Abstract
There is a need among patients suffering from drug‐resistant epilepsy (DRE) for more efficient and less toxic treatments. The objective of the present study was to assess the safety, feasibility, and potential efficacy of autologous bone marrow cell transplantation in pediatric patients with DRE. Two females and two males (11 months to 6 years) were enrolled and underwent a combined therapy consisting of autologous bone marrow nucleated cells (BMNCs) transplantation (intrathecal: 0.5 × 109; intravenous: 0.38 × 109–1.72 × 109) followed by four rounds of intrathecal bone marrow mesenchymal stem cells (BMMSCs) transplantation (18.5 × 106–40 × 106) every 3 months. The BMMSCs used were a unique population derived from CD271‐positive cells. The neurological evaluation included magnetic resonance imaging, electroencephalography (EEG), and cognitive development assessment. The characteristics of BMMSCs were evaluated. Four intravenous and 20 intrathecal transplantations into the cerebrospinal fluid were performed. There were no adverse events, and the therapy was safe and feasible over 2 years of follow‐up. The therapy resulted in neurological and cognitive improvement in all patients, including a reduction in the number of epileptic seizures (from 10 per day to 1 per week) and an absence of status epilepticus episodes (from 4 per week to 0 per week). The number of discharges on the EEG evaluation was decreased, and cognitive improvement was noted with respect to reactions to light and sound, emotions, and motor function. An analysis of the BMMSCs' characteristics revealed the expression of neurotrophic, proangiogenic, and tissue remodeling factors, and the immunomodulatory potential. Our results demonstrate the safety and feasibility of BMNCs and BMMSCs transplantations and the considerable neurological and cognitive improvement in children with DRE. stem cells translational medicine 2018;7:20–33
Keywords: Cell therapy, Bone marrow, Mesenchymal stem cells, Drug‐resistant epilepsy, Transplantation
Significance Statement.
This study, to the authors' knowledge, for the first time demonstrates safety, feasibility, and considerable neurological and cognitive improvement in children with drug‐resistant epilepsy after bone marrow nucleated cells and bone marrow mesenchymal stem cells transplantations. Multiple transplantations were performed, causing no adverse events during 2 years of follow‐up. The authors observed neurological and cognitive improvement in all patients, significant reduction in a number of epileptic seizures, and absence of status epilepticus episodes. The therapy decreased the number of discharges in electroencephalography evaluation and caused cognitive improvement in the sphere of reaction to light and sound, in the sphere of emotions, and in the sphere of motor function.
Introduction
Epilepsy is a serious neurological disorder that affects 65 million people worldwide and is characterized by excessive electrical discharges in the brain that cause progressive neural cell damage and subsequent loss 1, 2. Different types of epilepsy are recognized. Because of etiology, it is divided into four categories: idiopathic, symptomatic, provoked, and cryptogenic. We can also divide the epilepsy because of its localization (e.g., temporal lobe epilepsy). Twenty to forty percent of all epilepsy patients suffer from drug‐resistant epilepsy (DRE) 3. DRE is defined as recurrent seizures that are refractory to regimens of two or more antiepileptic drugs 4. DRE is difficult to treat, causing serious clinical burdens, including disability, mortality, comorbidities, social stigmas, as well as psychological and economic costs 3.
Additionally, some patients with DRE experience status epilepticus (SE), a life‐threatening condition characterized by seizures lasting 30 minutes or longer. SE requires prompt recognition and immediate action 5, 6; however, even with adequate treatment, the overall mortality of SE is as high as 30%. SE survivors have serious morbidities, including developmental delays, cognitive impairments, chronic epilepsy, and recurrent SE 7, 8, 9, 10.
The established standard treatment for DRE and SE includes corticosteroids, adrenocorticotropic hormone, immunoglobulins, plasmapheresis, and monoclonal antibodies 11. All of the existing alternative SE treatments, including hippocampal resection surgery, a ketogenic diet, and use of cannabinols, are connected with serious side effects. Causal treatments that target the dynamic and complex process of epileptogenesis have not been developed for SE 12.
It is well known that seizures cause neuronal death, leading to the development of an inflammatory response and an immunological reaction. In addition, the loss of gamma‐amino butyric acid (GABA)‐ergic interneurons and anticonvulsant glial‐derived neurotrophic factor (GDNF)‐expressing astrocytes results in a decreased threshold of neuronal excitation 13, 14. Thus, to target DRE both in the context of SE and as an independent entity, basic pathological and physiological processes should be addressed, including chronic immunological reactions, inflammation, neuroprotection, and the restoration of the proper excitation threshold. The lack of efficient antiepileptic drug therapy and the undesirability of alternative methods, especially neurosurgery for intractable epilepsy, make it necessary to develop novel therapeutic approaches.
Different types of cell therapies have been developed in recent decades, with many therapies demonstrating clinical efficiency, for example, in myocardial infarction, wound healing, and graft‐versus‐host disease (GVHD). Indeed, some of these therapies have sparked hope for the treatment of previously incurable diseases 15, 16. Cell therapy was also shown to induce considerable improvement in cases of peripheral nervous system regeneration after spinal cord injuries 17. Following encouraging results in the peripheral nervous system, other cell therapy approaches have been reported to improve central nervous system (CNS) regeneration 18.
Cell therapy for the treatment of epilepsy comprises a few primary approaches. The first includes the surgical insertion of fetal hippocampal neurons or GABA‐producing interneurons. The aim of these therapies is to increase the level of inhibitory neurotransmitters and thereby raise seizure thresholds or dampen seizure propagation; however, such approaches do not address the loss of neurons due to inflammation 19. The second approach includes the viral delivery of inhibitory neurotransmitters 20 and supportive growth factors, including brain‐derived neurotrophic factor (BDNF), basic fibroblast growth factor (FGF‐2) 21, GDNF 22, and vascular endothelial growth factor (VEGF) 23. Lastly, the cell transplantation approach, which employs the intravenous, intraperitoneal, or intrahippocampal implantation of BMNCs and mesenchymal stem cells (MSCs), aims to influence the microenvironment of the injured region and stimulate regeneration 24, 25, 26, 27, 28, 29, 30, 31, 32, 33. Preclinical studies have demonstrated the usefulness of all cell therapy approaches in reducing the number of seizures, with some treatments also demonstrating cognitive benefits 28, 29, 30.
The first report of the transplantation of any cell type to treat epilepsy appeared in 1998 and described a 40% reduction in seizures frequency in one patient after porcine GABA neuron implantation 34. The results of a clinical trial using the same porcine GABA neurons demonstrated a moderate reduction of seizure number in three patients 35.
Preclinical epilepsy trials have demonstrated the antiepileptic potential of human cells 33, 36. However, there have been no reports describing human cell or tissue transplantation in patients with DRE.
The implantation of BMNCs and MSCs appears to be among the only proposed methods to truly address the root cause of the pathology. BMNCs and MSCs are well known to secrete a number of growth factors (e.g., VEGF, GDNF, hepatocyte growth factor [HGF]) that promote neurogenesis 37. In addition, the pathomorphological analysis of animals undergoing MSCs transplantations has confirmed the anti‐inflammatory effect of transplanted cells, which is considered to be one of the mechanisms leading to the observed clinical improvement.
Recent findings from the field of spinal cord injury suggest that MSCs have a pleiotropic nature and may profoundly impact central nervous system regeneration by activating neurogenesis. For example, the positive influence of MSCs on neurite sprouting, synapse integrity, angiogenesis stimulation, and immunomodulation could lead to decreased demyelination by T cells and to microglia polarization toward an M2‐like regenerative phenotype 38.
All previous preclinical and clinical trials using MSCs use an adherence‐based cell isolation procedure, followed by the in vitro expansion of a variable population of cells. In our cell therapy approach, we used a unique population of MSCs, with our initial cultures consisting of isolated CD271+ mesenchymal stem cells. Our studies 39 and those of others 40, 41, 42 have demonstrated a higher proliferation potential, level of produced cytokines, immunosuppressive abilities, vascularization stimulation, and chondral repair of MSCs derived from CD271+ BMNCs compared with those isolated with adherence‐based methods. This unique MSC population has already demonstrated great clinical potential in the treatment of spinal cord injury patient 17. Based on preclinical data and our previous experience, we performed a pilot study to ascertain whether a cell therapy approach consisting of BMNCs and CD271+ bone marrow mesenchymal stem cells (BMMSCs) implantation would be safe, feasible, and therapeutically effective in patients with DRE.
Materials and Methods
Basic Study Information
The presented study was a prospective, longitudinal experiment conducted from 2012 to 2014 at the University Children's Hospital in Cracow, Poland. The Jagiellonian University Medical College Bioethical Committee (KBET/241/L/2011) approved the pilot study. The parents of the enrolled pediatric patients provided written informed consent according to the Helsinki Declaration. However, the study has not been registered at NIH ClinicalTrials.gov. The general data collected before the experimental therapy consisted of age; sex; past medical history; the type of encephalopathy, including its etiology, the type of epileptic seizures, and their frequency; and a description of previous medical treatments using anti‐epileptic drugs.
Enrollment Criteria
Pediatric patients up to 6 years of age were included in the study. The study included patients with neural infection‐related (bacterial or viral) and postnatal encephalopathy, including hypoxic ischemic encephalopathy confirmed by magnetic resonance imaging (MRI). The diagnosis of DRE was supported by an electrophysiology examination EEG and ineffective drug therapy. The latter was defined as (a) the failure of an adequate trial of two tolerated, appropriately chosen antiepileptic drug schedules, with medication compliance or (b) having failed at least to two antiepileptic drugs, with at least one seizure per month for the previous interval between epileptic episodes or 12 months. All of the patients also presented with repeated episodes of SE.
Only children who had received no alternative treatments were included in the study. Children with a minimum of 6 months between the diagnosis and the initiation of experimental therapy were enrolled. These patients were required to have shown no improvement in their medical condition or a reduction in seizure episode number after standard and obligatory therapeutic methods. Moreover, it was required that the patients did not exhibit deterioration or significant improvement in their general neurological state within the 1‐month period preceding the experimental therapy. Patients with focal CNS lesions (e.g., neoplastic lesions, untreated vascular malformations) or chronic diseases (e.g., systemic diseases) that would require long‐term pharmacotherapy (corticosteroids, antibiotics, chemotherapeutics) were excluded from the study. The same neurologist examined all of the patients prior to the treatment.
Cells Preparation
Autologous bone marrow (BM) was harvested from the posterior superior iliac crest under general anesthesia, through aspiration from multiple punctures of the iliac ala. Bone marrow nucleated cells (BMNCs) and BMMSCs isolation procedures were described previously 17. Briefly, an isolation of BMNCs from BM suspension was performed using 10% hydroxyethyl starch solution. Sterility of the material was confirmed. BMNCs were isolated from the BM sample by a density gradient centrifugation. CD271+ BMMSCs population was isolated according to the manufacturer's instructions (Stem Cells Technology, Vancouver, Canada, https://www.stemcell.com). Cells were plated into tissue culture flasks in Dulbecco's‐modified Eagle medium supplemented with 10% fetal bovine serum (FBS), platelet‐derived growth factor (PDGF) and epidermal growth factor. After 7 days, medium was exchanged. At confluence, the adherent cells were detached and re‐seeded at 0.075 × 106 cells/75 cm2 flasks and incubated again until confluence. BMMSCs phenotype was analyzed with antibodies specific for MSCs (CD73, CD90, CD105) and lymphocytes (CD3, CD45) using FACSCalibur cytometer with Cellquest software (Becton Dickinson, Franklin Lakes, NJ, https://www.bd.com). Cytogenetic stability of cultured BMMSCs was confirmed via GTG banding and array comparative genomic hybridization (aCGH) analysis. During the neurosurgical procedure, the BMMSCs were administered intrathecally via lumbar puncture. All patients were subsequently transplanted with multiple rounds of cultured BMMSCs at passage 3 every 3–4 months using the same methodology.
Evaluation of the Cytogenetic Stability of Cultured Mesenchymal Stem Cells
Cytogenetic stability of cultured BMMSCs was confirmed via GTG banding. Metaphases were analyzed under an Olympus BX51 microscope with a camera (Olympus Corporation, Tokyo, Japan, https://www.olympus-global.com) to document photomicrographs. A Cytovision program (Leica Microsystem, Inc., Buffalo Grove, IL, https://www.leica-microsystems.com) was used to arrange chromosomes into a karyogram.
Molecular karyotyping was performed through aCGH with the SurePrint G3 CGH ISCA v2 Microarray Kit 8 × 60K (Agilent Technologies, Santa Clara, CA, https://www.agilent.com) according to the manufacturer's protocol. DNA for aCGH analysis was isolated from samples of cells prepared for autologous transplantations. DNA was extracted using the machine and isolation QuickGene DNA tissue S kit (Kurabo Industries, Osaka, Japan, http://www.kurabo.co.jp/). Arrays were analyzed through the Agilent scanner and the Feature Extraction software (v8.1). Graphical overview was obtained using the Agilent CytoGenomics software (v2.9.2.4).
Quantitative Real Time Polymerase Chain Reaction Analysis
Total RNA was extracted using Universal RNA purification Kit (EURx, Gdansk, Poland, https://eurx.com.pl/) according to manufacturer's protocol. The reverse polymerase transcription of mRNA was performed using Moloney Murine Leukemia Virus Reverse Transcriptase (M‐MLV RT) (Promega Corporation, Madison, WI, https://www.promega.com) as provided by vendor.
Genes expression were determined by Real‐Time quantitative Reverse Transcription Polymerase Chain Reaction (qRT‐PCR) analysis on ABI PRISM 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, https://www.thermofisher.com/us/en/home/brands/applied-biosystems.html) using Blank qPCR Master Mix (EURx) and the following Taq‐Man probes (Applied Biosystems) for human genes: glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) ‐ Hs02758991_g1, BDNF ‐ Hs02718934_s1, GDNF ‐ Hs01931883_s1, ciliary neurotrophic factor (CNTF) ‐ Hs04194755_s1, nerve growth factor (NGF) ‐ Hs01113193_m1, HGF ‐ Hs00300159_m1, PDGF‐A ‐ Hs00964426_m1, vascular endothelial growth factor receptor 1 (FLT‐1) ‐ Hs01052937_m1, VEGF‐A ‐ Hs00173626_m1‐, heparin‐binding growth factor 1 (FGF‐1) ‐ Hs01092738_m1, FGF‐2 (Hs00266645_m1), angiopoietin 1 (ANGPT‐1) ‐ Hs00375822_m1, metalloproteinase‐2 (MMP‐2) ‐ Hs00234422_m1, metalloproteinase‐9 (MMP‐9) ‐ Hs00234579_m1, indoleamine 2,3‐dioxygenase (IDO1) ‐ Hs00984148_m1, and tumor necrosis factor‐inducible gene 6 (TSG‐6) ‐ Hs01113602_m1. mRNA expression levels were quantified by the 2 −ΔCt calculation using housekeeping GAPDH as a control.
Western Blot Analysis
Protein was isolated with M‐PER lysing buffer (Thermo Fisher Scientific, Waltham, Massachusetts; https://www.thermofisher.com/pl/en/home.html) containing protease and phosphatase inhibitors (Sigma‐Aldrich, St. Louis, MO, https://www.sigmaaldrich.com). Western blot was performed with anti‐GAPDH rabbit mAb (Cell Signaling Technology, Danvers, MA, https://www.cellsignal.com) and anti‐VEGF rabbit pAb (Santa Cruz Biotechnology Inc., Santa Cruz, CA, https://www.scbt.com), as described previously 43.
Cytokines Stimulations
BMMSCs were seeded into 6‐well plate. After reaching 85% of confluence, standard medium was replaced by stimulation medium (2% FBS) in the absence or presence of interleukin 1 β (Il‐1β; Sigma‐Aldrich) or tumor necrosis factor α (TNF‐α), interferon γ (INF‐γ; PeproTech Inc., Rocky Hill, NJ, https://www.peprotech.com). IDO1 induction cells were treated with 100 ng/ml INF‐γ for 48 hour and for TSG‐6 were treated with 50 ng/ml Il‐1β or TNF‐α for 72 hours. Optimized stimulation protocols were described before 44, 45.
Implantation Procedures and Regimen
All of the BM‐derived cell implantation procedures were performed when the patients were stable, without contraindications for general anesthesia based on the opinion of internal medicine and cardiology and without any serious infectious diseases, including sepsis, immediately prior to the procedure. The BMNCs were transplanted intravenously (i.v.) and intrathecally, whereas the BMMSCs were injected only intrathecally. The intrathecal injections were performed into the cerebrospinal fluid via lumbar puncture. For i.v. infusions, the cells were suspended in 0.9% NaCl (B. Braun). For intrathecal infusions, the cells were suspended in a 5% glucose solution (Fresenius Kabi, Kutno, Poland, https://www.fresenius-kabi.com).
The patients received a single BMNCs transplantation (0.5 × 109 cells intrathecally and between 0.38 × 109 and 1.72 × 109 i.v.) followed by four intrathecal passage 3 BMMSCs transplantations (dose range: 18.5 × 106–40 × 106) every 3 months (Table 1).
Table 1.
Therapy schedule and implanted cells number
| Patient | Number of transplantations | Number of cells administrated i.v. or via LP | |||
|---|---|---|---|---|---|
| BMNCs [×109] | BMMSCs in 5% Glc [×106] | ||||
| LP | IV | LP | |||
| Patient 1 | 5 | First | 0.5 | 1 | — |
| Second | — | — | 25 | ||
| Third | — | — | 32.1 | ||
| Fourth | — | — | 32.8 | ||
| Fifth | — | — | 26 | ||
| Patient 2 | 5 | First | 0.5 | 0.38 | — |
| Second | — | — | 22.8 | ||
| Third | — | — | 22 | ||
| Fourth | — | — | 32 | ||
| Fifth | — | — | 33 | ||
| Patient 3 | 5 | First | 0.5 | 1.72 | — |
| Second | — | — | 36.8 | ||
| Third | — | — | 40 | ||
| Fourth | — | — | 36 | ||
| Fifth | — | — | 25 | ||
| Patient 4 | 5 | First | 0.5 | 0.4 | — |
| Second | — | — | 20 | ||
| Third | — | — | 34 | ||
| Fourth | — | — | 18.5 | ||
| Fifth | — | — | 23 | ||
Abbreviations: —, no data; BMNCs, bone marrow nucleated cells; BMMSC, bone marrow mesenchymal stem cell; i.v., intravenously; LP, lumbar puncture.
Cell Therapy Safety Evaluation Criteria
The safety criteria for the transplantation procedure included the appearance of infection, fever, headache, pain, an increased level of C‐reactive protein (CRP), increased leukocytosis, allergic reaction/shock, and peritransplantation complications (e.g., analgesia‐related complications, infections of the wound) for 7–14 days after each procedure. The safety criteria for using BMNCs and BMMSCs included infection, pain, cancer development, and the deterioration of the neurological state. The patients were assessed for these signs, symptoms, and pathologies over a 2‐year follow‐up period.
Assessment of Treatment Follow‐Up
The follow‐up evaluation consisted of a neurological examination that assessed changes in general neurological status, palsy or paresis, muscle tension, coordination, ataxia, and the presence of pathological extrapyramidal signs. The physical examination consisted of MRI studies, which were performed 12 months after first BMMSCs administration, and an EEG examination, which was always performed 10 weeks after BMMSCs implantation using a standard scalp EEG (Elmico Digi Truck EEG). An imaging‐based neurological assessment using a prolonged 12‐hour Holter EEG examination could not be performed.
Additionally, a neurodevelopmental evaluation was performed. Advanced neurological deficits were ascertained in a neuropsychological examination. The neuropsychological diagnosis was based on behavioral and functional changes. The childhood developmental scale (CDS), was used to assess younger children (Patients 1, 2, and 3) but provides only a qualitative evaluation 46. The CDS is a well‐standardized and normalized test that is used to assess psychomotor neurodevelopment in children aged 2 months to 3 years. This scale assesses the domains of perception, memory, spatial imagination, language processes, social development, motor development, fine motor skills, object manipulation, and visual movement coordination. Orientation ability was assessed for the domain of perception, including following an object visually, following the source of a sound, following an object, and looking at the drawing. Nonverbal reactions were estimated for the speech domain, including attempts to babble and the imitation adult vocalizations. With respect to social behaviors, reactions to contact with adults and several adult behaviors were evaluated, including speaking, laughter, and disappearance. Also considered were smiles in response to another's smile, interest in one's own reflection, and looking in the same direction as an adult. Gross motor activity was assessed by movement in the horizontal position on the back and belly, attempts to turn the head, turning from the back to a side, and attempts to sit, crawl, and stand. With respect to object manipulation, the ability to grab objects within the sight of child was assessed (e.g., grabbing and manipulating with the rattle, pulling a string with an attached ring, grabbing a block moving close to the fingers, reaching out to the block, and grabbing a second block). The level of neurological impairment of the children from the trial was so significant that only the test suitable for children in their second and third months of life could be used; even so, most of the exercises were still impossible to perform.
Results
BMMSCs Evaluation
A phenotypic evaluation of cultured, passage 3, CD271+ BMMSCs allowed a comparison of their characteristics with those of gold standard criteria established by the International Society for Cellular Therapy. More than 95% of the analyzed cells were positive for MSC‐specific markers (CD73, CD90, and CD105), and fewer than 2% of the cells were positive for the lymphocyte markers CD3 and CD45 (Fig. 1A).
Figure 1.
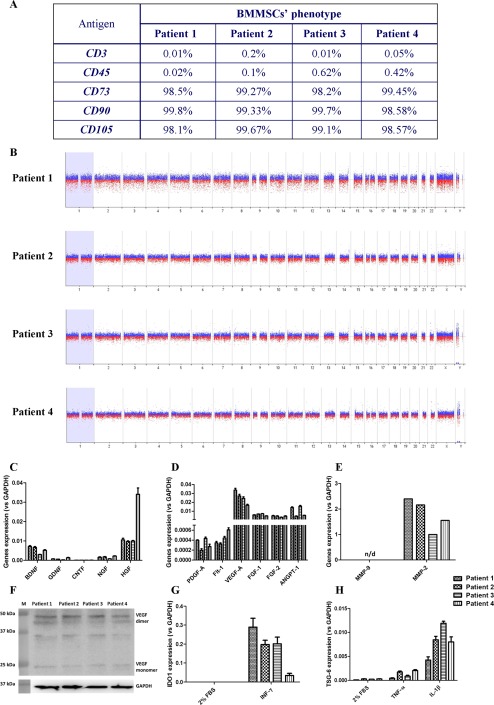
Molecular and functional analysis of BMMSCs. (A): Percentages of positive cells for subsequent surface markers are given in table. (B): Graphical overview of comparative genomic hybridization microarray results. Expression levels of mRNA for neurotrophic (C), proangiogenic (D), and tissue remodeling (E) factors in patients' BMMSCs. qRT‐PCR analysis revealed expression of BDNF, NGF, HGF, PDGF‐A, Flt‐1, VEGF‐A, FGF‐1, FGF‐2, ANGPT‐1, and MMP‐2. Slight expression of GDNF, CNTF, and NGF was observed. No expression of MMP‐9 was detected. The results are mean values ± SD (from triplicates) of mRNA expression relative to expression of housekeeping gene—GAPDH. (F): Western blot detection of VEGF in patients' BMMSCs lysates. Both forms of VEGF were identified: dimer (42 kDa) and monomer (21 kDa). The intermediary band is an effect of nonspecific reaction of antibody, observed also in manufacturer's specification. Standardized protein content in samples from different patients was confirmed by GAPDH detection. (G, H): qRT‐PCR analysis of IDO1 and TSG‐6 mRNA expression levels in patients' BMMSCs stimulated with proinflammatory cytokines. (G): Pretreatment of BMMSCs with INF‐γ caused manifestation of IDO1 expression. (H): Moreover, stimulation of BMMSCs with either TNF‐α or Il‐1β revealed elevated expression of TSG‐6. Pronounced effect was observed for Il‐1β. The results are mean values ± SD (from triplicates) of mRNA expression relative to expression of housekeeping gene—GAPDH. As a control in experiments, BMMSCs in stimulation medium only (2% FBS) were utilized. Abbreviations: ANGPT‐1, angiopoietin 1; BDNF, brain‐derived neurotrophic factor; BMMSC, bone marrow mesenchymal stem cell; CNTF, ciliary neurotrophic factor; FBS, fetal bovine serum; FGF, fibroblast growth factor; Flt‐1, vascular endothelial growth factor receptor 1; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; GDNF, glial‐derived neurotrophic factor; HGF, hepatocyte growth factor; IDO1, indoleamine 2,3‐dioxygenase; Il‐1β, interleukin 1 β; INF‐γ, interferon γ; MMP, metalloproteinase; NGF, nerve growth factor; PDGF, platelet‐derived growth factor; qRT‐PCR, real‐time quantitative reverse transcription polymerase chain reaction; TNF‐α, tumor necrosis factor α; TSG‐6, tumor necrosis factor‐inducible gene 6; VEGF, vascular endothelial growth factor.
The genomic stability of cultured CD271+ BMMSCs was confirmed via GTG banding (not shown) and molecular karyotyping (Fig. 1B). Both methods confirmed no chromosomal aberrations.
To shed light on the mechanism underlying the observed clinical improvement, we tested the mRNA levels of several genes in CD271+ BMMSCs from four patients. These analyses were performed using cells in the steady state as well as after stimulation with proinflammatory agents. These experiments revealed that CD271+ BMMSCs from all four patients expressed several neurotrophic factors (BDNF, GDNF, CNTF, NGF, and HGF; Fig. 1C). The BMMSCs were also observed to express genes encoding proangiogenic (PDGF‐A, Flt‐1, VEGF‐A, FGF‐1, FGF‐2, and ANGPT‐1; Fig. 1D) and tissue remodeling (MMP‐2; Fig. 1E) proteins. Furthermore, we detected VEGF dimers and monomers at the protein level in patients' CD271+ BMMSCs lysates (Fig. 1F). After stimulation with the proinflammatory agents INF‐γ, TNF‐α, and Il‐1β, we observed an increase in the expression of IDO1 (Fig. 1G) and TSG‐6 (Fig. 1H). These genes are involved in the modulation of various immunological responses. This analysis revealed certain differences in CD271+ BMMSCs characteristics between patients, both in terms of gene expression in the steady state conditions (i.e., HGF, PDGF‐A) and following proinflammatory stimulation. However, the impact of this variability on the ultimate clinical outcome requires further detailed analysis.
Patients' Neurological State Before Treatment
Four children, two males and two females, were enrolled in the study (Table 2). Both of the males had experienced acute hypoxia during an inflammatory episode caused by bacterial sepsis at the age of 7 months. The etiological factor was Streptococcus pneumoniae for the first male (Patient 1), and the cause was not identified for the second male (Patient 2). In both cases, the bacterial inflammation resulted in diffuse hypoxic destruction of white and grey matter and the nuclei basales, as revealed by MRI. Blood‐brain barrier damage resulted in irregular density of the brain cortex (Fig. 2A). Patient 1 developed signs of active hydrocephalus, dilatation of lateral and third ventricles, and slightly increased intracranial pressure, requiring the implantation of a ventriculoperitoneal shunt. CNS lesions resulted in mental and physical disability in this case. Patient 2, in whom the etiological factor was not identified, remained in a minimally conscious state.
Table 2.
Patients' characteristics, state at admission, epilepsy characteristic and MRI results before treatment
| Characteristics | BMNCs + BMMSCs | |||
|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Sex | M | M | F | F |
| Time interval from the illness to epilepsy | 7 m | 7 m | 2 m | 2 m |
| Cause of the illness | Bacterial sepsis/hypoxia | Bacterial sepsis/hypoxia | Bacterial sepsis/hypoxia | Bacterial sepsis |
| Etiological factor | Streptococcus pneumoniae | Unidentified | Klebsiella pneumoniae | Escherichia coli |
| Age (at the time of beginning therapy) | 15 m | 24 m | 11 m | 6 y |
| Epilepsy type | Drug resistant | Drug resistant | Drug resistant | Drug resistant |
| State at admission | Minimal conscious state | Mental and physical disability | Mental and physical disability | Progressive, severe mental and physical disability |
| MRI | White and grey matter impairment, nuclei basales destruction, hydrocephalus | White and grey matter impairment, nuclei basales destruction | White and grey matter impairment, nuclei basales destruction, periventricular leucomalacia, white matter malacia | Diffuse hyperintensive angiogenic and demyelinisation regions in white matter in frontotemporal lobes, focal changes in nuclei basales |
Abbreviations: BMNCs, bone marrow nucleated cells; BMMSC, bone marrow mesenchymal stem cell; F, female; M, male; m, months; MRI, magnetic resonance imaging; y, years.
Figure 2.
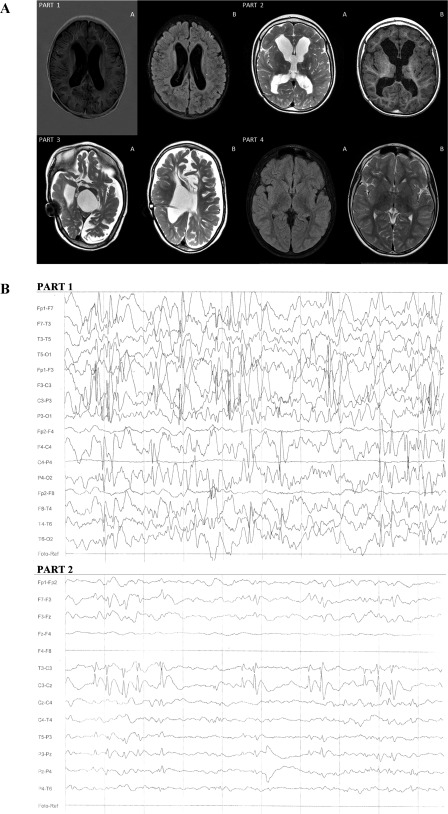
Magnetic resonance imaging (MRI) analysis and electroencephalography (EEG) evaluation. (A): MRI analysis. Part 1A: Patient 1 MRI. T1W turbo inversion recovery magnitude (TIRM) signs of active hydrocephalus; dilatation of lateral ventricles appeared with slightly increased intracranial pressure. Part 1B: Signs of blood‐brain barrier damage resulted in irregular density of the brain cortex, slightly increased intracranial pressure. Part 2A: Patient 2 MRI. T1W, hydrocephalus—winded lateral ventricles and third ventricle, without signs of increased intracranial pressure; blood‐brain barrier damage resulted in irregular density of the brain cortex. Part 2B: T2W, hydrocephalus without signs of activity, without increased intracranial pressure; signs of the destruction of the nuclei basales. Part 3A: T1W diffuse hypoxic destruction of white and grey matter and nuclei basales—post inflammatory vast areas of periventricular white matter malacia; vast areas of white matter and cortex atrophy. Part 3B: T1W—signs of active hydrocephalus with wide lateral, third, and fourth ventricles and increased intracranial pressure after implantation of ventriculoperoneal shunt; vast areas of white matter and cortex atrophy. Part 4A: T2 trim dark fluid‐destruction of nuclei basales; no signs of increased intracranial pressure. Part 4B: T2 TSE—diffuse hyperintensive angiogenic and demyelination regions in white matter, especially in frontotemporal lobes, and minimal focal changes in nuclei basales; no signs of increased intracranial pressure. (B): EEG evaluation. Part 1: Patient 2 EEG taken before treatment—hypersynchronous sleep EEG activity with groups and series of slow theta waves, single and groups of sharp waves, groups of spike‐and‐slow‐wave complexes (1–2 seconds duration), delta waves discharge located on right sight; the spike‐and‐slow‐wave complexes had higher amplitude, even 200 µV, with tendency to generalization; photostimulation and hyperventilation did not affect EEG activity. Part 2: Patient 2 EEG taken after last round of bone marrow mesenchymal stem cells showed reduction focal discharges—sharp waves of spike‐and‐slow‐wave complexes percentage reduction with curtailment of tendency to generalization, smaller percentage of delta waves discharge located on right sight; photostimulation and hyperventilation did not affect EEG activity.
A female child with a history of Klebsiella pneumoniae infection (Patient 3) experienced two episodes of hypoxia in her first two days of life and developed hydrocephalus as a neurological infection complication. MRI revealed diffuse hypoxic destruction of the white and grey matter and nuclei basales. Following the inflammatory episode, there were large volumes of periventricular white matter malacia (Fig. 2A). The examination showed signs of active hydrocephalus with widened lateral, third, and fourth ventricles and increased intracranial pressure, which required a ventriculoperitoneal shunt implantation. The second female child (Patient 4) experienced sepsis stemming from an Escherichia coli urinary tract infection at the age of 2 months. The destruction of central nervous system resulted in severe and progressive neuropsychological disability. The MRI examination revealed diffuse hyperintense angiogenic and demyelinated regions in the white matter, especially in the frontotemporal lobes; minimal focal changes were observed in the nuclei basales (not shown).
The first three patients underwent neurosurgical treatment of postinfectious hydrocephalus with ventriculoperitoneal shunts. The males were enrolled in the experimental study after 6 months of drug treatment in Patient 1 and 17 months in Patient 2, with no observed improvement in their epileptic seizures. Both of these male patients were treated with multiple drug therapy with high doses of valproic acid, phenobarbital and levetiracetam. Patients 3 and 4 both were treated with multidrug therapy, with no positive results for 7 months in Patient 3 and 5 years in Patient 4. The primary anti‐epileptic drugs used in these patients were valproic acid, levetiracetam, and lamotrigine. The older female child (Patient 4) was additionally treated with clobazam, topiramate, and corticosteroid therapy. All of these data are summarized in Table 3.
Table 3.
Epileptic seizure characteristic before and after treatment, EEG evaluation and epileptic drugs reduction after treatment
| Patient | Before experimental therapy | After experimental therapy | ||||
|---|---|---|---|---|---|---|
| Epileptic seizure character | Drugs | EEG | Epileptic seizure character | Drugs | EEG | |
| Patient 1 | Polymorphic, focal, hemilateral, grand mal. Status epilepticus. | Valproic acid, phenobarbital, levetiracetam. | Epileptic activity | Focal, nocturnal seizures | Phenobarbital | Reduced epileptic activity |
| Patient 2 | Polymorphic, usually partial seizures, myoclonic with secondary generalization, nocturnal myoclonic seizures. Status epilepticus. | Valproic acid, phenobarbital, levetiracetam. | Epileptic activity | Partial seizure, myoclonic | Valproic acid, levetiracetam | Reduced epileptic activity |
| Patient 3 | Partial‐onset seizures, tonic with secondary generalization, vocal. Status epilepticus. | Valproic acid, levetiracetam lamotrigine. | Epileptic activity | Partial onset, tonic | Valproic acid, levetiracetam | Reduced epileptic activity |
| Patient 4 | Polymorphic, tonic‐clonic, isolated tonic, absences with myoclonic of eyelids, atonic. Status epilepticus. | Valproic acid, levetiracetam, lamotrigine, clobazam, topiramate, corticosteroides. | Epileptic activity | Tonic, myoclonics | Lacosamide | Reduced epileptic activity |
Abbreviation: EEG, electroencephalography.
Therapy Schedule
All of the children who received this treatment protocol were administered with two types of cells: BMNCs and CD271+ BMMSCs. Each patient underwent five implantation procedures. The first procedure was a single implantation of BMNCs administered intrathecally (0.5 × 109 cells) and intravenously (Patient 1: 1 × 109 cells; Patient 2: 0.38 × 109 cells; Patient 3: 1.72 × 109 cells; Patient 4: 0.4 × 109 cells). Beginning 1 month after this implantation, the patients received 4 intrathecal CD271+ BMMSCs implantations (range 18.5 × 106–40 × 106 cells), which were performed every 12 weeks. In total, the patients received 4 implantations of BMNCs and 16 implantations of BMMSCs. All of the children underwent intense neurorehabilitation within 4 weeks after every procedure (Table 1).
Implantation and Therapy Safety Assessment
Early safety criteria of the transplantation procedure included assessment for the following signs: infection, fever, pain, irritation, headache, increased C‐reactive protein levels, increased leukocytosis, and allergic reaction shock. These criteria were assessed 7–14 days after the procedure (supplemental online Table 1). The considered safety‐related criteria of the therapy included cancer development, deterioration of the neurological state with increased epileptic episodes and epileptiform discharges on EEG, and infections or intracerebral hemorrhage in the 2‐year follow‐up period. Our observations revealed only minor side effects, namely a slight elevation of body temperature to 38°C in all treated children a few hours after transplantation. The treatment was generally safe, and no serious late complications were observed (supplemental online Table 1).
Reduction of Epileptic Seizures and SE Frequency
In Patient 1, epileptic seizures initially occurred 20–40 times per week. The first improvement appeared after the second round of BMMSCs transplantation, when epileptic episodes decreased to 14 episodes per week, with an average of 2 per day. Further improvement was reported after the third BMMSCs implantation, when the number of noticeable epileptic seizures dropped to only one per day. After the fourth round of BMMSCs treatment, the seizures almost disappeared, occurring sporadically with fever or pain (Table 4).
Table 4.
Reduction of epileptic seizures and SE frequency
| Treatment stage | Epileptic seizure frequency (number per week) | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient 1 | SE | Patient 2 | SE | Patient 3 | SE | Patient 4 | SE | |
| Before treatment | 20–40 | 2 | 30–60 | 1 | 14–21 | 2 | 10–40 | 4 |
| BMNCs | 20–40 | 2 | 30–60 | 1 | 14–21 | 2 | 10–40 | 4 |
| BMMSCs I | 20–40 | 2 | 30–40 | 1 | 14–21 | 2 | 10–40 | 3 |
| BMMSCs II | 14 | 1 | 20 | 0 | 14–21 | 1 | 10–30 | 3 |
| BMMSCs III | 7 | 1 | 2 | 0 | 2–3 | 0 | 10–14 | 2 |
| BMMSCs IV | 1/0 | 0 | 1 | 0 | 1 | 0 | 7 | 2 |
Abbreviations: BMNCs, bone marrow nucleated cells; BMMSC, bone marrow mesenchymal stem cell; SE, status epilepticus.
In Patient 2, the initial number of seizures was 30–60 per week. After the second round of BMMSCs implantation, the number of epileptic seizures decreased to 20 per week (estimated initially at 3–5 episodes per day). After the third and fourth rounds of BMMSCs treatment, the seizures almost ceased, occurring once or twice a week (Table 4).
A significant reduction in the number of epileptic seizures was observed after the third round of BMMSCs treatment in Patients 3 and 4. In the younger female child (Patient 3), the number of seizures episodes decreased from 21 per week to 2–3 times per week. In the case of the older female child (Patient 4), the decline was not as significant; after the third round of BMMSCs treatment, the number of episodes decreased from 40 to 30 times per week. Notably, a significant reduction in the duration of epileptic seizures was observed in this patient. The greatest reduction in seizures in both cases was seen after the fourth BMMSCs implantation. In Patient 3, the seizure number was reduced to one episode per week, while there were only seven episodes per week in Patient 4, with most episodes appearing during fever or infection.
In all cases, SE episodes were extremely rare after the treatment (zero to one per week) in comparison with the beginning of the treatment (one to four per week), especially in Patients 1, 2, and 3. In Patient 4, SE episodes occurred once or twice a month but were shorter in duration and not characterized by breathing difficulty (Table 4).
Epileptic Seizure Characteristics Before and After Treatment
Patient 1 developed epilepsy 2 months after the precipitating infection, at the age of 9 months. The seizures had different characteristics, varying from those located unilaterally to general tonic‐clonic seizures (Table 3). The seizures were polymorphic, usually represented by partial‐onset, unilateral seizures and myoclonic seizures with secondary generalization. After the treatment, the characteristics of the seizures changed, becoming shorter and usually focal. Night episodes of seizures have disappeared.
In Patient 2, epileptic episodes before treatment were polymorphic, partial, and non‐generalized, unilateral or myoclonic, and appearing during the night. After the second round of BMMSCs treatment, the seizures become shorter and partial or myoclonic, without secondary generalization. Nocturnal myoclonic seizures completely disappeared (Table 3).
In Patient 3, the seizures developed in the second month of life. The patient presented with partial‐onset epileptic seizures, generally tonic with secondary generalization (i.e., tonic‐clonic seizures). Occasionally, isolated seizures manifested vocally. In Patient 4, the epilepsy was polymorphic in character, with tonic‐clonic seizures and isolated tonic seizures episodes, absence episodes with myoclonic seizures of the eyelids, as well as atonic seizures. Some of the episodes occurred during the night as classic nocturnal seizures. In both cases, the characteristics of the epileptic seizures changed after treatment, becoming more frequently partial, myoclonic, and tonic, with rare generalization (Table 3).
EEG Analysis Evaluation
In Patient 1 and Patient 2, an EEG performed before the treatment revealed hypersynchronous sleep EEG activity with (a) groups and series of slow theta waves, (b) single sharp waves and groups of sharp waves, (c) groups of spike‐and‐slow‐wave complexes of 1–2 seconds in duration, and (d) delta wave discharges (Table 3). In Patient 1, most of the pathological epileptiform abnormalities were registered on both occipital lobes and the left temporal lobe. In Patient 2, these changes dominated on the right lobe, without any specific focal changes. The spike‐and‐slow‐wave complexes had higher amplitudes (as high as 200 μV) with a tendency towards generalization. In both cases, photostimulation and hyperventilation did not affect EEG activity. EEG analysis after the treatment showed a reduction in focal discharges of sharp waves and spike‐and‐slow‐wave complexes, and a decreased tendency toward generalization (Fig. 2B; Table 3).
In Patient 3, the EEG showed a weak presentation of sleep activity. While falling asleep and during irregular sleeping, desynchronized bioelectric activity was registered. The background was composed of a prevalence of high‐voltage delta waves (3–4 Hz) with a maximal amplitude of 180 μV. Most of these discharges appeared in both frontal regions, with alternating dominancy between the left and right sides. The base bioelectrical activity was mixed with numerous beta waves (15–16 Hz) with amplitude 90 µV. In frontotemporal regions, single and diffuse sharp waves were registered. After treatment, the EEG showed a reduction in polymorphic, high‐voltage delta waves and focal discharges of sharp waves in frontotemporal regions (Table 3).
In Patient 4, the first EEG performed before treatment revealed weak differentiation of base activity. The dominating activity was unclear and could not be distinguished from the background. The bioelectrical sleep pattern was only weakly visible, with weak and sporadic sleep spindles. Over this background, long epileptic focal series of mid‐ and high‐voltage slow waves mixed with sharp waves, spike waves, polyspike activity complexes, or slow wave complexes were observed. The epileptiform abnormalities were registered primarily on both frontotemporal lobes, with dominance on the right side. The experimental treatment significantly altered these EEG results, with subsequent analysis showing decreased epileptic discharges, particularly a reduction in continuous mid‐ and high‐voltage delta waves, a small percentage of typical and atypical sharp waves, and an absence of the spike‐waves and polyspike waves seen before the treatment. The focal changes were still located primarily on the right frontotemporal lobe (Table 3).
Neuropsychological and Cognitive Improvement
Patient 1 and Patient 3 showed severe impairment in every domain of the CDS. In the perception domain, they initially did not react to light, did not track objects or attendants, and did not turn towards sound. In addition, these patients did not show any verbal activity and could not project any sounds. With respect to motor activity, their hands stayed clenched at all times, they did not reach for objects, and they did not make voluntary movements. These children presented severe social development impairment as well, not reacting to others' voices. Moreover, there was no emotional connection between the patient and others, no interest in relatives' faces, and no laughter in response to another's behavior.
In Patient 1, inflammation caused severe CNS destruction, resulting in the necessity of oxygen supplementation with a tracheotomy tube. Regarding feeding, a loss of the swallowing reflex required an enteral nutrition tube. After the first BMMSCs implantation, the child required only partial oxygen supplementation, and after the second round of implantation, the patient's swallowing reflex was partially restored. It then became possible to feed him orally with semi‐liquid meals. In Patient 3, damage of central nervous system integrity resulted in severe mental and physical disability, with prevalent pyramidal symptoms and limited destruction of the optic nerve and auditory tract.
In both cases, the patients' eyes reacted to light after the first round of BMMSCs implantation. Moreover, the children sporadically attempted to track objects or relatives, and a single reaction to sound manifested as turning the head toward the sound source or freezing. Further improvement occurred after the second and third BMMSCs implantations. Patient 1 used the enteral nutrition tube only sporadically, and the tracheotomy tube remained open most of the time. Both children attempted to cry due to their emotional state. With respect to motor development, the hands stayed more relaxed. After the fourth BMMSCs implantation, Patient 1 began reaching for objects but without further manipulation of the objects. Patient 3 began to reach for objects earlier, after the third round of BMMSCs implantation. Emotional reactions (primarily cries), more dramatic facial expressions, and simple vocalizations appeared in both patients after the first BMMSCs implantation.
The two other children, Patients 2 and 4, initially reacted to light but did not track objects or attendants. Moreover, they could not concentrate their sight on objects; they did not turn towards sounds, but they could express emotions sporadically as a response to a strong impulse and could vocalize simple syllables. In Patient 2, the hands stayed clenched at all times. Both of the children could project simple sounds, such as cooing and gurgling. The primary neurological symptoms in these cases were full‐blown pyramidal and extrapyramidal symptoms, with very intense spasticity, especially in the lower limbs. Additionally, Patient 4 exhibited heavy ataxia of the limbs and trunk; she also expressed her emotions more easily than the other children in the group.
Slight neurological improvement was reported after the first BMMSCs implantation. All of the patients began to concentrate their sight longer on relatives and objects, reacted more to sound signals, began to vocalize more syllables, and exhibited higher levels of activity. Further improvement in all patients appeared after the second, third, and fourth rounds of BMMSCs; the children began reaching for objects, controlling their trunk and head, and assuming a crawling position. In addition, spasticity was significantly reduced, and a reduction in ataxia was noted in Patient 4. Data from the neuropsychological and functional evaluations are summarized in Table 5.
Table 5.
Neuropsychological evaluation and functional improvement
| Patient | Treatment stage | The sphere of perception |
Emotional reaction |
The sphere of speech | ||||
|---|---|---|---|---|---|---|---|---|
| Reaction to the light | Eye leading after the objects or attendant | Head response to sound signal |
Reaching the object |
Hands | ||||
| Patient 1 | At admission | No | No | No | No | Stay clenched | — | No sound, aphonia, no crowing gurgling |
| BMNCs | No | No | No | No | Stay clenched | — | No sound, aphonia, no crowing gurgling | |
| BMMSCs I | No | No | No | No | Stay clenched | — | No sound, aphonia, no crowing gurgling | |
| BMMSCs II | Appeared | An attempt to lead the eye | Yes | No | Stay clenched | Simple | An attempt to cry | |
| BMMSCs III | Appeared | An attempt to lead the eye | Yes | No | Relaxed | Simple | An attempt to cry | |
| BMMSCs IV | Appeared | Leading after interesting objects | Yes | Try to reach | Relaxed | Simple | Opening mouth for a sound signal, gurgling | |
| Patient 2 | At admission | Yes | No | No | No | Stay clenched | Impaired | Simple sound signals, loud crying |
| BMNCs | Yes | No | No | No | Stay clenched | Impaired | Simple sound signals, loud crying | |
| BMMSCs I | Yes | An attempt to lead the eye | No | Try to reach | Stay clenched | Simple, concentrate easier | Simple sound signals, loud crying | |
| BMMSCs II | Yes | An attempt to lead the eye | No | Try to reach | Relaxed | Simple, concentrate easier | Simple sound signals, loud crying | |
| BMMSCs III | Yes | Leading after interesting objects, relatives | Yes | Try to reach | Relaxed | Simple, concentrate easier | Opening mouth for a sound signal, gurgling | |
| BMMSCs IV | Yes | Leading after interesting objects, relatives | Yes | Reaching the objects normally | Normal, relaxed | Express emotions as response on intense stimulus | Further improvement in complex sound signals, crying as a result of even minimal emotional impact | |
| Patient 3 | At admission | No | No | No | No | Stay clenched | — | No sound, aphonia, no crowing gurgling |
| BMNCs | No | No | No | No | Stay clenched | — | An attempt to cry | |
| BMMSCs I | No | No | No | No | Stay clenched | — | An attempt to cry | |
| BMMSCs II | Appeared | An attempt to lead the eye | Yes | No | Stay clenched | Simple | Opening mouth for a sound signal, gurgling | |
| BMMSCs III | Appeared | An attempt to lead the eye | Yes | No | Relaxed | Simple | Opening mouth for a sound signal, gurgling | |
| BMMSCs IV | Appeared |
Leading after interesting objects |
Yes | Try to reach | Relaxed | Further | Simple sound signals, loud crying | |
| Patient 4 | At admission | Yes | No | No | Reaching the objects normally | Relaxed most of the time | Impaired | Simple sound signals, loud crying |
| BMNCs | Yes | No | No | Reaching the objects normally | Relaxed most of the time | Impaired | Simple sound signals, loud crying | |
| BMMSCs I | Yes | An attempt to lead the eye | No | Reaching the objects normally | Relaxed most of the time | Simple | Simple sound signals, loud crying | |
| BMMSCs II | Yes | An attempt to lead the eye | Yes | Reaching the objects normally | Normal, relaxed | Simple | Simple sound signals, loud crying | |
| BMMSCs III | Yes | An attempt to lead the eye | Yes | Reaching the objects normally | Normal, relaxed | Simple | Simple sound signals, loud crying | |
| BMMSCs IV | Yes | Leading after interesting objects, relatives | Yes | Reaching the objects normally | Normal, relaxed | Further | Further improvement in complex sound signals, crying as a result of even minimal emotional impact | |
Abbreviation: —, no data; BMNCs, bone marrow nucleated cells; BMMSC, bone marrow mesenchymal stem cell.
Clinical Results Summary
Collectively, 4 intravenous and intrathecal BMNC implantations and 16 intrathecal BMMSC implantations were performed without complications. No signs of infection, fever, pain, headache, increased CRP levels, increased leukocytosis, allergic reactions, or other post‐transplantation reactions were observed during the 2‐year follow‐up period.
All of the children demonstrated neurological improvement, with significant reductions in the number of both epileptic seizures and SE episodes. The characteristics of the seizures also changed following treatment. Improvements were also noted in various clinical observations and confirmed via EEG examinations. These analyses revealed decreased epileptiform discharges and significant reductions in sharp waves and epileptic complexes. The observed neurological and neuropsychological symptoms amelioration led to improvements in the patients' and families' quality of life. Meaningful improvement in clinical and quality of life domains appeared after the third round of implantations.
Discussion
Different cell therapy approaches to treat epilepsy have been proposed and tested on animal models, including surgical insertion of fetal hippocampal neurons or GABA‐producing interneurons 19, viral delivery of inhibitory neurotransmitters 20, and supportive growth factors 21, 22, as well as intravenous, intraperitoneal, or intrahippocampal transplantation of BMNCs and MSCs 24, 25, 26, 27, 28, 29, 30, 31, 32.
Bone marrow collection is a standard procedure performed prior to autologous or allogenic BM cell transplantation 47, 48. The safety of intravenous delivery of BMNCs and MSCs was previously demonstrated in clinical trials for many conditions, such as GVHD 49, multiple sclerosis 50, amyotrophic lateral sclerosis 51, and systemic lupus erythematosus 48, as well as cardiovascular 52 and liver 53 diseases. The safety and feasibility of intrathecal BMNCs and MSCs transplantation was also demonstrated for different adult and pediatric patients 17, 51, 54, 55, 56.
In our study, we evaluated the safety, feasibility, and potential efficacy of a cell therapy approach with single BMNCs and multiple BMMSCs transplantations as a treatment for pediatric DRE.
Here, we demonstrate that BM collection and the subsequent intrathecal BMNCs and BMMSCs transplantations were safe and feasible for pediatric patients with DRE, with only transient fever being observed after implantation. Furthermore, we report that the applied therapy itself is safe, showing no negative effects and resulting in no deterioration of patient clinical status in a 2‐year follow‐up period. Moreover, the experimental cell therapy was performed in combination with pharmacological therapy, which was also determined to be safe.
The cell therapy approach appeared to result in significant clinical improvement, manifesting as a marked reduction in seizure number in all of the patients (from 14–60 to 1–7 per week). The first reduction appeared after the second BMMSCs transplantation, followed by a gradual decrease in frequency after this point. The reduction of seizure number as a result of cell or gene therapy has been demonstrated in animal models of chronic temporal lobe epilepsy 13, 27, 28, 29, 30, 36, 57 as well as CA3‐selective epileptogenesis 33. Such improvements in seizure number have also been reported following the viral delivery of FGF‐2 and BDNF 21 or GDNF 22. Our observations demonstrate that an autologous cell transplantation strategy can be effective in treating epilepsy in human patients.
The range of the seizure number reduction observed in our patients was as high as those reported in animal trials 13, 21, 22, 27, 28, 29, 30, 33, 57. Moreover, the observed benefit in this outcome was much better than the only previously published attempt of intracerebral transplantation of porcine fetal GABAergic cells to treat an epileptic patient with intractable partial‐onset seizures 34 or patients with focal epilepsy 35. Notably, the improvement began gradually and persisted over consecutive transplantations, demonstrating the need for multiple injections to achieve the observed range of improvement. In fact, this observation confirms our and others' earlier reports showing the importance of a multiple implantation strategy in obtaining considerable improvement in spinal cord regeneration 17. Importantly, a decrease in the number of seizures was not the only beneficial result of our therapy strategy. The occurrence of SE episodes, which are life‐threatening, ceased completely in three patients and considerably decreased in the fourth.
Interestingly, not only the number but also the types of seizures changed with treatment. At the beginning of the treatment, the seizures were polymorphic, often tonic‐clonic, and with secondary generalization. After the treatment, the seizures became less polymorphic and more tonic, and secondary generalization was almost never observed. This observation indicates that bioelectric activity of the brain underwent partial normalization. This result was in accordance with preclinical studies and reports showing that MSCs can change the convulsive threshold, as demonstrated by shortening of the tonic seizure duration 32, a decrease in the frequency and amplitude of sharp waves in the kindling model of epilepsy 31, and a reduction in epileptiform waves 58.
All our patients were alive at the end of the study, and we were not able to perform any histological evaluation to ascertain the mechanism of action. However, the mechanism of improvement after human BMNCs or BMMSCs transplantations was already elucidated using an animal epilepsy model. Chronic inflammation is believed to be responsible for a vicious circle, resulting in the further death of neurons and continued neurodegeneration 59. MSCs are known for their immunomodulatory actions 15 and have been previously reported to reduce inflammation in rat hippocampal epilepsy 26, 29, 30.
BMMSCs have been demonstrated to secrete various neurotrophic factors, such as BDNF and GDNF, which promote neurite outgrowth and survival 60, 61, as well as HGF, which has been shown to stimulate myelination 62. BMMSCs are also capable of suppressing T‐cell proliferation 63. All of these factors would have an impact on neurons, stimulating their proliferation, differentiation, and, subsequently, the regeneration of injured brain regions. In fact, the neuroprotective properties of MSCs against excitotoxic cell death were already demonstrated in an in vitro and in vivo preclinical model 24.
Although we were not able to perform any histology studies, we confirmed the transcript expression of the growth factors BDNF, NGF, CNTF, HGF, VEGF‐A, FGF‐2, as well as that of MMP‐2, in the transplanted BMMSCs in all of our patients. VEGF‐A expression was also detected at the protein level in both monomer and dimer forms. Moreover, we demonstrated the immunomodulatory potential of patients' BMMSCs following in vitro treatment with proinflammatory cytokines. Based on preclinical data and the characteristic of the transplanted cells, we believe that the reduced numbers of seizures and SE episodes indicate an elimination or reduction of inflammation in the affected area as well as the neuroprotective action of the transplanted cells on neighboring neurons. In this way, the vicious circle that was responsible for further neuronal death and continued neurodegeneration was broken. Additionally, the partial normalization of bioelectrical activity suggests that the transplanted cells stimulated regeneration of the injured region or altered the excitation threshold of the injured neurons.
The range of the improvement we observed for patients with DRE shows the strong potential of our therapeutic strategy. Interestingly, the degree of improvement increased over sequential transplantations even after the cessation of the multi‐therapy regimen. We believe that one of the reasons for the success of cell therapy may be the unique populations of MSCs we used for the implantations. Previous animal epilepsy trials and clinical trials using MSCs collected the MSCs using an adherence‐based isolation procedure. However, our group cultures a pure CD271+ mesenchymal stem cell population. Our studies 39 and those of others 40, 41, 42 have demonstrated that CD271+ BMMSCs have a higher proliferation potential, produce more cytokines, exhibit stronger immunosuppressive abilities, and promote more vascularization and chondral repair than MSCs isolated with adherence‐based methods. However, a direct comparison would need to be performed to confirm our hypothesis.
We are aware that the results of this pilot study should be confirmed using a larger group of patients. Those studies should include clearly defined endpoints: for example, efficacy, safety measurements, or feasibility. As different numbers of patients will be necessary for feasibility, treatment tolerability, and efficacy studies, these trials should also include proper power calculations for sample size justification. Appropriate statistical methods should be planned ahead to prove clinical and statistical goals. For example, if efficacy will be defined as a percentage of reduction, statistical analysis will use methods for continuous dependent variable. It seems appropriate to also apply multivariate regression models.
Additionally, the cell dose and the frequency of transplantation should be optimized for the best clinical effect. The possibility cannot be excluded that engineering the cells to express even higher levels of neuroprotective cytokines would increase their clinical efficiency. Whether it will be possible to obtain total seizure and SE withdrawal as well as improved bioelectrical activity normalization remains an open question. Similarly, longer follow‐up periods are needed to determine whether patients require repeat transplantations to maintain the improvement or if the benefits will persist even after stopping the transplantation procedure.
Conclusion
We report here, for the first time to our knowledge, the safety and feasibility of using a single BMNCs and multiple BMMSCs implantations as a cell therapy treatment for a small group of DRE pediatric patients. Our results demonstrate that there are no early or late adverse events associated with the transplantation procedure or the therapy itself. Additionally, the treatment resulted in substantial neurological improvement, which manifested as a reduced number of seizures and SE episodes as well as partially normalized bioelectrical activity in the brain. Thus, we demonstrated the strong potential of our therapeutic approach to treat epilepsy patients. However, because of the low number of patients in the current study, further studies are necessary to move this experimental treatment into standard clinical use.
Author Contributions
O.M.: conception and design, financial support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing; D.J.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; A.S.‐F.: collection and/or assembly of data, data analysis and interpretation; S.K.: financial support, provision of study material or patients, collection and/or assembly of data; B.B.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; M.M.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Note Added in Proof
This article was published online on 10 December 2017. Minor edits have been made that do not affect data. This notice is included in the online and print versions to indicate that both have been corrected 28 December 2017.
Supporting information
Supplemental online Table 1. Safety criteria: early and late treatment complications and side effects
Acknowledgments
This work was partially financed by Jagiellonian University School of Medicine Grants K/DSC/003093 and K/ZDS/005693, by Leading National Research Center (KNOW) scheme supported by the Polish Ministry of Science and Higher Education, and by the “Dar Nadziei” Foundation.
References
- 1. Zanirati G, Azevedo PN, Marinowic DR et al. Transplantation of bone marrow mononuclear cells modulates hippocampal expression of growth factors in chronically epileptic animals. CNS Neurosci Ther 2015;21:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodarzi P, Aghayan HR, Soleimani M et al. Stem cell therapy for treatment of epilepsy. Acta Med Iran 2014;52:651–655. [PubMed] [Google Scholar]
- 3. Laxer KD, Trinka E, Hirsch LJ et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav 2014;37:59–70. [DOI] [PubMed] [Google Scholar]
- 4. Kwan P, Arzimanoglou A, Berg AT et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 5. Riviello JJ Jr, Ashwal S, Hirtz D et al. Practice parameter: Diagnostic assessment of the child with status epilepticus (an evidence‐based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2006;67:1542–1550. [DOI] [PubMed] [Google Scholar]
- 6. McMullan JT, Knight WA, Clark JF et al. Time‐critical neurological emergencies: The unfulfilled role for point‐of‐care testing. Int J Emerg Med 2010;3:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeLorenzo RJ, Hauser WA, Towne AR et al. A prospective, population‐based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46:1029–1035. [DOI] [PubMed] [Google Scholar]
- 8. Chin RF, Neville BG, Peckham C et al. Incidence, cause, and short‐term outcome of convulsive status epilepticus in childhood: Prospective population‐based study. Lancet 2006;368:222–229. [DOI] [PubMed] [Google Scholar]
- 9. Raspall‐Chaure M, Chin RF, Neville BG et al. Outcome of paediatric convulsive status epilepticus: A systematic review. Lancet Neurol 2006;5:769–779. [DOI] [PubMed] [Google Scholar]
- 10. Martinos MM, Yoong M, Patil S et al. Early developmental outcomes in children following convulsive status epilepticus: A longitudinal study. Epilepsia 2013;54:1012–1019. [DOI] [PubMed] [Google Scholar]
- 11. Melvin JJ, Huntley Hardison H. Immunomodulatory treatments in epilepsy. Semin Pediatr Neurol 2014;21:232–237. [DOI] [PubMed] [Google Scholar]
- 12. Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 2011;10:173–186. [DOI] [PubMed] [Google Scholar]
- 13. Waldau B, Hattiangady B, Kuruba R et al. Medial ganglionic eminence‐derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells 2010;28:1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rüschenschmidt C, Koch PG, Brüstle O et al. Functional properties of ES cell‐derived neurons engrafted into the hippocampus of adult normal and chronically epileptic rats. Epilepsia 2005;46:174–183. [DOI] [PubMed] [Google Scholar]
- 15. Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem Cytobiol 2006;44:215–230. [PubMed] [Google Scholar]
- 16. Compagna R, Amato B, Massa S et al. Cell therapy in patients with critical limb ischemia. Stem Cells Int 2015;2015:931420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jarocha D, Milczarek O, Wedrychowicz A et al. Continuous improvement after multiple mesenchymal stem cell transplantations in a patient with complete spinal cord injury. Cell Transplant 2015;24:661–672. [DOI] [PubMed] [Google Scholar]
- 18. Parr AM, Tator CH, Keating A. Bone marrow‐derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 2007;40:609–619. [DOI] [PubMed] [Google Scholar]
- 19. Shetty AK. Hippocampal injury‐induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: Can early neural stem cell grafting intervention provide protection? Epilepsy Behav 2014;38:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kullmann DM, Schorge S, Walker MC et al. Gene therapy in epilepsy‐is it time for clinical trials? Nat Rev Neurol 2014;10:300–304. [DOI] [PubMed] [Google Scholar]
- 21. Bovolenta R, Zucchini S, Paradiso B et al. Hippocampal FGF‐2 and BDNF overexpression attenuates epileptogenesis‐associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflammation 2010;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanter‐Schlifke I, Georgievska B, Kirik D et al. Seizure suppression by GDNF gene therapy in animal models of epilepsy. Mol Ther 2007;15:1106–1113. [DOI] [PubMed] [Google Scholar]
- 23. Nicoletti JN, Lenzer J, Salerni EA et al. Vascular endothelial growth factor attenuates status epilepticus‐induced behavioral impairments in rats. Epilepsy Behav 2010;19:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voulgari‐Kokota A, Fairless R, Karamita M et al. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol 2012;236:161–170. [DOI] [PubMed] [Google Scholar]
- 25. Uccelli A, Prockop DJ. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin Immunol 2010;22:768–774. [DOI] [PubMed] [Google Scholar]
- 26. Agadi S, Shetty AK. Concise review: Prospects of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsy. Stem Cells 2015;33:2093–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abdanipour A, Tiraihi T, Mirnajafi‐Zadeh J. Improvement of the pilocarpine epilepsy model in rat using bone marrow stromal cell therapy. Neurol Res 2011;33:625–632. [DOI] [PubMed] [Google Scholar]
- 28. Costa‐Ferro ZS, Vitola AS, Pedroso MF et al. Prevention of seizures and reorganization of hippocampal functions by transplantation of bone marrow cells in the acute phase of experimental epilepsy. Seizure 2010;19:84–92. [DOI] [PubMed] [Google Scholar]
- 29. Venturin GT, Greggio S, Marinowic DR et al. Bone marrow mononuclear cells reduce seizure frequency and improve cognitive outcome in chronic epileptic rats. Life Sci 2011;89:229–234. [DOI] [PubMed] [Google Scholar]
- 30. Costa‐Ferro ZS, Souza BS, Leal MM et al. Transplantation of bone marrow mononuclear cells decreases seizure incidence, mitigates neuronal loss and modulates pro‐inflammatory cytokine production in epileptic rats. Neurobiol Dis 2012;46:302–313. [DOI] [PubMed] [Google Scholar]
- 31. Huicong K, Zheng X, Furong W et al. The imbalanced expression of adenosine receptors in an epilepsy model corrected using targeted mesenchymal stem cell transplantation. Mol Neurobiol 2013;48:921–930. [DOI] [PubMed] [Google Scholar]
- 32. Tamura BP, Almeida DC, Felizardo RJ et al. Convulsive seizure protection after hippocampal transplantation of mesenchymal cells from adipose tissue in mice. J Stem Cell Res Ther 2014;4:196. [Google Scholar]
- 33. Li T, Ren G, Kaplan DL et al. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3‐selective epileptogenesis. Epilepsy Res 2009;84:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schachter S, Schomer D, Blume H et al. Porcine fetal GABA‐producing neural cell transplants for human partial‐onset seizures: safety and feasibility. Epilepsia 1998:39:67. 9578015 [Google Scholar]
- 35. Edge AS. Current applications of cellular xenografts. Transplant Proc 2000;32:1169–1171. [DOI] [PubMed] [Google Scholar]
- 36. Costa‐Ferro ZS, de Borba Cunha F, de Freitas Souza BS et al. Antiepileptic and neuroprotective effects of human umbilical cord blood mononuclear cells in a pilocarpine‐induced epilepsy model. Cytotechnology 2013;66:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma S, Xie N, Li W et al. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ropper AE, Thakor DK, Han I et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix‐assisted hMSC implantation. Proc Natl Acad Sci USA 2017;114:E820–E829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jarocha D, Lukasiewicz E, Majka M. Adventage of mesenchymal stem cells (MSC) expansion directly from purified bone marrow CD105+ and CD271+ cells. Folia Histochem Cytobiol 2008;46:307–314. [DOI] [PubMed] [Google Scholar]
- 40. Kuçi S, Kuçi Z, Kreyenberg H et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment‐promoting properties. Haematologica 2010;95:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cuthbert RJ, Giannoudis PV, Wang XN et al. Examining the feasibility of clinical grade CD271+ enrichment of mesenchymal stromal cells for bone regeneration. PLoS One 2015;10:e0117855 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hermida‐Gómez T, Fuentes‐Boquete I, Gimeno‐Longas MJ et al. Bone marrow cells immunomagnetically selected for CD271+ antigen promote in vitro the repair of articular cartilage defects. Tissue Eng Part A 2011;17:1169–1179. [DOI] [PubMed] [Google Scholar]
- 43. Lukasiewicz E, Miekus K, Kijowski J et al. Inhibition of rhabdomyosarcoma's metastatic behavior through downregulation of MET receptor signaling. Folia Histochem Cytobiol 2009;47:485–489. [DOI] [PubMed] [Google Scholar]
- 44. Ries C, Egea V, Karow M et al. MMP‐2, MT1‐MMP, and TIMP‐2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007;109:4055–4063. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Z, Han Y, Song J et al. Interferon‐γ regulates the function of mesenchymal stem cells from oral lichen planus via indoleamine 2,3‐dioxygenase activity. J Oral Pathol Med 2015;44:15–27. [DOI] [PubMed] [Google Scholar]
- 46. Matczak A, Jaworowska A, Ciechanowicz A et al. DSR Dziecięca Skala Rozwojowa. Skala Wykonaniowa, Skala Obserwacyjna. Warszawa: Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego, 2007. [Google Scholar]
- 47. Hare JM, Fishman JE, Gerstenblith G et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang J, Zhang H, Hua B et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann Rheum Dis 2010;69:1423–1429. [DOI] [PubMed] [Google Scholar]
- 49. Ringdén O, Uzunel M, Rasmusson I et al. Mesenchymal stem cells for treatment of therapy‐resistant graft‐versus‐host disease. Transplantation 2006;81:1390–1397. [DOI] [PubMed] [Google Scholar]
- 50. Connick P, Kolappan M, Crawley C et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open‐label phase 2a proof‐of‐concept study. Lancet Neurol 2012;11:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oh KW, Moon C, Kim HY et al. Phase I trial of repeated intrathecal autologous bone marrow‐derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med 2015;4:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heldman AW, DiFede DL, Fishman JE et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC‐HFT randomized trial. JAMA 2014;311:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peng L, Xie D, Lin BL et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short‐term and long‐term outcomes. Hepatology 2011;54:820–828. [DOI] [PubMed] [Google Scholar]
- 54. Jarocha D, Milczarek O, Kawecki Z et al. Preliminary study of autologous bone marrow nucleated cells transplantation in children with spinal cord injury. Stem Cells Transl Med 2014;3:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sharma A, Sane H, Gokulchandran N et al. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res Treat 2014;2014:234095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharma A, Sane H, Gokulchandran N et al. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: A new frontier. Stem Cells Int 2015;2015:905874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hattiangady B, Rao MS, Shetty AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol 2008;212:468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Long Q, Qiu B, Wang K et al. Genetically engineered bone marrow mesenchymal stem cells improve functional outcome in a rat model of epilepsy. Brain Res 2013;1532:1–13. [DOI] [PubMed] [Google Scholar]
- 59. Gao HM, Hong JS. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol 2008;29:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008;132:645–660. [DOI] [PubMed] [Google Scholar]
- 61. Kaspar BK. Mesenchymal stem cells as trojan horses for GDNF delivery in ALS. Mol Ther 2008;16:1905–1906. [DOI] [PubMed] [Google Scholar]
- 62. Bai L, Lennon DP, Caplan AI et al. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci 2012;15:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Batten P, Sarathchandra P, Antoniw JW et al. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo‐responses via the TH2 pathway: Relevance to tissue engineering human heart valves. Tissue Eng 2006;12:2263–2273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental online Table 1. Safety criteria: early and late treatment complications and side effects


