Abstract
Islet engraftment after transplantation is impaired by high rates of islet/β cell death caused by cellular stressors and poor graft vascularization. We studied whether cotransplantation of ex vivo expanded autologous bone marrow‐derived mesenchymal stem cells (MSCs) with islets is safe and beneficial in chronic pancreatitis patients undergoing total pancreatectomy with islet autotransplantation. MSCs were harvested from the bone marrow of three islet autotransplantation patients and expanded at our current Good Manufacturing Practices (cGMP) facility. On the day of islet transplantation, an average dose of 20.0 ± 2.6 ×106 MSCs was infused with islets via the portal vein. Adverse events and glycemic control at baseline, 6, and 12 months after transplantation were compared with data from 101 historical control patients. No adverse events directly related to the MSC infusions were observed. MSC patients required lower amounts of insulin during the peritransplantation period (p = .02 vs. controls) and had lower 12‐month fasting blood glucose levels (p = .02 vs. controls), smaller C‐peptide declines over 6 months (p = .01 vs. controls), and better quality of life compared with controls. In conclusion, our pilot study demonstrates that autologous MSC and islet cotransplantation may be a safe and potential strategy to improve islet engraftment after transplantation. (Clinicaltrials.gov registration number: NCT02384018). stem cells translational medicine 2018;7:11–19
Keywords: Bone marrow, Cell death, Chronic pancreatitis, Glycemic control, Islet transplantation, Mesenchymal stem cell
Significance Statement.
This pilot study demonstrates for the first time that intrahepatic infusion of autologous bone marrow‐derived mesenchymal stem cells (MSCs) during islet transplantation may be safe and may have the potential to improve islet engraftment, glycemic control, and quality of life. This work extends the current paradigm of MSCs as an immune regulatory factor and reveals important additional functions of MSCs in promoting islet engraftment after transplantation in islet autotransplant patients. This study proves justification for a larger and randomized clinical trial, as MSCs have the potential to reduce inflammatory damage and support angiogenesis in transplanted islets.
Introduction
Autologous islet transplantation is currently performed in patients with chronic pancreatitis (CP) to avoid type 3c diabetes after total pancreatectomy. Allogeneic islet transplantation offers promising therapy for patients with type 1 diabetes. The long‐term efficiency and effectiveness of islet transplantation are problematic. Stresses induced during islet harvesting and after transplantation led to death of more than half of the transplanted islets even under optimal conditions 1, 2. Numerous factors including the instant blood‐mediated inflammatory reaction (IBMIR), proinflammatory cytokines, and hypoxia contribute to poor islet engraftment and β cell death during the peritransplantation period 3, 4, 5, 6. Engrafted islets may be further destroyed by chronic β cell exhaustion and autoimmune responses, which decreases the survival and function of transplanted islets and eventually leads to impaired glycemic control in patients. Insulin independent rates are known to be low in both autologous and allogeneic islet recipients 3, 7, 8, 9, 10. Strategies that can increase islet engraftment after transplantation may enhance the effectiveness of islet transplantation 11, 12.
Mesenchymal stem cells (MSCs) are adult stem cells that can be harvested from bone marrow, adipose, and dental pulp. They attach to standard plastic cell culture plates, express specific cellular markers such as CD73, CD90, and CD105, and can be differentiated into osteoblasts, adipocytes, and chondroblasts 13. They possess a remarkably diverse array of tissue protective and immunosuppressive characteristics 14, 15, 16, 17, 18.
In murine models of diabetes, systemic infusion of MSCs delays the onset of diabetes, improves glycemic control, promotes pancreatic tissue regeneration, reduces pancreatic insulitis, and prevents autoimmune destruction 17, 19, 20. Intravenously infused MSCs are known to migrate to pancreatic islets or the injured pancreas 21, 22.
The immunological properties of MSCs show great potential in organ transplant therapies and are associated with lower rates of organ rejection and infection following transplant. Cotransplantation of MSCs and islets protects the islets from injury in rodent models. In streptozotocin (STZ)‐induced diabetic mice, cotransplantation of syngeneic MSCs with a marginal mass of allogeneic islets under the kidney capsule resulted in prolonged normoglycemia 23. In the STZ‐induced diabetic rodents, cotransplantation of syngeneic MSC in the omentum 24 and kidney capsule 23, 25, 26 significantly prolonged islet graft survival compared with animals receiving islets alone. In nonhuman primates, intraportal cotransplantation of donor MSCs and islets in an allogeneic islet transplantation setting provided a distinct advantage in promoting early islet engraftment 27.
The major mechanism of MSC protection may be in their paracrine secretion of protective factors 27, 28. MSCs promote angiogenesis based on their ability to secrete vascular endothelial growth factor, hepatocyte growth factor, TGF‐β and others. These factors are required for angiogenesis, and local increase in concentration by MSCs may improve islet implantation 29, 30, 31. Based on these attributes, we hypothesize that cotransplantation of autologous MSCs can enhance islet engraftment, survival, and function in CP patients undergoing islet autotransplantation (IAT).
MSCs are currently being tested in clinical trials for the treatment of type 1 and type 2 diabetes, diabetes complications, and other diseases (clinicaltrials.gov). A recent survey indicated that more than 1,000 humans have received MSCs for various indications. No adverse events (AEs) during or after MSC infusion have been reported, and no ectopic tissue formation has been noted 32. We have validated that the phenotype and functional integrity of MSCs from CP patients are comparable to those from healthy donors (Wang, unpublished data), suggesting that MSCs from CP patients are suitable for cellular therapy. In this pilot clinical trial, we evaluated the safety and efficacy of cotransplantation of autologous MSCs and islets in patients who undergo total pancreatectomy and islet transplantation (TP‐IAT). We compared adverse events, glycemic control, and quality of life (QOL) of MSC treated patients with 101 matched historical patients who received islet transplantation alone.
Materials and Methods
Patent Selection
Diabetes‐free adult chronic pancreatitis patients scheduled for total pancreatectomy with islet autotransplantation were enrolled in this case‐control study. Participants were enrolled if they had normal renal function, normal coagulation parameters, and normal liver function as measured by serum levels of alanine aminotransferase, aspartate aminotransferase, and total bilirubin. Patients who had undergone prior transduodenal sphincteroplasty or pancreatic head resection procedures were eligible for enrollment, but other resection or pancreatic drainage procedures precluded enrollment. All patients signed informed consent approved by the Medical University of South Carolina (MUSC) Institutional Review Board. The clinicaltrial.gov registration number is NCT02384018.
Study Design
This study was designed as a phase I, open‐label, single‐center, pilot study to evaluate the safety and efficacy of cotransplantation of autologous bone marrow‐derived MSCs with islets in CP patients undergoing TP‐IAT. The safety and efficacy of infusion of MSCs immediately after islet transplantation were assessed through adverse events, onset of diabetes, glycemic control, pain relief, and quality of life indexes. Data were compared with those from 101 adult patients who were diabetes‐free prior to surgery and had TP‐IAT at MUSC from 2009 to 2015 who completed 6‐ and 12‐month follow‐up visits.
MSC Preparation
A bone marrow aspirate (10–15 ml) was harvested from the iliac crest from patients under local anesthesia 21 days before scheduled islet transplantation. Samples were placed on ice and transferred to the MUSC class 10,000 current Good Manufacturing Practice (cGMP) compliant clean room. Bone marrow mononuclear cells (MNCs) were isolated by Ficoll density gradient (density 1.077 g/cm3) centrifugation. Washed cells were resuspended in serum‐free alpha‐MEM supplemented with gentamicin (VWR, Radnor, PA, USA, https://us.vwr.com/store/). MNCs were plated at a density of 10,000–20,000 cells/cm2. Cells were cultured in alpha‐MEM supplemented with pooled human platelet lysate (from Emory University) at 37°C in a humidified atmosphere containing 5% CO2. When the cultures reach near confluence (>80%), cells were detached by treatment with TrypLE (Gibco, Gaithersburg, MD, USA, https://www.thermofisher.com/us/en/home/brands/gibco.html) and re‐plated at a density of 1,000 cells/cm2 in flasks. MSCs at passage 4 were used for transplantation. Release criteria of MSCs were as follows: (>95%) CD73, CD90, CD105, and (<5%) for CD14, CD31, CD45 and HLA‐DR, viability >70% (by Trypan blue dye exclusion assay), negative gram stain of the infusion product, negative bacterial, fungal, endotoxin, and mycoplasma tests. We obtained an average of 26 ± 9.7 × 106 MSCs at passage 4. Cells were re‐suspended into 4 × 106 cells/ml in Plasmalyte A for infusion. Each patient received 20 × 106 (± 15%) cells without heparin. The number of MSCs infused was determined based on numbers of MSCs used in other clinical trials and a nonhuman primate study in which MSCs and islet were cotransplanted into streptozotocin‐induced diabetic cynomolgus monkeys 27.
Islet Harvest and Transplantation
The pancreatectomy was performed by open laparotomy at the MUSC Hospital. After devascularization, the pancreas was transferred to the MUSC cGMP facility where islets were harvested from the pancreas using the modified Ricordi method 33. Total unpurified islets were resuspended in 5% human albumin with heparin (70 U/kg body weight) for infusion. Endotoxin assays, gram stains, and bacterial and fungal cultures were measured in the final products and used as sterility indicators. Non‐purified islets were infused into the portal vein via the mesenteric vein of the patient who was under general anesthesia. MSCs were infused right after islet infusion via the same route. No other anticoagulation was used during and after islet infusion. Hepatic pressures were measured before, during, and after infusion.
Safety Tests
Patients were put on insulin treatment for a target glucose level of 100 mg/dl during the perioperative period before being discharged. All AEs were recorded and classified according to their severity. The independent Data Safety Monitoring Committee (DSMB) reviewed serious AEs (SAEs). All autologous islet transplant patients are scheduled to return to MUSC at 1, 2, 3, and 6 months, and then yearly for checkup after hospital discharge.
Measurement of Clinical Outcomes
Diabetes onset and insulin requirements after surgery were measured and compared between groups. Fasting plasma glucose, C‐peptide, hemoglobin A1C (HbA1c), and indexes from a mixed‐meal tolerance test (MMTT) including serum C‐peptide levels evaluated glycemic control. Insulin independence was defined 34 as no‐insulin therapy, HbA1c <6.5%, fasting blood glucose <126 mg/dl and 2 h postprandial blood glucose <180 mg/dl. Daily oral morphine equivalents and QOL as measured by the SF‐12 questionnaire were recorded.
MMTT
The MMTT was performed at 6 months postoperatively. Patients were asked to drink “BOOST,” which contains a mixture of protein, fat and carbohydrates, at a dose of 6 mL per kilogram body weight after fasting overnight. Patient's blood was drawn prior to the BOOST and then at 15, 30, 60, 90, 120, 180, and 240 minutes (±5 minutes) after ingestion. Glucose and C‐peptide levels in serum were measured using standard methods. The mixed meal stimulation index was calculated as described 35, and results were compared with the only four historical patients who had done the MMTTs at 6 months postoperatively.
Statistics
Two‐tailed independent sample t tests were used to compare mean differences between groups, and variances were conservatively assumed to be unequal. Glucose and C‐peptide values after MMTT over time were compared between groups using general linear mixed models. All values are presented as mean and standard deviation unless otherwise specified. p < .05 was denoted as statistically significant.
Results
Characteristics of Patients
We report on the first three patients who received MSCs as part of their islet autotransplantation and compared demographic variables with the 101 historical patients referred as “CTRs”. There were no significant differences in demographic variables, including patient age, body weight, body mass index, HbA1c, and years of chronic pancreatitis (Table 1). MSCs were collected and ex vivo expanded for 21 days prior to scheduled islet transplantation (Fig. 2A). No differences in islet product weight, total islet equivalent numbers (IEQ) infused, and IEQ transplanted per kilogram of body weight were seen. MSC patients received an average of 20.0 ± 2.6 × 106 MSCs (Table 1). The total volume of cells infused was 5.36 ± 0.75 ml. Hepatic pressures increased to similar degrees after infusion in both MSC and CTR patients (Table 1).
Table 1.
Patient characteristics at diagnosis and islet and MSC infusion
| Average of MSC subjects (n = 3) | Horizontal control subjects (n = 101) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Mean | SD | Mean | SD | p value |
| Age (yr) | 26 | 29 | 46 | 33.7 | 10.8 | 42.4 | 11.4 | .29 |
| Body weight (kg) | 76 | 109 | 79 | 88.0 | 18.2 | 74.4 | 20.6 | .33 |
| BMI (kg/m2) | 23 | 44 | 25 | 30.7 | 11.6 | 26.4 | 6.3 | .59 |
| HbA1C pre‐op (%) | 5.4 | 5.6 | 5.4 | 5.5 | 0.1 | 5.7 | 0.9 | .11 |
| Years of CP | 9 | 11 | 10 | 10.0 | 1.0 | 8.2 | 5.8 | .06 |
| Islet product weight (g) | 7 | 50 | 30 | 29.0 | 21.5 | 15.6 | 11.9 | .39 |
| Total islet infused IEQ | 221,124 | 704,244 | 470,888 | 465,152 | 241,598 | 277,986 | 221,211 | .31 |
| IEQ/kg | 2,909 | 6,461 | 5,950 | 5,107 | 1,920 | 3,863 | 3,127 | .38 |
| MSC (×106) | 22 | 17 | 21 | 20.0 | 2.6 | 0 | 0 | N/A |
| Hepatic pressure (mmHg) | ||||||||
| Pre‐infusion | 7 | 11 | 6 | 8 | 2.6 | 7.9 | 3.6 | N/A |
| During infusion | 7 | 19 | 13 | 13 | 6 | 13 | 5.8 | N/A |
| Post‐infusion | 8 | 29 | 16 | 17.7 | 10.6 | 16 | 8.3 | N/A |
Abbreviations: BMI, body mass index; CP, chronic pancreatitis; HbA1C, hemoglobin A1C; IEQ, islet equivalent number; MSC, mesenchymal stem cell; N/A, not applicable; yr, years; SD, standard deviation.
Figure 2.
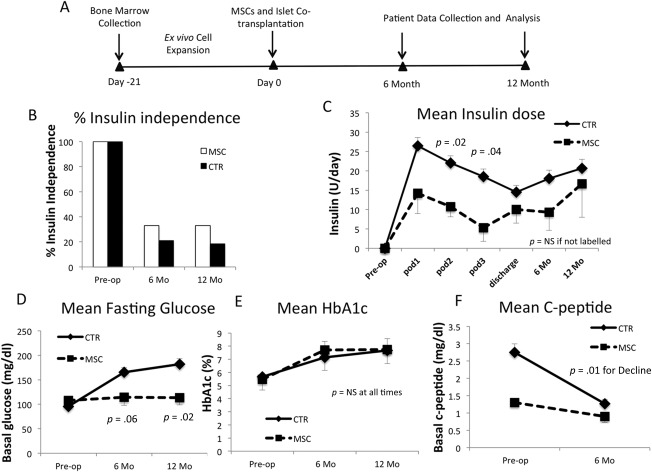
MSC patients have reduced insulin requirements and lower blood glucose levels after transplantation. (A): Schematic diagram of MSC preparation, islet transplantation, data collection, and analysis. (B): Percentages of patients that were insulin‐independent at preoperative period (preop) and 6 months and 12 months after TP‐IAT. (C): Daily insulin requirement of MSC (n = 3) and control patients (n = 101), preop, postoperatively on days 1 (POD1), 2 (POD2), 3 (POD3), at discharge, and 6 Mo and 12 Mo after islet transplantation. Mean fasting blood glucose levels (D), HbA1c (E), and C‐peptide levels (F) at preoperative, 6‐month, and 12‐month visits. p values evaluated by Student's t tests and assumed unequal variances. Error bars represent standard errors. Abbreviations: CTR, historical patients; Mo, month; MSC, mesenchymal stem cell; NS, not significant; POD1, postoperatively on day 1; POD2, postoperatively on day 2; POD3, postoperatively on day 3; TP‐IAT, total pancreatectomy with islet autotransplantation.
AEs and SAEs
Adverse events are summarized in Table 2. All three patients who received MSCs tolerated the procedure well. The first patient did not have any AE. The second patient had dehydration, bacterial pneumonia, a pleural effusion, and a positive purified protein derivative. This patient was also treated for a possible non‐occlusive portal vein thrombosis (PVT). The diagnosis was suggested by a contrasted dual phase computed tomography image that showed a filling defect not observed at the time of follow‐up visits. The possibility of incomplete contrast timing could not be excluded. The third patient had emesis, upper gastrointestinal bleeding and left PVT. All AEs and SAEs resolved within 8 weeks. All of the above AEs have also been observed in historical control patients, and none of them were determined by the DSMB to be directly related to MSC infusion. PVTs were seen in 8 of 100 historical patients. In these patients, the chance of PVT is positively correlated with total islet pellet weight: the average islet pellet weight of historical patients with PVT was 28.3 ± 4.2 g versus 13.8 ± 4.0 g in those without PVT (Fig. 1). During the first 12 months after islet and MSC cotransplantation, no tumor or ectopic tissue formation was observed in any MSC patient.
Table 2.
Adverse events of MSC patients
| Subject ID | AE Description | Start Date | End Date | Severity | Casual Relationship | Action Taken | SAE? | SAE Reason | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| MSC1 | None | ||||||||
| MSC2 | Dehydration | 4/1/15 | 4/13/15 | 1 | 1 | 2 | Y | 2 | 1 |
| MSC2 | Portal vein thrombosis | 4/22/15 | 1 | 1 | 2 | Y | 6 | 1 | |
| MSC2 | Pneumonia | 4/29/15 | 5/1/15 | 1 | 1 | 2 | Y | 2 | 1 |
| MSC2 | Pleural effusion | 6/20/15 | 6/26/15 | 1 | 1 | 1 | Y | 2 | 1 |
| MSC2 | Latent TB infection w/fever | 7/15/15 | 7/26/15 | 1 | 1 | 1 | Y | 2 | 1 |
| MSC2 | Right pleural effusion | 8/19/15 | 8/20/15 | 1 | 1 | 1 | Y | 2 | 1 |
| MSC3 | Left portal vein thrombosis | 6/1/15 | 6/20/15 | 1 | 1 | 2 | Y | 6 | 1 |
| MSC3 | Emesis | 6/11/15 | 6/18/15 | 1 | 1 | 2 | Y | 2 | 1 |
| MSC3 | Upper GI bleed | 6/11/15 | 6/18/15 | 3 | 1 | 3 | Y | 2 | 1 |
| Key | ||||
|---|---|---|---|---|
| Severity | Casual Relationship | Action Taken | SAE Reason | Outcome |
| Grade 1 = Mild | 1 = None | 1 = Life‐ threatening | 1 = Recovered | |
| Grade 2 = Moderate | 1 = Unlikely | 2 = Medication | 2 = Required hospitalization | 2 = Ongoing |
| Grade 3 = Severe | 2 = Possibly | 3 = Other | 3 = Prolonging existing hospitalization | 3 = Recovered with sequela |
| Grade 4 = Life‐ threatening | 3 = Probably | 4 = Resulting in persistent significant disability or incapacity | 4 = Fatal | |
| Grade 5 = Death | 5 = Congenital anomaly or birth defect | 5 = Unknown | ||
| 6 = Medically significant or important medical condition | ||||
| 7 = Death | ||||
Abbreviations: AE, adverse event; GI, gastrointestinal; MSC, mesenchymal stem cell; SAE, serious adverse events; TB, tuberculosis; Y, yes.
Figure 1.
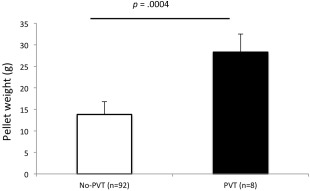
Patients who had PVT showed higher average islet pellet weight compared with patients without PVT. Islet pellet weights in patients who had PVT or didn't have PVT (no‐PVT) after islet transplantation. Error bars represent standard errors; p values are from Student's t test assuming unequal variances. Abbreviation: PVT, portal vein thrombosis.
MSC Patients Have Improved Glycemic Control
We first compared diabetes onset and mean insulin requirements of MSC and control patients. One in 3 (33%) MSC patients was insulin‐free, compared with 22.2% and 20.0% of historical control patients at 6 and 12 months, respectively (Fig. 2B). MSC patients required less daily insulin than historical controls on postoperative day 2 (10.7 ± 4.5 U vs. 22.0 ± 18.7 U, MSC: control, p = .02) and day 3 (5.3 ± 6.1 U vs. 18.7 ± 19.1 U, p = .04). Daily exogenous insulin usages still trended lower in MSC group at 6 months (9.3 ± 8.1 U vs. 18.1 ± 21.5 U, p = .19) and 12 months postoperatively (16.7 ± 15.0 U vs. 20.7 ± 23.1U, p = .69; Fig. 2C.
There was no difference in fasting blood glucose levels preoperatively. However, MSC patients had lower fasting blood glucose levels compared with controls at both 6 months (114.3 ± 28.4 mg/dl vs. 165.3 ± 95.2 mg/dl, p = .06) and 12 months (113.7 ± 26.0 mg/dl vs. 182.1 ±110.8 mg/dl, p = .02; Fig. 2D). No differences in HbA1c levels were observed between MSC patients and controls at any time (Fig. 2E). Patients in the historical group had significantly higher C‐peptide levels preoperatively compared with MSC patients (2.3 ± 2.3 ng/ml vs. 1.3 ± 0.1 ng/ml, p < .001). However, the 6‐month mean C‐peptide decline of controls dropped 1.5 ± 2.7 ng/ml, while C‐peptide levels in MSC group dropped 0.4 ± 0.4 ng/ml (p = .01; Fig. 2F).
Mixed meal tolerance tests were performed in the MSC and 4 historical control patients at 6 months postoperatively. There was no difference in peak C‐peptide level during the MMTT (2.2 ± 2.3 ng/ml vs. 1.9 ± 0.9 ng/ml, MSC vs. CTR, p = .87; Fig. 3A, 3B), C‐peptide area under the curve (24.9 ± 11.2 vs. 17.7 ± 19.5, MSC vs. CTR, p = .5; Fig. 3C), or Mixed Meal Stimulation Index (0.36 ± 0.22 vs. 0.22 ± 0.32, MSC vs. CTR, p = .5) between MSC and control populations, respectively (Fig. 3D).
Figure 3.
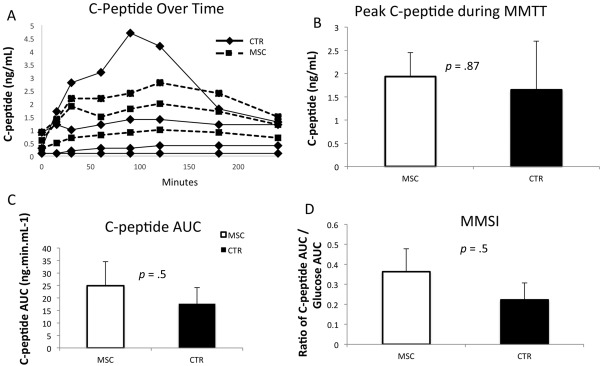
Results of MMTT at 6 months after islet transplantation in MSC patients (n = 3) and controls (n = 4). (A): Individual patient responses in C‐peptide to a MMTT during a 240 minutes after drinking the BOOST. Solid lines are from controls and dotted lines from MSC patients. (B): Peak C‐peptide level during an MMTT. (C): Mean C‐peptide area under the curve from control and MSC patients. (D): MMSI at 240 mins after MMTT. Error bars represent standard errors, p values are from Student's t test assuming unequal variances. Abbreviations: AUC, area under the curve; CTR, historical patients; MMSI, mixed meal stimulation index; MMTT, mixed‐meal tolerance test; MSC, mesenchymal stem cell.
Quality of Life
One in 3 patients in the MSC group required narcotics for long‐term pain relief, compared with 79% and 74% of the historical controls at both 6 and 12 months, respectively. MSC patients required much less morphine and equivalent at both time points (Fig. 4A). MSC patients showed significantly higher physical QOL with better pain relief at 6 months compared with controls (Fig. 4B, 4C). There were no differences in psychological QOL between MSC and control patients (Fig. 4D).
Figure 4.
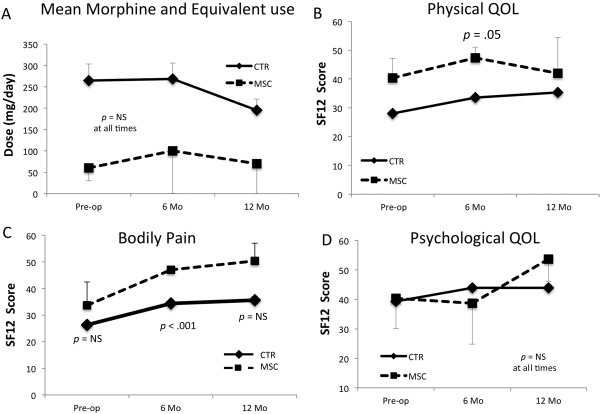
MSC (n = 3) cases' and historical control patients' (n = 101) morphine use and quality of life (QOL). Daily morphine and equivalent use (A), physical QOL (B), bodily pain (C), and psychological QOL (D) of MSC cases (dotted lines) and historical control patients (solid line) at preop and 6 Mo and 12 Mo postop. Error bars represent standard errors; p values are from Student's t test assuming unequal variances. Abbreviations: CTR, historical patients; Mo, month; MSC, mesenchymal stem cell; NS, not significant; QOL, quality of life.
Discussion
Although limited in the number of patients treated, our pilot study demonstrates for the first time that intrahepatic infusion of autologous bone marrow‐derived MSCs during islet transplantation may be safe and may have the potential to improve islet engraftment, glycemic control, and quality of life. This work extends the current paradigm of MSCs as an immune regulatory factor and reveals important additional functions of MSCs in promoting islet engraftment after transplantation in TP‐IAT patients.
It seems that MSC infusion via the portal vein was safe and well tolerated by our three islet transplant patients. Increasing numbers of clinical trials are evaluating the therapeutic effects of MSC in a wide range of diseases and conditions related to autoimmune disorders, inflammatory diseases, and regenerative disorders. Data from MSC‐treated patients have demonstrated the safety of this procedure 36. Safety is particularly difficult to assess following an operation with known major morbidity such as pancreatectomy. Therefore, we relied on the independent DSMB to adjudicate all events as related or not related to MSC infusion. All AEs observed were determined as not related to MSC infusion.
One of the common complications of islet transplantation is PVT. The slowed portal vein flow by cellular occlusion is in part responsible, and this complication may be related to the volume of cells infused into the portal vein. It has been reported that higher islet tissue volume is associated with higher portal pressure and complications during autologous islet transplantation 37. We also found that PVT occurrence is positively correlated with islet pellet weight (Fig. 1) and hepatic pressure (Table 1). Because Patients 2 and 3 in the MSC group had high pellet weights and higher hepatic pressures during product infusion, they had higher chance of PVT. In future studies, purification may be used to reduce the total islet tissue volume and the complications it may cause 37. Whether MSC infusion increases the chance of thrombosis is controversial since these cells are significantly smaller than islets. However, microvascular obstruction after intracoronary delivery has been described 38. In addition, MSCs may have innate procoagulant activity. One previous study showed that cultured MSCs trigger an IBMIR when injected in a higher dose (>1 × 106 per kg) 39. Other studies indicate that local MSC injection may restore a thrombosed vein by clot recanalization and may stimulate angiogenesis 40. Although studies have shown that partial thrombosis did not affect islet function 41, further studies with more patients are needed to exclude the possibility that MSC infusion increases the chance of thrombosis in islet transplantation patients.
Another finding of this study is that MSC infusion improved glycemic control in CP patients. Although we did not observe differences in HbA1c and C‐peptide levels, MSC patients required less exogenous insulin and had lower blood glucose levels at 12 months. Tempering the enthusiasm is the heterogeneity of 6‐month MMTT responses that will need much larger cohorts to define the correlates of islet cell survival and function.
Two potential mechanisms might have contributed to the improvement in glycemic control. First, as suggested in the nonhuman primate and mouse islet transplantation models, MSC cotransplantation increased islet engraftment by inhibition of islet cell death and enhanced graft revascularization after transplantation 27. Second, infused MSCs increased insulin sensitivity in IAT patients and therefore led to a better glycemic control, as observed in mouse models of obesity and diabetes 42. In addition, MSC might also have played a role in pain relief and therefore quality of life in this patient population, as observed in other studies 43, 44. Further studies are needed to test this possibility.
In addition to the small cohort size, a major limitation of this study is the large difference in islet number and islet numbers per kg body weight transplanted between MSC patients and controls. Several studies showed a positive correlation between high islet cell yields and postoperative insulin independence 45, 46. When initial yields are high, islets are subject to damage and loss during and after transplantation.
There are still many unanswered questions that can be resolved in further trials. The first is to determine an ideal number of MSC needed for the islet transplant patients. Although the large number and types of currently registered MSC clinical trials highlight the safety and tolerability of MSCs infusions, the ideal dosage of MSC for a certain disease is unknown. In graft versus host disease trials, patients have been given intravenous doses of 100 × 106 cells/kg MSCs 47. In Crohn's disease, patients have received intravenous infusions of allogeneic MSCs (2 × 106 cells/kg body weight) weekly for 4 weeks 48. In an allogeneic islet transplantation model in the nonhuman primate, 5 × 106 MSCs were mixed with 10,000–20,000 IEQ islets and cotransplanted intraportally into recipients. The islet cell and MSC ratios ranged from 3.7 to 13.4 [27]. The MSCs we used were autologous in nature, delivered fresh, and not cryopreserved as in other studies. By delivering autologous cells, we hoped to prolong MSC survival in our patients and produce a better clinical response. Another benefit of delivering fresh cells is that they are more metabolically active than those being thawed and infused at the bedside, and, consequently, DMSO‐associated side effects following infusion would be absent.
The duration of MSC survival and ultimate differentiation pathways remain unknown in humans. In the allogeneic nonhuman primate study, MSCs injected via the portal vein were observed in the pancreas and lung of one monkey but not the other at 1 month after cell infusion 27. In lupus patients, the beneficial effect of MSC lasted from 6 months to >2 years after infusion of MSC 49. In the future, it will be possible to study cell trafficking by the infusion of labeled cells with cell tracer.
The mechanisms by which MSCs confer islet protection also remain unknown. As shown in a nonhuman primate islet transplantation model, the most probable mechanism is that MSCs stay in proximity to islets at the time of transplantation and provide vascularization and regenerative signals that contribute to graft survival 27. The local balance of the proinflammatory and anti‐inflammatory microenvironment may be altered, as is suggested in other diseases 50. In addition, MSCs cotransplanted with islets have the potential to differentiate into insulin‐secreting cells, because MSCs have high plasticity. This has been shown in diabetic rat models in vitro and in vivo 51, 52.
To the best of our knowledge, this is the first clinical trial showing that intrahepatic cotransplantation of autologous MSCs with islets improves glycemic control and quality of life of IAT patients. Although only three patients have undergone this novel treatment approach, we did observe improvements in MSC patients compared with 101 matched historical patients.
Conclusion
Autologous MSC and islet cotransplantation may be a promising approach to improve the success rates of autologous islet transplantation and to enhance current autologous islet transplantation protocols. The results of this pilot study show that cotransplantation of MSC with islets may be a safe and feasible strategy to improve the efficacy of islet transplantation. This procedure can also serve as a platform to solve more complex allogeneic islet transplantation issues and treat patients with type 1 diabetes.
Author Contributions
H.W.: conception and design, performance of some of the study, data analysis and interpretation, manuscript writing; C.S.: data analysis and interpretation, manuscript writing; P.N.: conception and design, data analysis and interpretation, manuscript writing; J.W., C.C., S.O., T.D., B.S.: performance of the study; T.T.: data analysis and interpretation, manuscript writing; G.G., L.L.: conception and design; K.H., J.F.: evaluation of treatment safety; D.A., K.M.: performance of surgery, patient care, manuscript writing.
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Note Added in Proof
This article was published online on 21 November 2017. Minor edits have been made that do not affect data. This notice is included in the online and print versions to indicate that both have been corrected 28 December 2017.
Acknowledgments
We thank Dr. Jacque Galipeu (University of Wisconsin, Madison) for kindly offering human platelet lysate. We thank the Reeves family for their research support. This study was supported in part by the National Institutes of Health (EB015744, DK097544, DK105183, and DK099696 to H.W.). Dr. Nietert's work on this project was supported in part by the National Center for Advancing Translational Science (UL1 TR000062), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR062755), and the National Institute of General Medical Sciences (U54‐GM104941). H.W. is the guarantor of this study and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
References
- 1. Montaña E, Bonner‐Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest 1993;91:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davalli AM, Scaglia L, Zangen DH et al. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 1996;45:1161–1167. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017;13:268–277. [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Sun Z, Gou W et al. α‐1 antitrypsin enhances islet engraftment by suppression of instant blood‐mediated inflammatory reaction. Diabetes 2017;66:970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naziruddin B, Iwahashi S, Kanak MA et al. Evidence for instant blood‐mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014;14:428–437. [DOI] [PubMed] [Google Scholar]
- 6. Sakata N, Hayes P, Tan A et al. MRI assessment of ischemic liver after intraportal islet transplantation. Transplantation 2009;87:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmad SA, Lowy AM, Wray CJ et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005;201:680–687. [DOI] [PubMed] [Google Scholar]
- 8. Ryan EA, Paty BW, Senior PA et al. Five‐year follow‐up after clinical islet transplantation. Diabetes 2005;54:2060–2069. [DOI] [PubMed] [Google Scholar]
- 9. Argo JL, Contreras JL, Wesley MM et al. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg 2008;74:530–536; discussion 536–537. [PubMed] [Google Scholar]
- 10. Anazawa T, Balamurugan AN, Bellin M et al. Human islet isolation for autologous transplantation: Comparison of yield and function using SERVA/Nordmark versus Roche enzymes. Am J Transplant 2009;9:2383–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hering BJ, Kandaswamy R, Ansite JD et al. Single‐donor, marginal‐dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830–835. [DOI] [PubMed] [Google Scholar]
- 12. Rickels MR, Liu C, Shlansky‐Goldberg RD et al. Improvement in β‐cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013;62:2890–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 14. Ge W, Jiang J, Arp J et al. Regulatory T‐cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3‐dioxygenase expression. Transplantation 2010;90:1312–1320. [DOI] [PubMed] [Google Scholar]
- 15. Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42–48. [DOI] [PubMed] [Google Scholar]
- 16. Casiraghi F, Azzollini N, Cassis P et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 2008;181:3933–3946. [DOI] [PubMed] [Google Scholar]
- 17. Fiorina P, Jurewicz M, Augello A et al. Immunomodulatory function of bone marrow‐derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009;183:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol 2011;10:649–656. [DOI] [PubMed] [Google Scholar]
- 19. Lee RH, Seo MJ, Reger RL et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA 2006;103:17438–17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Urbán VS, Kiss J, Kovács J et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells 2008;26:244–253. [DOI] [PubMed] [Google Scholar]
- 21. Ezquer FE, Ezquer ME, Parrau DB et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 2008;14:631–640. [DOI] [PubMed] [Google Scholar]
- 22. Sordi V, Malosio ML, Marchesi F et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005;106:419–427. [DOI] [PubMed] [Google Scholar]
- 23. Sakata N, Chan NK, Chrisler J et al. Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation 2010;89:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solari MG, Srinivasan S, Boumaza I et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long‐term islet allograft survival and sustained normoglycemia. J Autoimmun 2009;32:116–124. [DOI] [PubMed] [Google Scholar]
- 25. Figliuzzi M, Cornolti R, Perico N et al. Bone marrow‐derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc 2009;41:1797–1800. [DOI] [PubMed] [Google Scholar]
- 26. Ito T, Itakura S, Todorov I et al. Mesenchymal stem cell and islet co‐transplantation promotes graft revascularization and function. Transplantation 2010;89:1438–1445. [DOI] [PubMed] [Google Scholar]
- 27. Berman DM, Willman MA, Han D et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 2010;59:2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeung TY, Seeberger KL, Kin T et al. Human mesenchymal stem cells protect human islets from pro‐inflammatory cytokines. PLoS One 2012;7:e38189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrara N, Davis‐Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4–25. [DOI] [PubMed] [Google Scholar]
- 30. Neufeld G, Cohen T, Gengrinovitch S et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9–22. [PubMed] [Google Scholar]
- 31. Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Translational Medicine 2013;2:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin I, Baldomero H, Bocelli‐Tyndall C et al. The survey on cellular and engineered tissue therapies in Europe in 2009. Tissue Eng Part A 2011;17:2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang H, Desai KD, Dong H et al. Prior surgery determines islet yield and insulin requirement in patients with chronic pancreatitis. Transplantation 2013;95:1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bellin MD, Beilman GJ, Dunn TB et al. Sitagliptin treatment after total pancreatectomy with islet autotransplantation: A randomized, placebo‐controlled study. Am J Transplant 2017;17:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Faradji RN, Tharavanij T, Messinger S et al. Long‐term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation 2008;86:1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei X, Yang X, Han ZP et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 2013;34:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsumoto S, Takita M, Shimoda M et al. Impact of tissue volume and purification on clinical autologous islet transplantation for the treatment of chronic pancreatitis. Cell Transplant 2012;21:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gleeson BM, Martin K, Ali MT et al. Bone marrow‐derived mesenchymal stem cells have innate procoagulant activity and cause microvascular obstruction following intracoronary delivery: Amelioration by antithrombin therapy. Stem Cells 2015;33:2726–2737. [DOI] [PubMed] [Google Scholar]
- 39. Moll G, Rasmusson‐Duprez I, von Bahr L et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012;30:1565–1574. [DOI] [PubMed] [Google Scholar]
- 40. Maiborodin IV, Morozov VV, Markevich YV et al. Acceleration of angiogenesis after paravasal injection of mesenchymal stem cells at the site of modeled venous thrombosis. Bull Exp Biol Med 2015;159:128–133. [DOI] [PubMed] [Google Scholar]
- 41. Kawahara T, Kin T, Kashkoush S et al. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am J Transplant 2011;11:2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cao M, Pan Q, Dong H et al. Adipose‐derived mesenchymal stem cells improve glucose homeostasis in high‐fat diet‐induced obese mice. Stem Cell Res Ther 2015;6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siniscalco D, Giordano C, Galderisi U et al. Human mesenchymal stem cells as novel neuropathic pain tool. J Stem Cells Regen Med 2010;6:127. [PubMed] [Google Scholar]
- 44. Das AK, Bin Abdullah BJ, Dhillon SS et al. Intra‐arterial allogeneic mesenchymal stem cells for critical limb ischemia are safe and efficacious: Report of a phase I study. World J Surg 2013;37:915–922. [DOI] [PubMed] [Google Scholar]
- 45. Matsumoto S, Noguchi H, Takita M, Shimoda M, Tamura Y, Olsen G, Naziruddin B, Onaca N, Levy MF. Super‐high‐dose islet transplantation is associated with high SUITO index and prolonged insulin independence: a case report. Transplant Proc 2010;42:2156–2158. [DOI] [PubMed] [Google Scholar]
- 46. Johnston PC, Lin YK, Walsh RM, Bottino R, Stevens TK, Trucco M, Bena J, Faiman C, Hatipoglu BA. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off‐site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015;100:1765–1770. [DOI] [PubMed] [Google Scholar]
- 47. Muroi K, Miyamura K, Okada M et al. Bone marrow‐derived mesenchymal stem cells (JR‐031) for steroid‐refractory grade III or IV acute graft‐versus‐host disease: A phase II/III study. Int J Hematol 2016;103:243–250. [DOI] [PubMed] [Google Scholar]
- 48. Forbes GM, Sturm MJ, Leong RW et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol 2014;12:64–71. [DOI] [PubMed] [Google Scholar]
- 49. Sun L, Akiyama K, Zhang H et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009;27:1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel AN, Genovese J. Potential clinical applications of adult human mesenchymal stem cell (Prochymal®) therapy. Stem Cells Cloning 2011;4:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khorsandi L, Nejad‐Dehbashi F, Ahangarpour A et al. Three‐dimensional differentiation of bone marrow‐derived mesenchymal stem cells into insulin‐producing cells. Tissue Cell 2015;47:66–72. [DOI] [PubMed] [Google Scholar]
- 52. Haidara MA, Assiri AS, Youssef MA et al. Differentiated mesenchymal stem cells ameliorate cardiovascular complications in diabetic rats. Cell Tissue Res 2015;359:565–575. [DOI] [PubMed] [Google Scholar]


