysis was used to measure linear correlations among determined factors. PCA was used to define the most important parameters, which could be used as key factors for individual variations.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
The authors thank all the members of environmental toxicology laboratory for their help in collecting brain tissue samples at the College of Animal Science and Technology, Northeast Agricultural University.
Abbreviations
- Se
selenium
- Pb
lead
- (CH3COO)2Pb)
lead acetate
- Na2SeO3
sodium selenite
- mRNA
messenger RNA
- GPx
glutathione peroxidase
- SOD
superoxide dismutase
- Txnrd
thioredoxin reductases
- Dio
iodothyronine deiodinases
- SelT
selenoprotein T
- Sepn1
selenoprotein n1
- Sep15
15-kDa selenoprotein
- SPS2
selenophosphate synthetases 2
- Bcl-2
B-cell lymphoma-2
- MDA
malondialdehyde
- Bax
Bcl-2 associated X protein
- Cyt c
cytochrome c
- PC 12
rat adrenal medulla pheochromocytoma cells
- Cd
cadmium
- IC50, 50%
inhibitory concentration
- PBS
phosphate buffered saline
- DMEM
dulbecco's modified eagle's medium
- PCR
polymerase chain reaction
- PCA
principal component analysis
- ANOVA
analysis of variance
- H2O2
hydrogen peroxide
Author contributions
Xiaohua Teng conceived and designed experiment. Yihao Zhu, Xiaoyan Jiao, and Yang An performed the experiment. Yihao Zhu analyzed data and wrote manuscript. Xiaohua Teng and Shu Li revised the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This study was funded by the Heilongjiang Province on Natural Fund Project of China (No. C201420).
REFERENCES
- 1.Onianwa PC, Fakayode SO. Lead contamination of topsoil and vegetation in the vicinity of a battery factory in Nigeria. Environ Geochem Health. 2000;22:211–218. [Google Scholar]
- 2.Binkowski ŁJ, Sawicka-Kapustab K. Lead poisoning and its in vivo biomarkers in Mallard and Coot from two hunting activity areas in Poland. Chemosphere. 2015;127:101–108. doi: 10.1016/j.chemosphere.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Counter SA, Buchanan LH, Ortegae F. Zinc protoporphyrin levels, blood lead levels and neurocognitive deficits in Andean children with chronic lead exposure. Clin Biochem. 2008;41:41–47. doi: 10.1016/j.clinbiochem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Khalil N, Morrow LA, Needleman H, Talbott EO, Wilson JW, Cauley JA. Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology. 2009;23:10–19. doi: 10.1037/a0013757. [DOI] [PubMed] [Google Scholar]
- 5.Tavernier P, Roels S, Baert K, Hermans K, Pasmans F, Chiers K. Lead intoxication by ingestion of lead shot in racing pigeons (Columba livia) Vlaams Diergeneeskd Tijdschr. 2004;73:307–309. [Google Scholar]
- 6.Dey PM, Burger J, Gochfeld M, Reuhl KR. Developmental lead exposure disturbs expression of synaptic neural cell adhesion molecules in herring gull brains. Toxicology. 2000;146:137–147. doi: 10.1016/s0300-483x(00)00171-2. [DOI] [PubMed] [Google Scholar]
- 7.Nan A, Zhou XK, Chen LJ, Liu ML, Zhang N, Zhang L, Luo YW, Liu ZZ, Dai LJ, Jiang YG. A transcribed ultraconserved noncoding RNA, Uc.173, is a key molecule for the inhibition of lead-induced neuronal apoptosis. Oncotarget. 2016;7:112–124. doi: 10.18632/oncotarget.6590. https://doi.org/10.18632/oncotarget.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye F, Li XY, Li LL, Lyu L, Yuan J, Chen J. The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem Toxicol. 2015;86:191–201. doi: 10.1016/j.fct.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Flora SJ, Gautam P, Kushwaha P. Lead and ethanol co-exposure lead to blood oxidative stress and subsequent neuronal apoptosis in rats. Alcohol Alcohol. 2012;47:92–101. doi: 10.1093/alcalc/agr152. [DOI] [PubMed] [Google Scholar]
- 10.Rahman MM, Ukiana J, Uson-Lopez R, Sikder MT, Saito T, Kurasaki M. Cytotoxic effects of cadmium and zinc co-exposure in PC12 cells and the underlying mechanism. Chem Biol Interact. 2017;269:41–49. doi: 10.1016/j.cbi.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L. Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. 2016;90:1193–209. doi: 10.1007/s00204-015-1547-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZW, Bi MY, Liu Q, Yang J, Xu SW. Meta-Analysis of the correlation between selenium and incidence of hepatocellular carcinoma. Oncotarget. 2016;7:77110–77116. doi: 10.18632/oncotarget.12804. https://doi.org/10.18632/oncotarget.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Fu HJ, Xiao YM, Ai BM, Wei Q, Wang SY, Liu T, Ye LQ, Hu QS. Effects of low-level organic selenium on lead-induced alterations in neural cell adhesion molecules. Brain Res. 2013;1530:76–81. doi: 10.1016/j.brainres.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wu YC, Luo K, Liu YX, Zhou M, Yan S, Shi H, Cai YQ. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol. 2013;58:61–67. doi: 10.1016/j.fct.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Li XJ, Xing MY, Chen MH, Zhao JX, Fan RF, Zhao X, Cao CY, Yang J, Zhang ZW, Xu SW. Effects of selenium-lead interaction on the gene expression of inflammatory factors and selenoproteins in chicken neutrophils. Ecotoxicol Environ Safety. 2017;139:447–453. doi: 10.1016/j.ecoenv.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG. Gene Expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of Se-deficient chicks. J Nutr. 2013;143:613–619. doi: 10.3945/jn.112.172395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messaoudi I, Banni M, Saïd L, Saïd K, Kerkeni A. Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem Biol Interact. 2010;188:94–101. doi: 10.1016/j.cbi.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhao WC, Liu W, Chen X, Zhu YH, Zhang ZW, Yao HD, Xu SW. Four endoplasmic reticulum resident selenoproteins may be related to the protection of selenium against cadmium toxicity in chicken lymphocytes. Biol Trace Elem Res. 2014;161:328–333. doi: 10.1007/s12011-014-0135-0. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrenner H, Alili L, Bilgic E, Sies H, Brenneisen P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med. 2006;40:1513–1523. doi: 10.1016/j.freeradbiomed.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Abdollahi M, Rahmat-Jirdeh N, Soltaninejad K. Protection by selenium of lead-acetate-induced alterations on rat submandibular gland function. Hum Exp Toxicol. 2001;20:28–33. doi: 10.1191/096032701667736070. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Xu FP, Yang ZJ, Li M, Min YH, Li S. Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: mechanisms of oxidative stress and apoptosis. Biol Trace Elem Res. 2014;160:340–351. doi: 10.1007/s12011-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 22.Gao H, Liu CP, Song SQ, Fu J. Effects of dietary selenium against lead toxicity on mRNA levels of 25 selenoprotein genes in the cartilage tissue of broiler chicken. Biol Trace Elem Res. 2016;172:234–241. doi: 10.1007/s12011-015-0579-x. [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Xu Z, Zhao X, Chen MH, Xu SW. The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere. 2017;180:259–266. doi: 10.1016/j.chemosphere.2017.03.130. [DOI] [PubMed] [Google Scholar]
- 24.Jiao XY, Yang K, An Y, Teng XJ, Teng XH. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res. 2017;24:7555–7564. doi: 10.1007/s11356-016-8329-y. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Wang K, Huang H, Gu XH, Teng XH. Alleviative effect of selenium on inflammatory damage caused by lead via inhibiting inflammatory factors and heat shock proteins in chicken testes. Environ Sci Pollut Res. 2017;24:13405–13413. doi: 10.1007/s11356-017-8785-z. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Wang Y, An Y, Tian YG, Li S, Teng XH. Selenium for the mitigation of toxicity induced by lead in chicken testes through regulating mRNA expressions of HSPs and selenoproteins. Environ Sci Pollut Res. 2017;24:14312–14321. doi: 10.1007/s11356-017-9019-0. [DOI] [PubMed] [Google Scholar]
- 27.Cao CY, Fan RF, Zhao JX, Zhao X, Yang J, Zhang ZW, Xu SW. Impact of exudative diathesis induced by selenium deficiency on LncRNAs and their roles in the oxidative reduction process in broiler chick veins. Oncotarget. 2017;8:20695–20705. doi: 10.18632/oncotarget.14971. https://doi.org/10.18632/oncotarget.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 29.Yao HD, Liu W, Zhao WC, Fan RF, Zhao X, Khoso PA, Zhang ZW, Xu SW. Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. RSC Adv. 2014;4:64032–64042. [Google Scholar]
- 30.Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta. 2013;1830:3112–3120. doi: 10.1016/j.bbagen.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Yang TS, Zhao ZP, Liu TQ, Zhang ZW, Wang PZ, Xu SW, Lei XG, Shan AS. Oxidative stress induced by Se-deficient high-energy diet implicates neutrophil dysfunction via Nrf2 pathway suppression in swine. Oncotarget. 2017;8:13428–13439. doi: 10.18632/oncotarget.14550. https://doi.org/10.18632/oncotarget.14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao HD, Fan RF, Zhao X, Zhao WC, Liu W, Yang J, Sattar H, Zhao JX, Zhang ZW, Xu SW. Selenoprotein W redox-regulated Ca2+ channels correlate with selenium deficiency-induced muscles Ca2+ leak. Oncotarget. 2016;7:57618–57632. doi: 10.18632/oncotarget.11459. https://doi.org/10.18632/oncotarget.11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Lin SQ, Li ZF, Yang DB, Wang ZY. Protective effects of puerarin on experimental chronic lead nephrotoxicity in immature female rats. Hum Exp Toxicol. 2013;32:172–185. doi: 10.1177/0960327112462729. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Wang H, Hu MZ, Cao J, Chen DW, Liu ZP. Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. 2009;83:417–427. doi: 10.1007/s00204-009-0425-z. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZC, Gao XJ, Guo MY, Jiang HC, Cao YG, Zhang NS. The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol Trace Elem Res. 2017;175:129–135. doi: 10.1007/s12011-016-0731-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhao PP, Guo Y, Zhang W, Chai HL, Xing HJ, Xing MW. Neurotoxicity induced by arsenic in Gallus Gallus: Regulation of oxidative stress and heat shock protein response. Chemosphere. 2017;166:238–245. doi: 10.1016/j.chemosphere.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Yang B, Cheng Y, Lin H. Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biol Trace Elem Res. 2015;167:308–319. doi: 10.1007/s12011-015-0314-7. [DOI] [PubMed] [Google Scholar]
- 38.Schlorff EC, Husain K, Somani SM. Dose- and time-dependent effects of ethanol on plasma antioxidant system in rat. Alcohol. 1999;17:97–105. doi: 10.1016/s0741-8329(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 39.Ognjanović BI, Marković SD, Pavlović SZ, Zikić RV, Stajn AS, Saicić ZS. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res. 2008;57:403–411. doi: 10.33549/physiolres.931197. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Wang H, Li JG, Chen DW, Liu ZP. Simultaneous effects of lead and cadmium on primary cultures of rat proximal tubular cells: interaction of apoptosis and oxidative stress. Arch Environ Contam Toxicol. 2011;61:500–511. doi: 10.1007/s00244-011-9644-4. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi A, Masuda A, Sun M, Centonze VE, Herman B. Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm) Brain Res Bull. 2004;62:497–504. doi: 10.1016/j.brainresbull.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Kudryavtseva AV, Krasnov GS, Dmitriev AA, Alekseev BY, Kardymon OL, Sadritdinova AF, Fedorova MS, Pokrovsky AV, Melnikova NV, Kaprin AD, Moskalev AA, Snezhkina AV. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7:44879–44905. doi: 10.18632/oncotarget.9821. https://doi.org/10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JJ, Guo WH, Zhou H, Luo N, Nie CL, Zhao XY, Yuan Z, Liu XY, Wei YQ. Mitochondrial p53 phosphorylation induces Bak-mediated and caspase-independent cell death. Oncotarget. 2015;6:17192–17205. doi: 10.18632/oncotarget.3780. https://doi.org/10.18632/oncotarget.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billard C. Apoptosis inducers in chronic lymphocytic leukemia. Oncotarget. 2014;5:309–325. doi: 10.18632/oncotarget.1480. https://doi.org/10.18632/oncotarget.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou AH, Lin AC, Hong KY, Hu SH, Chen YL, Chen JY, Wang HL. p53 activation mediates polyglutamine-expanded ataxin-3 upregulation of Bax expression in cerebellar and pontine nuclei neurons. Neurochem Int. 2011;58:145–152. doi: 10.1016/j.neuint.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, Ichimiya M, Sengupta S, Mechanic L, Okamura S, Hofseth LJ, Moake M, Nagashima M, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhang MM, Luo JR, Zhang CY, Cao HB, Xia B, Hu GL. Alterations in antioxidant function and cell apoptosis in spleens of duck exposed to molybdenum or/and cadmium. J Vet Sci. 2017;18:193–200. doi: 10.4142/jvs.2017.18.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin S, Cui HM, Peng X, Fang J, Zuo ZC, Deng JL, Wang X, Wu BY, Guo HR. Toxic effect of NiCl2 on development of the bursa of Fabricius in broiler chickens. Oncotarget. 2015;7:125–139. doi: 10.18632/oncotarget.6591. https://doi.org/10.18632/oncotarget.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng H, Kuang P, Cui H, Chen L, Fang J, Zuo Z, Deng J, Wang X, Zhao L. Sodium fluoride induces apoptosis in cultured splenic lymphocytes from mice. Oncotarget. 2016;7:67880–67900. doi: 10.18632/oncotarget.12081. https://doi.org/10.18632/oncotarget.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang QY, Huang JQ, Zhang H, Lei XG, Du ZY, Xiao C, Chen SL, Ren FZ. Selenium deficiency-induced apoptosis of chick embryonic vascular smooth muscle cells and correlations with 25 selenoproteins. Biol Trace Elem Res. 2017;176:407–415. doi: 10.1007/s12011-016-0823-z. [DOI] [PubMed] [Google Scholar]
- 51.Zhou YJ, Zhang SP, Liu CW, Cai YQ. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK 1 cells. Toxicol In Vitro. 2009;23:288–294. doi: 10.1016/j.tiv.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Vengris VE, Maré CJ. Lead poisoning in chickens and the effect of lead on interferon and antibody production. Can J Comp Med. 1974;38:328–335. [PMC free article] [PubMed] [Google Scholar]
- 53.Klaassen C. Casarett & Doull's Toxicology: The Basic Science of Poisons. McGraw-Hill; New York: 2013. [Google Scholar]
- 54.Liu C, Sun ZP, Xu Z, Liu TQ, Pan TR, Li S. Down-regulation of microRNA-155 promotes selenium deficiency-induced apoptosis by tumor necrosis factor receptor superfamily member 1B in the broiler spleen. Oncotarget. 2017;8:58513–58525. doi: 10.18632/oncotarget.17222. https://doi.org/10.18632/oncotarget.17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Pan T, Wan N, Sun ZP, Zhang ZW, Li S. Cadmium-induced endoplasmic reticulum stress in chicken neutrophils is alleviated by selenium. J Inorg Biochem. 2017;170:169–177. doi: 10.1016/j.jinorgbio.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.