Summary
The California Institute for Regenerative Medicine has formed a group of clinics called the Alpha Stem Cell Clinics Network. Its goal is to accelerate clinical trials of stem cell‐based therapies for diseases with unmet medical needs. In this report, we describe our experience in establishing an Alpha Stem Cell Clinic at City of Hope. Implementation and integration of the clinic into the existing institutional structures required collaboration and cooperation with clinical trial units, nursing administration, and creation of new positions. The highlight of this process and the centerpiece to our success has been the definition of the role of the “hybrid nurse,” a person with nursing competencies in both clinical care and research. stem cells translational medicine 2018;7:6–10
Abstract Video Link: https://youtu.be/WOeZrNyXkGU
Significance Statement.
It is increasingly important that stem cell therapies be evaluated in competent facilities and, in view of the unmet medical needs that could be provided by such treatments, that the work be accelerated. For this reason, the California Institute for Regenerative Medicine has formed a network of clinics called the Alpha Stem Cell Clinics Network. Establishing a stem cell clinic comes with challenges, but integration into the existing clinical and research structures is feasible, and nursing competencies play a crucial role in the success of this process.
Introduction
The California Institute for Regenerative Medicine (CIRM) proposed the concept of an Alpha Stem Cell Clinic (ASCC), also known as Alpha Clinic, in 2012. The ASCC was envisioned as a specialized clinic dedicated to accelerating the development of stem cell‐based therapeutic trials for patients with unmet medical needs 1. Without such an effort, the public remains increasingly vulnerable to the use of untested stem cell therapies 2. CIRM launched the actual ASCC Network in 2015 with three clinics, which are centers of excellence located at City of Hope (COH) National Medical Center, University of California (UC) San Diego, and at UC Los Angeles in collaboration with UC Irvine 3.
For the past two and a half years, each clinic has been expanding California's capacity for realizing the promise of regenerative or curative therapies using stem cells for targeted diseases. In addition, the ASCC Network maintains a portfolio of preclinical projects to guide and accelerate basic and translational research. The overall goal is to bring development, manufacturing, and clinical capacity to bear on intractable diseases using stem cell treatments.
In describing clinical operations, each of these state‐of‐the‐art clinics gives considerable focus to the infrastructure required to manufacture and deliver cell products following good manufacturing practice and good clinical practice (GCP). At COH, we have capitalized on the existing highly trained personnel involved in management of cell products and the delivery of patient‐centric care for hematopoietic cell transplantation (HCT). Deployment of these capacities within the clinic setting depends on our nurses and the ability to implement novel cell‐based therapies within a discrete nursing unit. This approach provided the nursing expertise required for management of very sick patients, but was challenged by the need to develop research competencies among clinical staff.
This report describes our experience in setting up the ASCC at COH, involving its integration into the hospital administrative structure while maintaining an alignment with the host research organization. An important outcome has been the erasing of borders between nursing and research, thus creating (a) new nursing competencies, which result in high patient satisfaction and support the efficiency of the overall process and (b) the role of “hybrid nurse” with a set of core competencies for successful delivery of stem cell treatments.
Integration of the ASCC into Existing Institutional Research Systems
In funding the ASCC, CIRM asked for the efficient implementation of stem cell‐based research protocols and development of sustainable clinical activities within the 5‐year funding period. For such novel therapies, it was necessary to have a specialized outpatient facility with a business plan to incorporate coordination of clinical care and education of nurses and patients, while maintaining usual data collection, biospecimen processing, and regulatory activities essential to research trials. COH ASCC operations became integral to the existing systems of inpatient and outpatient clinical care as well as to the clinical research unit/clinical trials office (CRU/CTO) (Fig. 1). Importantly, the ASCC was administered not as a subunit of the CRU/CTO, but as a clinical nursing unit of the COH Day Hospital, which specializes in outpatient autologous HCT. This is because the ASCC primarily oversees cell‐based clinical trials, and the CRU/CTO primarily oversees investigational drug trials.
Figure 1.
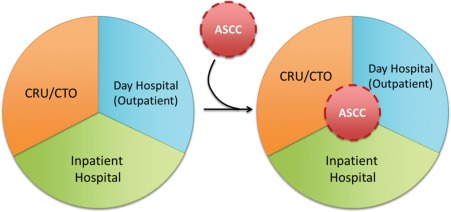
Integration of the City of Hope (COH) ASCC into the existing clinical research and inpatient and outpatient clinics of COH. Abbreviations: ASCC, Alpha Stem Cell Clinic; CRU, clinical research unit; CTO, clinical trials office.
Since the treatment methods used in these novel stem cell‐based trials likely will be part of any eventual “package insert” describing instructions for outpatient use of the approved product, it made sense to test them in the actual site of such eventual therapy. Thus, the ASCC was housed in the Day Hospital, and in doing this, available nurses used their clinical insight and technical skills to provide valuable input to the cell‐based trial protocol designs, ensuring consistency with clinical practice requirements.
Creation of this adjunct location for the provision of care for patients participating in complex stem cell research studies introduced a “disruptive” activity into the normal workflow within our organization. Not only did the ASCC take patients from the ambulatory clinics that had supported their particular treatment, but also competed with the CRU/CTO for these novel first‐in‐human trials. The challenge was to show the main stakeholders, the Principal Investigators (PI) and their protocol management teams (PMT), the CRU/CTO, and the hospital administration, that the ASCC did not threaten their goals. To mitigate this disruptive change, the ASCC staff and the PMT defined the protocol‐related responsibilities and distributed them appropriately. This process results in a matrix model of roles versus responsibilities that are established during each protocol intake and could differ depending on the needs of the PMT. The selling point to attract new protocols was the promise that the ASCC could provide highly desired nursing expertise derived from outpatient transplantation and incorporate this into new protocol requirements. ASCC also offered other support for trial implementation, including patient scheduling, education, and navigation, biospecimen processing, and regulatory assistance, all of which are essential aspects for accelerating stem cell clinical studies.
The PMTs became used to working with the ASCC and eventually sought out the resources and skills available. For example, the nurse to patient ratio in the COH ASCC, based on patient acuity, varies from 1:1, during any stem cell‐based therapy infusion, to lower ratios depending on monitoring, decision‐making, care coordination, and technical skills necessary to deliver care. The Day Hospital is open 7 days per week and has nine bays, of which the ASCC uses approximately 3 daily; this utilization depends on the transplant volume and patient acuity. After discharge, ASCC patients are provided a card that identifies and prioritizes their status as ASCC patients. If they need emergent care, they call for nursing triage who facilitates communication with the PMT and appropriate hospital services. The primary goal is to stabilize the patient and then directly transfer to the appropriate level of care.
Data collected using the Press Ganey survey technique (http://www.pressganey.com/) show that patient satisfaction constantly ranks high in the COH Day Hospital and ASCC. Nursing engagement ranks at or above the 75th percentile in 11 of 17 engagement categories surveyed by a Gallup poll at COH. No staff member resigned since the opening of the Day Hospital and staff numbers grew from 4 FTE in 2013 to 14.67 FTE currently. The ASCC unit focuses also on eventual financial sustainability by maintaining the high standards of the Foundation for the Accreditation of Cellular Therapy, an accreditation required for patient care reimbursement from many government agencies and health insurance companies. By linking accredited procedures with future cellular therapies approved for reimbursement by third party medical insurance, ASCC hopes to accelerate the market penetration of new standard of care treatments.
Stem Cell Therapies Require Specific Nursing Competencies
An ASCC must be prepared for a variety of procedures ranging from classical HCT, brain or spinal cord injection of neural stem cells, retinal, liver, lung, or cardiac delivery of stem cells, and infusion of chimeric antigen receptor (CAR) T cells. Some procedures include viral vector infusions for in vivo targeted stem cell sites and others require preparative chemotherapy for lymphodepletion. Our nurses’ ability to coordinate these specialized, complex outpatient activities is paramount to safe and efficient conduct of clinical trials. The initial staffing of the COH ASCC utilized transplantation‐experienced nurses from our Day Hospital and was managed by the COH Nursing Division. These nurses have extensive experience in stem cell infusion, chemotherapy administration, acute symptom management, and care for highly complex patients. However, they were not all familiar with every stem cell procedure and lacked credentials in human research protection.
The COH ASCC created the role of “hybrid nurse,” that is, a nurse with patient care experience and confidence as well as clinical research training. This skill set allows for successful integration of novel research protocols into the daily work of a busy outpatient clinical nursing unit. The program took clinical nurses and transformed them into “hybrid nurses” by training them in human research protection and GCP using modules provided by the Collaborative Institutional Training Initiative [collaborative institutional training initiative; https://www.citiprogram.org]. Key to this innovation was the establishment of a role called Patient Care Coordinator (PCC) who is responsible for developing an educational program to teach nurses about the novel research cell products that they would be handling (see a list of training and assessment documents in Table 1).
Table 1.
List of training documents for “hybrid nurses” currently used at City of Hope Alpha Stem Cell Clinic
| Document title | Brief description |
|---|---|
| Alpha Clinic CVI | Validation of minimum nursing skills/knowledge performed initially upon hire and then annually |
| Protocol overview for nurses | Concise description of protocol background and scientific rationale for the study |
| HCT Department CVI | Transplant‐specific competency inventory of minimum skills required to perform autologous and allogeneic transplants |
| QRG | Concise, but detailed, stand‐alone reference guide for nursing instructions, procedures, or supply/equipment usage |
| Alpha Clinic Patient Call Flow Chart | The algorithm for triaging any active ASCC patient outside of scheduled appointments |
| General stem cell and CAR T cell educational materials | International Society for Stem Cell Research approved “Stem Cell Facts” and “Patient Handbook on Stem Cell Therapies” [with permission from ISSCR] |
Abbreviations: ASCC, Alpha Stem Cell Clinic; CAR, chimeric antigen receptor; CVI, Competency Validation Inventory; HCT, hematopoietic cell transplantation; QRG, Quick Reference Guide.
To ensure necessary core competencies for our diverse portfolio of trials, each nurse is required to do an initial and an annual self‐assessment of skills and knowledge using a Core Competency Validation Inventory (CVI). The CVI, developed at COH by its Nursing Professional Practice Leader Group for stem cell transplantation, provides an inventory of validated nursing skills that are actionable, modified to specific ASCC nursing activities, and include human research protection training, as well as patient management of possible and expected side effects. This validation includes cognitive, psychomotor, and affective assessments. Scoring is based on Benner's novice‐to‐expert framework 4, in which the competence level is rated as 0 for not familiar, 1 for novice, 2 for advanced beginner, 3 for competent, 4 for proficient, and 5 for expert.
To embrace this new role, a “hybrid nurse” must have a conceptual understanding of the mission and goals of both the ASCC and CIRM and be educated on the ethical responsibilities of doing first‐in‐human research while maintaining high‐level clinical performance. Nurses need to understand the intricacies of each protocol and their role in stem cell therapy administration, care management, and patient education. For this purpose, we used the COH electronic research protocol database (Clinical Trials On Line) linked to the electronic medical record, allowing nurses to navigate protocol structures and glean protocol imperatives.
Education about the unique care needs of patients participating in stem cell‐based trials is incorporated into the mandatory annual education event conducted by the COH Division of Nursing. This process ensures that every nurse knows how to access electronic copies of the various protocols, standard research orders, consent forms, and Investigator's Brochures. In particular, the restricted medication list for each study is available online in the event patients present to any area of the hospital for care outside the ASCC. Nursing competencies are demonstrated for research treatments such as neural stem cell infusions into the brain and infusions of CAR T‐cells, viral vectors, and HCT, all done in the outpatient Day Hospital (case studies discussed in Table 2). Nurses quickly assimilated to these settings and acquired competencies needed for patient care and assessment and reporting of adverse side effects, such as the recognition of cytokine‐release syndrome triggered by CAR T‐cell therapies.
Table 2.
Two case studies illustrating competencies, challenges, and solutions with performing stem cell therapy clinical trials in the Alpha Stem Cell Clinic
| Case study A | |
| Therapy | Gene‐modified NSC |
| Disease target | Glioma |
| Sponsor | Academia |
| Required competencies | High acuity nursing care including certification in chemotherapy administration and biotherapy infusion; HCT department CVI; CITI certification for human subjects research |
| Challenges with study implementation |
Technical issues: CSF specimen collection from microcontainer (1 ml) at bedside; use of research pumps for CNS infusion; Nursing issues: care of very sick patients with low Karnofsky status within an outpatient setting; extended nursing care hours; novel documentation for infusion of NSC |
| Solutions and new methods | The PCC provided support in the form of educational materials and guidelines, adequate staffing based patient acuity mix, enhancement of teamwork among nurses, modification of existing FACT‐compliant blood stem cell documentation templates, creation of ASCC guidelines for all stem cell infusions implemented at the bedside |
| Performance metric | Press Ganey survey of hospital unit; CVI assessments; tracking of protocol deviations reported to IRB |
| Case study B | |
| Therapy | Gene modified HSPC |
| Disease target | HIV‐1 infection |
| Sponsor | Academia |
| Required competencies | High acuity nursing care including certification in chemotherapy administration and biotherapy infusion; HCT department CVI; CITI certification for human subjects research |
| Challenges with study implementation | Complexity of the study regarding PK‐based chemotherapy adjustment and correlative studies; need for HCT recipient and caregiver education for outpatient post HCT |
| Solutions and new methods | PCC provided physical support, prepared educational materials and guidelines, adequate staffing, enhancement of teamwork among nurses, improved communication with all clinical disciplines involved with patient care |
| Performance metrics | Press Ganey survey of hospital unit; CVI assessments; tracking of protocol deviations reported to IRB |
Abbreviations: ASCC, Alpha Stem Cell Clinic; CITI, collaborative institutional training initiative; CNS, central nervous system; CSF, cerebrospinal fluid; CVI, competency validation inventory; FACT, Foundation for the Accreditation of Cellular Therapy; HCT, hematopoietic cell transplantation; HSPC, hematopoietic stem/progenitor cells; IRB, institutional review board; NSC, neural stem cells; PCC, patient care coordinator; PK, pharmacokinetics.
The ASCC Patient Care Coordinator Role
To improve study execution, CIRM requires an essential role for a PCC to assure subject safety and retention by focus on patient/caregiver education and ease of patient navigation through the various phases of trials. The PCC is the lead nurse in ASCC, responsible for educating nursing staff and caregivers, patient and family, hospital‐wide triage nurses, nursing house supervisors, and, at times, the PI who might not be familiar with hospital requirements outside of the CRU/CTO.
When patients are enrolled in ASCC trials, the PCC usually becomes their primary point person. The PCC is the bridge connecting the research participant with clinic nurses and research staff, personalizing the patient's journey by developing necessary education materials. This reduces research participant/caregiver anxiety by providing personalized patient calendars, maps, procedure descriptions or plan of care so that 24/7 support is available as needed. In our experience, these initiatives play a key role in building patient trust and confidence, ensuring patient adherence to particularly demanding clinical trials. This results in protocol compliance that is the key to success.
From the outset, the PCC participates in research protocol reviews, assesses the impact of study activation in the ASCC, anticipates obstacles and proposes solutions, and develops resources and tools to support trial implementation. To accomplish this, the PCC acts as “translator” of the study protocol for the nursing staff and develops information sheets describing the scientific basis and goals of each protocol, requirements for clinical assessment, lab specimen collection, treatment administration, monitoring parameters, and management of expected and unexpected side effects. Should a new skill or assessment be required, the PCC, in collaboration with the nursing leadership team, establishes the necessary education and competency review for the nurses in the ASCC.
Conclusion
Implementation of the CIRM‐funded COH ASCC has contributed to the strengthening of nursing competencies for cell therapies and the education of nursing personnel necessary for acceleration of novel cell therapy trials. By placing such activities on a nursing unit, the clinical care nurse can be brought into the realm of clinical research in a new role as “hybrid nurse.” The advantages of this union continue to be observed, but when such therapies are approved for general use, the nursing procedures and competencies necessary for broad implementation will already be available. The CIRM ASCC Network uses several metrics to evaluate the effort and to ensure acceleration of trials, safety of patients, and sustainability of this effort. It is anticipated that stem cell clinics or clinical research units such as the ASCC will become a model, directly contributing to the approval by the FDA of stem cell‐based therapies.
Author Contributions
M.P., T.K.: collection and/or assembly of data, manuscript writing; R.S., V.L.V.: manuscript writing; P.G., J.T.: collection and/or assembly of data; S.J.: conception and design, manuscript writing; G.P.L.: conception and design, and final approval of manuscript; J.A.Z.: conception and design, manuscript writing, and final approval of manuscript.
Disclosure of Potential Conflicts of Interest
S.J. is a consultant with Oncology Resource Consultants, Inc. G.L. has compensated research funding and CIRM provides funding to COH. J.A.Z. has research funding from California Institute for Regenerative Medicine grants.
Note Added in Proof
This article was published online on 03 November 2017. Minor edits have been made that do not affect data. This notice is included in the online and print versions to indicate that both have been corrected 28 December 2017.
Acknowledgment
This work has been funded by the California Institute for Regenerative Medicine Grant AC1–07659.
References
- 1. Trounson A, DeWitt ND, Feigal EG. The alpha stem cell clinic: A model for evaluating and delivering stem cell‐based therapies. Stem Cells Translational Medicine 2012;1:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner L. US stem cell clinics, patient safety, and the FDA. Trends Mol Med 2015;21:271–273. [DOI] [PubMed] [Google Scholar]
- 3. Lomax GP, Adams J, Jamieson AC et al. Proceedings: Accelerating stem cell treatments for patients: The value of networks and collaboration. Stem Cells Translational Medicine. Available at https://www.cirm.ca.gov/sites/default/files/files/about_cirm/SCTM_Proceedings.pdf. Accessed October 13, 2017. [Google Scholar]
- 4. Benner P. From Novice to Expert: Excellence and Power in Clinical Nursing Practice. 2nd ed Upper Saddle River, NJ: Prentice Hall Press, 2001. [Google Scholar]


