Abstract
Distal extremity wounds are a significant clinical problem in horses and humans and may benefit from mesenchymal stem cell (MSC) therapy. This study evaluated the effects of direct wound treatment with allogeneic stem cells, in terms of gross, histologic, and transcriptional features of healing. Three full‐thickness cutaneous wounds were created on each distal forelimb in six healthy horses, for a total of six wounds per horse. Umbilical cord‐blood derived equine MSCs were applied to each wound 1 day after wound creation, in one of four forms: (a) normoxic‐ or (b) hypoxic‐preconditioned cells injected into wound margins, or (c) normoxic‐ or (d) hypoxic‐preconditioned cells embedded in an autologous fibrin gel and applied topically to the wound bed. Controls were one blank (saline) injected wound and one blank fibrin gel‐treated wound per horse. Data were collected weekly for 6 weeks and included wound surface area, thermography, gene expression, and histologic scoring. Results indicated that MSC treatment by either delivery method was safe and improved histologic outcomes and wound area. Hypoxic‐preconditioning did not offer an advantage. MSC treatment by injection resulted in statistically significant increases in transforming growth factor beta and cyclooxygenase‐2 expression at week 1. Histologically, significantly more MSC‐treated wounds were categorized as pro‐healing than pro‐inflammatory. Wound area was significantly affected by treatment: MSC‐injected wounds were consistently smaller than gel‐treated or control wounds. In conclusion, MSC therapy shows promise for distal extremity wounds in horses, particularly when applied by direct injection into the wound margin. stem cells translational medicine 2018;7:98–108
Keywords: Mesenchymal stem cells, Tissue regeneration, Animal models, Cord blood, Hypoxia
Significance Statement.
Distal extremity wounds are a significant clinical problem in horses and humans and may benefit from mesenchymal stem cell (MSC) therapy. This study evaluated the effects of direct wound treatment with allogeneic stem cells. This study provides evidence that MSC therapy shows promise for distal extremity wounds in horses, particularly when applied by direct injection into the wound margin. Interestingly, hypoxic preconditioning did not offer an advantage in this study. These findings in a horse model may be directly applicable to chronic wound studies in human patients, and provide insights to cellular based approaches of treatment.
Introduction
Chronic wound management is a growing problem for human health care around the world 1, 2. Full thickness wounds and extensive burns are extremely detrimental to patients even with early intervention 3. Traumatic wounds also commonly occur in the distal limbs of horses and healing is often delayed, due to high skin tension, and minimal subcutaneous stroma for vascular and structural support, and an aberrant inflammatory response. The pathobiology occurring in equine distal limb wounds is characterized by prolonged but somewhat ineffectual inflammation 4, 5, persistent transforming growth factor beta 1 (TGFβ1) signaling leading to a fibroproliferative response 6, and regional hypoxia with microvascular occlusion 7, 8, 9, 10. Epithelialization is slow and there is minimal capacity for wound contraction in this location. Taken together, these factors result in a delayed and often dysplastic healing process 11, as is the case for many human patients as well. Both species demonstrate hypoxia and delayed or absent epithelialization in distal extremity wounds, and horses have been proposed as a translational model for human wound research 12. The development of new methods to augment healing in distal extremity wounds could have significant clinical impact for both horses and people.
Recent studies have demonstrated beneficial effects of mesenchymal stem cells (MSC) on wound repair in laboratory animals 13, 14, 15 and in one case series of horses 16. It has also been reported that umbilical cord derived MSCs have improved burn repair in rodent models 15, 17. Preconditioning MSCs in a hypoxic environment prior to tissue delivery appears to increase prosurvival and proangiogenic gene expression 18, 19. Since these cells may improve neovascularization, reduce inflammatory cytokines, and promote wound contraction 19, they may be of particular benefit in the inherently hypoxic and persistently inflamed distal limb wounds of horses. Several MSC delivery methods have been reported, including intradermal injection 13, 16, 20 and topical application of cells within a synthetic 21 or natural polymer construct 22, such as fibrin 3, 23, 24.
Autologous and allogeneic MSCs have been widely used in both research and clinical applications in the horse, particularly in the treatment of musculoskeletal tissues 25, 26. Recent studies have also reported stem cell therapy to improve equine wound healing in vitro and in vivo 16, 27, 28, 29. Multipotent cells have been sourced from equine peripheral blood 16, bone marrow, adipose tissue, tendon, umbilical cord blood and tissue 30, amnion 31 and skin 32, and pluripotent cells have been obtained from embryos or induced from fibroblasts and keratinocytes 33, 34, 35, 36, 37. Induced pluripotent cells of fetal origin have also been directed into keratinocyte lineage with a view toward cultured equine skin constructs 33. Umbilical cord blood MSCs have been well‐characterized in our laboratory 38, 39, 40 and were selected for this study based on their stability within fibrin gels during pilot studies and their nitric oxide production 39, which could prove advantageous in the hypoxic wound environment of the distal extremity.
In this study, we evaluated the application of allogeneic cord‐blood derived MSCs to experimentally created distal limb wounds in horses. We compared hypoxic to normoxic‐preconditioning of the cells, and also compared their delivery by direct injection versus topical application embedded within a fibrin gel. We hypothesized that MSC‐treated wounds would have improved outcomes in wound area, thermography, gene expression, and histology when compared to untreated controls. We further hypothesized that hypoxic‐preconditioned MSCs would be of greater benefit for wound healing than normoxic MSCs.
Methods
Animals
Six adult horses (three females, three castrated males) of varying breeds (four Quarter Horses, two Warmbloods) and ages (range: 5–19 years; mean: 14 years) were selected for the study. Horses were housed in outdoor individual stalls at the Center for Equine Health, University of California, Davis and were examined prior to enrollment to ensure they were healthy and free of any scars or skin disease on the distal limbs. The study was conducted according to an approved institutional animal care and use protocol.
Experimental Overview
Equine cord blood MSCs from one allogeneic donor were expanded and cultured exactly as previously described; 40 cells were trypsinized and prepared for wound treatment at passage 4–5. Cells were continued in culture or transferred into fibrin gels, and were then either exposed to hypoxic conditions or maintained in normoxia, before application to wounds (Fig. 1). In vitro preparation began approximately 2 weeks before wound treatment and data was collected weekly for 6 weeks thereafter (Supporting Information Fig. S1).
Figure 1.
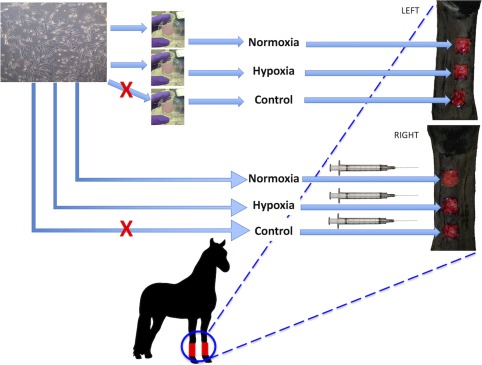
Experimental design. Banked cord blood mesenchymal stem cells from one donor were cultured and either embedded in fibrin gels or suspended in phosphate‐buffered saline for treatment of experimental wounds on the distal forelimbs of horses. Prior to wound application, one gel and one culture flask were exposed to hypoxia (2% O2) for 24 hours. Injected treatments were randomized in terms of right or left limb and proximodistal wound assignment, and the corresponding gel was applied to the corresponding wound on the opposite limb. Control wounds were treated with the corresponding blank vehicle (gel or saline). Treatment was applied 24 hours after wound creation.
MSCs in Fibrin Gels
Autologous fibrinogen was isolated from each study horse for fibrin gel preparation as described previously 41. Centrifugation was applied with minimal acceleration and deceleration and prostaglandin E1 (10 µg/ml) (Sigma, St. Louis, MO) was added to plasma to prevent platelet activation. Precipitated fibrinogen was dissolved in DPBS and quantified by a STA Compact Coagulation Analyzer (Diagnostica Stago, Parsippany, NJ). Fibrin gels were prepared in six‐well cell culture dishes (Falcon, Becton Dickinson, Franklin Lakes, NJ). Each gel was 2.375 ml total volume (approximately 2.5 mm thick), 9.5 cm2 in area, and contained 20 mg/ml fibrinogen and 1.66 × 106 MSCs. Blank control gels were prepared without MSCs. On day 7, half of the gels were transferred to a 5% CO2, 2% O2 hypoxic incubator, and grown for 24 hours before application to wounds. Normoxic and control gels remained in standard culture conditions.
Based on in vitro pilot studies and reported doubling times for cord blood cells 42, we estimated the cell population to be 10–20 × 106 cells per gel after 8 days of incubation (i.e., at the time of application to wounds). Cell viability in the gels was confirmed by visual assessment on a light microscope, based on the observation of elongated cell morphology and refractility; migration from the gel margin was also confirmed during pilot studies (Fig. 2A). On the day of wound treatment, gels were lifted from wells in the laboratory hood (Fig. 2B) and transferred onto a Telfa pad (Telfa pad, Covidien, Minneapolis, MN) for transport to the horse housing facility.
Figure 2.
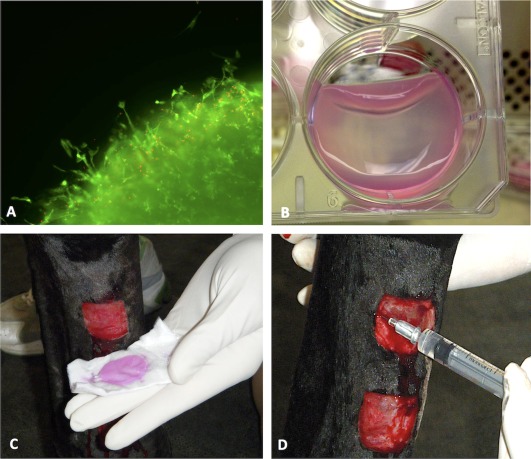
Experimental execution. Cord blood mesenchymal stem cells (MSCs) from one donor were suspended in autologous fibrinogen from each experimental subject to create fibrin gels. Pilot studies confirmed cell viability within the gels (calcein‐ethidium staining) and migration from the gel margins at 48 hours after gels were released from wells (A). On the morning of wound treatment, gels were released from the well just prior to wound application (B). (Pink staining is a result of phenol red in cell culture medium.) Wounds were treated by topical application of MSCs in fibrin gels (C) or by direct subcutaneous injection of MSCs in saline (D).
MSCs for Injection
Twenty‐four hours before wound treatment, half of the flasks were transferred to a 5% CO2, 2% O2 hypoxic incubator. Cells were trypsinized, washed, and resuspended to a final concentration of 17.5 × 106 cells per milliliter in saline. One milliliter of cells was injected for each treatment; 1 ml injectable saline was used as a vehicle control. The target cell dose was 15–20 × 106 cells; the cell concentration for the first horse's injection was 17.5 × 106 cells per milliliter and we therefore matched all subsequent injections to that same concentration.
Wound Creation
Surgical wounding was performed under field anesthesia: after placing a 14 g IV catheter in the left jugular vein, horses were treated with phenylbutazone (2 g IV) and then premedicated with xylazine (1 mg/kg IV). Anesthesia was induced with ketamine (3 mg/kg IV) and diazepam (0.1 mg/kg IV) and was maintained with repeat doses of xylazine (0.5 mg/kg IV) and ketamine (1.5 mg/kg IV) every 10 minutes thereafter. Horses were positioned in lateral recumbency and the dorsomedial aspect of the down limb was aseptically prepared. Three square full‐thickness skin wounds (2.5 cm × 2.5 cm, spaced 2.5 cm apart and vertically aligned; Fig. 1) were created using a sterile adherent template and a #10 scalpel blade. The limb was bandaged in standard fashion (Telfa pad, roll gauze (Kling, Johnson & Johnson, New Brunswick, NJ), cotton, brown gauze, VetWrap (3M, St. Paul, MN) and Elastikon (Johnson & Johnson, New Brunswick, NJ)). The procedure was repeated on the opposite forelimb.
Wound Treatment
Treatments were assigned as follows: MSC injection was randomly assigned to the right or left limb, and then control, normoxic MSCs, or hypoxic MSCs were each randomly assigned to one of the three wounds on that leg. On the opposite forelimb, corresponding MSC‐fibrin gel treatments (control, normoxic, hypoxic) were assigned to the corresponding wound (i.e., proximal, middle, distal, respectively) (Fig. 1). Treatments were applied on day 1 after wounding; horses were sedated with 0.01 mg detomidine IV. Gels were lifted from wells in the laboratory hood by gently rimming the margin with a P200 pipette tip and then passing the tip back and forth under one released edge. The pipette tip was moved across the well under the gel in an advancing manner as the gel was allowed to fold down on itself (Fig. 2B). Spatula‐tipped forceps were used to transfer the gel onto a Telfa pad (Telfa pad, Covidien, Minneapolis, MN) set within a sterile individual Petri dish for transport to the horse housing facility. Gels were applied directly to the wound surface by handling the Telfa pad (Fig. 2C), and were secured to the limb with a single circumferential wrap of Elastikon. The gels were placed to ensure full contact with the wound bed and no overlap with the wound margin. Injections were applied subcutaneously at the mid‐point of each border of the square wound: 0.25 ml was injected at each site using a 25 g needle (Fig. 2D). Control wounds received either saline injection or a blank fibrin gel with no MSCs.
Bandaging
For the first week post‐wounding, wounds were bandaged continuously, as previously described (changed on days 1 and 5). Bandages were also temporarily applied for 24 hours after each biopsy to control bleeding. For the remainder of the experiment, wounds were left unbandaged with the intent of minimizing exuberant granulation tissue (EGT) formation.
Thermography
Thermal images were acquired using a Flir Vet T‐420 thermography camera (FLIR Systems, Inc., North Billerica, MA) at a consistent distance of 1 m. A standard emissivity value of 0.98 was chosen based on recently published equine studies 43. Bandages were removed 15 minutes before acquisition of thermal images, in an environmentally controlled room. One dorsopalmar image of the metacarpus was acquired per limb with the thermography camera before treatment of day 1 wounds (Week 0, baseline), and at 1, 2, 3, 4, 5, and 6 weeks. Using the FLIR Systems' software program (FLIR Systems, Inc., Wilsonville, OR), standard delineated areas were superimposed on saved digital thermal images at specific wound sites (Fig. 3) to determine core and whole leg temperatures. The operator was blinded to the management of the wounds (gel vs. injection, normoxic vs. hypoxic).
Figure 3.
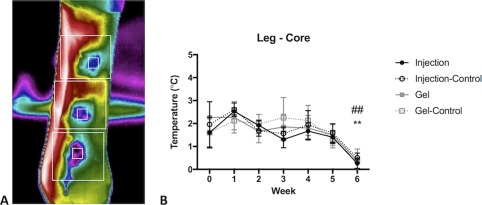
Thermography. (A): Thermographic data were analyzed according to core wound temperature (small square gates centered on wounds) and overall Leg temperature (larger square gates). (B): A significant effect of time was detected for all groups (p < .0001). By 6 weeks, the difference between core wound and surrounding leg temperature is nearly zero, that is, temperature of the wound bed has normalized. There was a significant decrease of temperature variation in wounds treated with mesenchymal stem cell (MSC) embedded gels and injected MSCs at Week 6. Data are displayed as mean (SE). Week 0 data was collected on day 1 after wounding, prior to treatment application. Symbols: * denotes statistically significant difference for Injection (*, p < .05; **, p < .01; ***, p < .001); # denotes statistically significant difference for Gel (#, p < .05; ##, p < .01).
Wound Surface Area
Digital photography of each forelimb was performed on seven occasions beginning the day after wound creation and then weekly for 6 weeks thereafter. On each occasion, a clipboard containing horse identification, date, limb identification, and a metric ruler was placed behind each limb and included in the photograph. Each picture was taken parallel to the ground and included all three wounds. Care was taken to ensure that the true margins of each wound were completely visible (i.e., not obscured by bleeding, exudate, or regrown hair); any obscuring exudate was gently removed using a saline‐soaked gauze prior to photography. Images were calibrated to scale and were analyzed using Image J 44 (www.imagej.net) to trace wound margins and calculate surface area. Each wound area was then expressed as a percentage of its original area on day 1 (just prior to treatment).
Wound Biopsy
Biopsies were obtained weekly for 6 weeks beginning on Week 1 (day 8), immediately following wound photography. Biopsies were taken beginning from the distal right vertical border on Week 1 and proceeded proximally on Weeks 2 and 3, such that there was no overlap with the previous biopsy site. On Weeks 4–6 the same procedures were repeated on the left vertical margin of the wound. Prior to biopsy, horses were sedated (0.01 mg/kg detomidine IV) and an assistant wearing latex gloves cleaned the wound margins and adjacent skin with saline‐soaked gauze. Surgeons wearing non‐sterile latex gloves injected local anesthetic (2% lidocaine without epinephrine, 2 ml total) subcutaneously through the intact skin proximal and adjacent to the upper wound corner above the intended biopsy site. Biopsies were then collected using sterile technique: the 8 mm skin biopsy punch was centered on the wound margin in order to obtain ½ wound bed and ½ adjacent intact skin in each biopsy. Samples were then cut in half transversely: ½ was placed in formalin for histology and the other ½ was used for PCR analysis. For the PCR samples, intact skin was removed and the remaining wound bed portion was cut into approximately 4 pieces, placed in cryovials containing RNAlater (Qiagen, Valencia, CA) and frozen at −80°C.
Gene Expression
Biopsy samples were thawed, minced, and washed with DPBS prior to cell lysis in modified (1% β‐Mercaptoethanol) RLT buffer (Qiagen). Tissues were then placed in a preheated (55°C) water bath sonicator (Branson, Shelton, CT) for 10 minutes. Lysed tissues were further run through a QIAshredder column (Qiagen) per manufacturer's instructions. Tissue lysate was centrifuged, supernatant was removed and subjected to RNA extraction with RNeasy mini‐kit (Qiagen) per manufacturer's instructions. Extracted RNA was then converted to cDNA using a First‐strand cDNA Synthesis kit (Origene, Rockville, MD) per manufacturer's instructions. Quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR) was performed using primers that were purchased from a commercial vendor (TGFβ1, Kingfisher Biotech, Saint Paul, MN) or designed via on‐line software (peptidylpropyl isomerase B, cyclooxygenase 2 [COX2], vascular endothelial growth factor [VEGF], and basic fibroblast growth factor 2 [FGF2], www.idtdna.com. See Supporting Information Table 1 for primers' details), and Fast SYBR Green Master MIX (AB Applied Biosystems, Foster City, CA) and run on a 7300 Real Time PCR System (AB Applied Biosystems) thermocycler. All PCR products sizes were confirmed by electrophoresing PCR products on a 2% agarose gel. Gene expression data were reported as fold‐change over corresponding controls from each time point.
Histology
Biopsies were processed for routine H&E‐stained tissue sections. Tissue sections were randomly assigned a number value and blindly evaluated by a board‐certified veterinary pathologist. Sections were scored 0–4 for each of four categories: degree of epithelialization, inflammation, vascularity, and fibrosis. For all scores, 0 was assigned to cases lacking the histologic feature, and 4 was assigned when the feature was prominent.
Statistical Analysis
Significance for all analyses was set at p < .05 and data were reported as mean +/– SE.
For wound area, thermography, and gene expression, three dependent variables were considered; treatment of wound by MSC, hypoxic preconditioning, and modality of MSC application. Data were analyzed using R software. Due to the small sample size of the current study, data was log‐transformed to achieve normality. A Wilk‐Shapiro test of normality revealed all transformed data sets had error distributions within a normal range (W > 0.95). An ANOVA using a mixed model procedure was performed to assess the array of treatments fixed effects by MSCs, oxygen and application. No effects of hypoxic preconditions were observed, therefore groups were combined to compare injection versus gel over a 6‐week time frame. Statistical outliers were removed, only if alterations were suspect of causation by auto‐mutilation. Finalized data were analyzed using GraphPad Prism 6 for Mac OS X. Ordinary two‐way ANOVA testing was performed to detect significant differences between treatment groups over time. Repeated measures could not be performed due to removal of experimental wounds from data sets caused by self‐mutilation by some study animals. Using the Sidak multiple comparison test, post‐tests were performed to first compare treatment groups at each time point and then to compare all time points within each treatment group.
Histologic scores were entered into a matrix and blindly categorized by hierarchical clustering based on scores across all four variables. Hierarchical clustering was performed using package GMD in R software. In addition, the number of horses within each group (MSC‐treated vs. control) was tabulated and a Fisher's exact test was performed using R software.
Results
Gross Wound Evaluation
All wounds healed and no adverse effects were noted specific to MSC therapy. Pilot studies using continuous bandaging resulted in significant EGT formation. This problem was largely eliminated using the short‐term and intermittent (i.e., after biopsy) bandaging protocol as described in “Methods” section. However, in the absence of protective bandages, fly irritation and self‐mutilation were confounding factors in some horses; four affected wounds (two Injection, one Gel, and one Injection‐Control) were excluded from data analysis. Notably, gels were not grossly visible in the wound bed at the first bandage change (day 5), suggesting proteolytic digestion.
No Effect of Hypoxic Preconditioning on Wound Healing Outcomes
Based on the existing literature and our pilot studies, which showed altered gene expression after hypoxic preconditioning, we expected to find a positive effect of hypoxic‐MSC treatment. Surprisingly, we did not detect significant differences in any outcome on the basis of hypoxic versus normoxic MSC culture conditions. Data were therefore combined and results are reported as four groups (Injection, Injection‐Control, Gel, Gel‐Control).
Thermographic Imaging Tracked Wound Healing In Situ
In order to noninvasively evaluate wound physiology in vivo, we used thermographic imaging to detect changes in blood flow post‐wounding. This complementary modality correlated well with histologic assessment of vascularization. Core wound temperature and leg temperature changed throughout the study as we expected: core wound temperature increased overall, as vascularization of the wound bed occurred. Surrounding leg temperature initially increased, during the acute inflammatory phase after wounding, and then decreased as healing progressed and inflammation subsided (Fig. 3). The difference between these two values (“Leg‐Core”) was calculated as an index of healing, that is, in the absence of a wound, there would be no difference in temperature at these two sites, and the Leg‐Core value would therefore equal zero. As healing progresses, therefore, the Leg‐Core value should trend toward zero. There was an overall significant effect of time (p < .0001), that is, as wounds healed, they returned to the temperature of the surrounding intact skin. MSC treated wounds began to normalize temperature (delta leg‐core approaching zero) at Week 6 in both gel and injection groups (p < .01).
MSC Treatment Accelerates Reduction in Wound Area
Wounds initially enlarged in size, as expected for equine limb wounds, and then reduced to 72% (controls), 78% (MSC‐gel), and 53% (MSC‐injected) of original wound area by Week 6 (Fig. 4). A significant treatment effect was detected (p = .039) but post hoc multiple comparisons failed to further clarify this effect. Nonetheless, mean wound area was smallest in the Injected group for all time points. A significant effect of time was also detected for treated wounds but not for control wounds: wound area was significantly less at Weeks 4, 5, and 6 for Injection and at Weeks 5 and 6 for Gel, in comparison to Week 1 values (largest wound areas).
Figure 4.
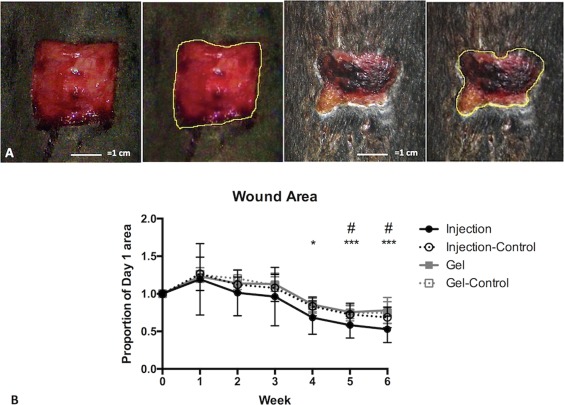
Wound area. Wound surface area was calculated from wound margins traced on calibrated digital images. (A): Example images of the same wound on day 1 (left) and day 36 (right), with corresponding outlines of the same images. (B): Wound area decreased significantly in Weeks 4–6 for mesenchymal stem cell (MSC) Injected wounds, compared to Week 1 values (largest wound area); wounds treated with MSC Gel followed suit at Weeks 5–6 but to a lesser extent. Data is displayed as mean (SE). Symbols: * denotes statistically significant difference for Injection (*, p < .05; **, p < .01; ***, p < .001); # denotes statistically significant difference for Gel (#, p < .05; ##, p < .01).
Gene Expression Varies After MSC Injection but Not Gel Application
Injected MSCs clearly impacted gene expression within the wound, suggesting that direct application into the tissue allowed immediate interaction and functionality within the wound environment. Alternatively, it is possible that the physical process of injection alone resulted in activation of the MSCs and altered their gene expression profile.
Transcriptional changes did not occur when cells were embedded within a three‐dimensional fibrin matrix and applied to the wounds.
Cyclooxygenase 2 (COX‐2) expression changed significantly based on time and treatment. Specifically, a 20‐fold increase in COX‐2 expression was observed at Week 1 after MSC injection (Fig. 5). This value was significantly greater than after MSC gel application at the same time point (p < .0001) and was also significantly greater than all subsequent time points for MSC injection (p < .0001). In contrast, there were no significant differences in COX‐2 expression with MSC gels.
Figure 5.
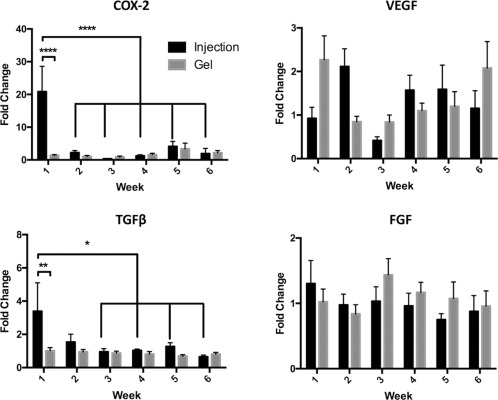
Gene expression. PCR Data (mean [SE]), expressed as fold‐change over corresponding controls. One week after treatment, COX‐2 and TGFβ gene expression were significantly greater after mesenchymal stem cell (MSC)‐Injection in comparison to MSC‐Gels. Week 1 expression after Injection was also significantly greater than that measured in all subsequent weeks (COX‐2) and Weeks 3–6 (TGFβ). No significant differences were detected for VEGF or FGF. Symbols: *, p < .05; **, p < .01; ****, p < .0001. Abbreviations: COX‐2, cyclooxygenase‐2; FGF, fibroblast growth factor; TGFβ, transforming growth factor beta; VEGF, vascular endothelial growth factor.
TGFβ1 expression was also significantly altered after MSC injection but not after gel application: MSC injection significantly increased expression of TGFβ1 at Week 1 only, as compared to gel application (p < .01). Expression of TGFβ1 at Week 1 was also significantly higher than Weeks 3–6 after MSC injection (p < .05). No alterations in TGFβ1 expression occurred after MSC‐gel application.
For VEGF and FGF, there were no significant differences based on treatment or time. There was a notable inversion of VEGF expression: MSCs embedded in gel were associated with a twofold increase in expression at Week 1 and returned to control levels at Week 2; MSC injection results were just the opposite. We were surprised not to see a greater impact on VEGF expression, particularly in light of improved histologic scores for vascularity with MSC treatment and the known nitric oxide production of cord blood MSCs 39.
MSC Treatment Improves Histologic Outcomes
Histologic appearances varied widely and ranged from early vascularization, fibrosis, and epithelialization to chronic inflammation and ulceration (Fig. 6). In order to identify trends within this large data set, histologic scores were distributed in a dendrogram with a heatmap matrix (Supporting Information Fig. 2) superimposed, using assigned colors to illustrate clusters of data. The following clusters of wound characteristics were identified: (1A) increased vascularity and epithelialization (1B) increased fibrosis (“Pro‐Healing”), (2A) lack of epithelialization, or ulceration, and (2B) increased inflammation (“Ulcerative/Pro‐Inflammatory”). Although significant differences could not be detected between Injected versus Gel treatment groups, there was a significantly greater number of MSC‐treated wounds (Injected and Gel combined) in the Pro‐Healing Group than in the Ulcerative/Pro‐Inflammatory Group; (Fig. 7; p = .0136).
Figure 6.
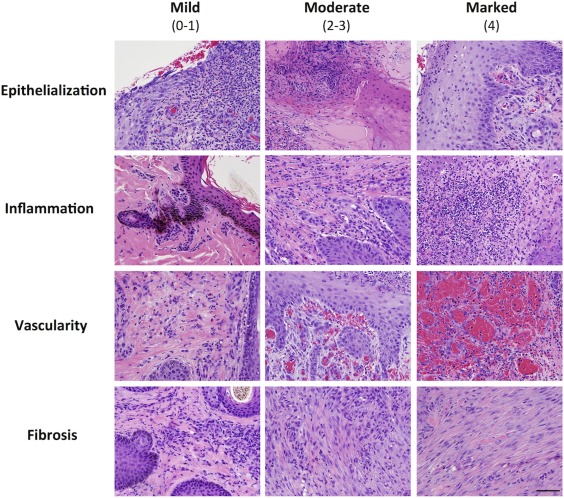
Histology. Representative photomicrographs demonstrating the histologic scoring criteria for epithelialization, inflammation, vascularity, and fibrosis. H&E staining, bar = 50 μm.
Figure 7.
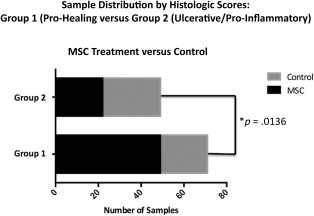
Histology results. Fisher's exact results comparing the proportion of mesenchymal stem cell (MSC)‐Treated versus control wounds classified as either Histologic Score Group 1 (Pro‐Healing) or Group 2 (Ulcerative/Pro‐Inflammatory). MSC‐treated wounds comprised a significantly greater proportion of the Pro‐Healing Group (p = .0136).
Discussion
Chronic wounds represent an enormous burden for the individual patient and society as a whole. The impact of this “silent epidemic” is felt on many levels, from lost work days to permanent disability (amputation) to death of the patient, and the economic implications of chronic wound care are alarming ($25 billion annually in the U.S.) 1. Impaired healing can occur in severely injured normal tissue, such as a burn, or as a result of comorbidities which create compromised tissue integrity, such as decubital sores or diabetic foot ulcers (DFU). Distal limb wounds in horses share many of the complexities of chronic wounds in people, in that they heal mainly by epithelialization, rather than contraction, and are persistently hypoxic and pro‐inflammatory during the healing period. This study adds to the accumulating evidence 16, 29, 45, 46, 47, 48, 49, 50, 51, 52 that allogeneic MSC therapy can facilitate wound healing: MSC‐treated wounds showed significant differences in wound area, gene expression and histologic scores. MSCs are believed to impact wound healing by paracrine immunomodulatory, antifibrotic 50, 51 and anti‐inflammatory effects 15, 39, 45, 46, enhanced neovascularization 15, 21, reversal of collagen dysregulation 52, and in some reports, by transdifferentiation and engraftment 13, 53.
We evaluated several variables in the preparation and application of MSCs to wounds, including hypoxic‐preconditioning and topical versus injectable delivery methods. In contrast to several other reports 18, 19, hypoxia during MSC culture was not advantageous in our study. Our pilot studies indicated an obvious in vitro effect of hypoxia on MSC gene expression (induction of VEGF expression), but this did not translate into a detectable effect in vivo. With regard to delivery method, we investigated direct injection of MSCs as the simplest means for applying these cells to tissue, but were also interested in the impact of MSCs in a hydrogel. Burns and DFUs are preferentially treated with some type of biological matrix to provide a physical barrier as well as to “instruct” or contribute to the wound bed directly with molecules such as hyaluronic acid 54, or conceivably, resident cells 49. We chose autologous fibrin to provide the most native provisional matrix possible for each wound, as well as naturally occurring adhesion motifs to allow MSC anchorage and interaction within the gel. The MSCs in gels were applied to wounds in what we believed to be a “primed” state for biological activity: they were elongated, attached to a matrix, migrating, and presumably interacting with other cells in the gel through signal transduction, in contrast to the dissociated, recently trypsinized cells used for injection. In a recent study, protein synthesis by MSC spheroids improved dramatically when embedded in an RGD‐modified alginate gels, presumably based on the ability of the cells to interact with the matrix 55. Another study on MSCs in a PEGylated fibrin system showed significant increases in vascularization in a burn model, and MSCs also transmigrated from the gel into the wound bed 49. However, in our study MSC‐injection appeared superior to topical application of MSC‐laden gels. Wounds treated by MSC‐injection showed an earlier decrease in wound area, and significant differences in early TGFβ1 and COX‐2 gene expression were detected in the injected but not the gel‐treated wounds. Injection was logistically easier when compared to gel preparation: significantly less time and materials are required for injection and the entire cell dose is delivered directly into the tissue. Our gels appeared to be digested rapidly within the wound, such that the MSCs may have been liberated faster than they could migrate into tissue; gel modification to reduce proteolytic destruction (such as PEGylation) may have changed our results.
We expected that MSC treatment would mitigate the prolonged inflammatory response that contributes to delayed healing of equine limb wounds. Indeed, MSC‐treated wounds had lower histologic scores for inflammation and appeared to progress through the stages of wound healing earlier than control wounds. It was therefore initially surprising to observe a dramatic increase in expression of COX‐2 in MSC‐injected wounds at Week 1. This increased expression was early and transient in nature. Although COX‐2 and its eicosanoid products are hallmarks of inflammation, they (specifically COX‐2 and Prostaglandin E2) are also recognized as critical components of early wound healing in multiple tissues including skin 56, 57, 58, 59. The increased COX‐2 gene expression in our study appears to represent an amplification of this key signal in the healing process, as stimulated by MSC injection. Further research into COX‐2 protein expression by MSCs or their paracrine targets during wound repair, including the timing and spatial localization of this event, may clarify the mechanism for our observed MSC treatment effect.
TGFβ1 dysregulation has been implicated in excessive scar formation in people and EGT in the horse. This pro‐fibrotic marker has been extensively studied in the equine wound literature 60, 61, 62, 63, and is known to peak early in normal healing and then subside. In contrast, TGFβ1 persists in wounds healing with EGT [6] and inhibits epithelialization 62. We found that TGFβ1 expression was significantly increased (threefold) at Week 1 after MSC injection, and then returned to control values. Interestingly, a more intense burst of TGFβ1 has been reported in wounds in ponies than in horses, and has been proposed as an explanation for the more rapid and uncomplicated healing that ponies exhibit 63. In that sense, an early but transient increase in TGFβ1, as observed here, may be a favorable event mediated by MSC treatment of wounds. A recent study by Fang et al. 50 documented paracrine suppression of the TGFβ/SMAD pathway by microsomal RNA released from umbilical cord tissue‐derived MSC exosomes. This fascinating phenomenon resulted in reduced myofibroblast function and ultimately reduced scar production in vivo. Future studies to investigate expression of the antifibrotic isoform, TGFβ3 [6, 64] may reveal additional favorable matrix effects of MSCs or MSC‐derived treatment in wounds, as reported in other species 23, 51. Clinically, this could translate into less EGT production and scarring, as suggested by other authors 27.
Extrapolation from acute, experimentally created wounds to naturally occurring, traumatic, chronic, and dysplastic wound encountered in the clinical patient is obviously an imperfect science, and is an inherent limitation of most wound studies. Nonetheless, the study of acute wounds can hopefully identify targets and timing for early therapeutic intervention. Any favorable modulation of acute wound events may prevent complications from developing in “at‐risk” wounds, such as complex traumatic wounds with extensive soft tissue deficit or wounds with reduced regenerative capacity (i.e., burns or DFUs). The horse may be a more critical translational model for wound research than the traditional murine model, based on the hypoxic nature and minimal contraction of wounds on their distal limbs. Environmental challenges to wound healing were also present in our study, as horses were housed outside during the summer months and wounds were left largely unbandaged to avoid the confounding effect of EGT. Fly irritation and self‐mutilation occurred in several wounds, which were eliminated from the data set and thereby reduced study power. A positive treatment effect was still detected despite these limitations, and this model perhaps better represented the complicated wounds for which we aim to improve treatment.
Our study employed allogeneic cord blood MSCs from one donor. We selected cord blood MSCs based on favorable culture characteristics, the perceived greater pluripotency of cord blood cells, nitric oxide production 39, greater gene expression changes with cord blood cells than with bone‐marrow‐derived MSCs during our pilot studies, and better physical characteristics and gel stability with cord‐blood cells than with bone‐marrow‐derived MSCs. The clinical safety of allogeneic MSCs has been confirmed in the horse 26, 38, 65 and comparable efficacy to autologous cells for wound healing has been demonstrated in other species 66. However, some limitations to allogeneic cell use may exist: a recent study reported a slight advantage of autologous MSCs over allogeneic cells in terms of treatment effect on equine wounds 27, and allogeneic cells do elicit some immune response 67, 68, 69. Nonetheless, allogeneic cells obviously provide logistical advantages, such as a readily available supply of banked cells (thereby preventing delays in treatment) and selection of cell lines with favorable and consistent growth characteristics. Future work in this field will no doubt be directed at optimizing donor‐recipient suitability and thereby, presumably, treatment response.
Summary
Allogeneic cord blood cells enhanced early healing in this critical wound model in horses. This effect was greater for cells delivered by injection than for cells applied topically in fibrin gel. Hypoxic preconditioning did not offer an advantage in this study.
Author Contributions
J.A.T.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; K.C.C.: collection and assembly of data, data analysis and interpretation, administrative support, other: animal work; N.J.W.: other: technical support, collection and assembly of data; F.A.A.: collection and assembly of data, other: animal work; A.K.: collection and assembly of data, data analysis and interpretation; K.D.W.: collection and assembly of data, data analysis and interpretation; S.S.L.: collection and assembly of data; A.B.: collection and assembly of data; A.D.: collection and assembly of data; S.N.G.: other: animal work; L.K.B.‐W.: other: animal work; D.L.B.: conception and design, financial support, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Note Added in Proof
This article was published online on 24 October 2017. Minor edits have been made that do not affect data. This notice is included in the online and print versions to indicate that both have been corrected 28 December 2017.
Supporting information
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Table 1
Acknowledgments
This study was supported by the Center of Equine Health and a gift from Dick and Carolyn Randall. Statistical analysis and consultation was provided by Dr. Neil Willits of the UC Davis Statistics Laboratory. We would like to thank the following student volunteers for their time and contributions to this study: Monica Plank, Lyndsey Marsh and Bailey McBride.
References
- 1. Sen CK, Gordillo GM, Roy S et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark RA, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Invest Dermatol 2007;127:1018–1029. [DOI] [PubMed] [Google Scholar]
- 3. Skardal A, Mack D, Kapetanovic E et al. Bioprinted amniotic fluid‐derived stem cells accelerate healing of large skin wounds. Stem Cells Translational Medicine 2012;1:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilmink JM, van Weeren PR, Stolk PW et al. Differences in second‐intention wound healing between horses and ponies: Histological aspects. Equine Vet J 1999;31:61–67. [DOI] [PubMed] [Google Scholar]
- 5. Wilmink JM, Veenman JN, van den Boom R et al. Differences in polymorphonucleocyte function and local inflammatory response between horses and ponies. Equine Vet J 2003;35:561–569. [DOI] [PubMed] [Google Scholar]
- 6. Theoret CL, Barber SM, Moyana TN et al. Preliminary observations on expression of transforming growth factors beta1 and beta3 in equine full‐thickness skin wounds healing normally or with exuberant granulation tissue. Vet Surg 2002;31:266–273. [DOI] [PubMed] [Google Scholar]
- 7. Dubuc V, Lepault E, Theoret CL. Endothelial cell hypertrophy is associated with microvascular occlusion in horse wounds. Can J Vet Res 2006;70:206–210. [PMC free article] [PubMed] [Google Scholar]
- 8. Lepault E, Celeste C, Dore M et al. Comparative study on microvascular occlusion and apoptosis in body and limb wounds in the horse. Wound Repair Regen 2005;13:520–529. [DOI] [PubMed] [Google Scholar]
- 9. Celeste CJ, Deschene K, Riley CB et al. Regional differences in wound oxygenation during normal healing in an equine model of cutaneous fibroproliferative disorder. Wound Repair Regen 2011;19:89–97. [DOI] [PubMed] [Google Scholar]
- 10. Deschene K, Celeste C, Boerboom D et al. Hypoxia regulates the expression of extracellular matrix associated proteins in equine dermal fibroblasts via HIF1. J Dermatol Sci 2012;65:12–18. [DOI] [PubMed] [Google Scholar]
- 11. Theoret CL, Olutoye OO, Parnell LK et al. Equine exuberant granulation tissue and human keloids: A comparative histopathologic study. Vet Surg 2013;42:783–789. [DOI] [PubMed] [Google Scholar]
- 12. Theoret CL, Wilmink JM. Aberrant wound healing in the horse: Naturally occurring conditions reminiscent of those observed in man. Wound Repair Regen 2013;21:365–371. [DOI] [PubMed] [Google Scholar]
- 13. Kim SW, Zhang HZ, Guo L et al. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PloS one 2012;7:e41105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim WS, Sung JH. Hypoxic culturing enhances the wound‐healing potential of adipose‐derived stem cells. Adv Wound Care 2012;1:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu L, Yu Y, Hou Y et al. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PloS one 2014;9:e88348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spaas JH, Broeckx S, Van de Walle GR et al. The effects of equine peripheral blood stem cells on cutaneous wound healing: A clinical evaluation in four horses. Clin Exp Dermatol 2013;38:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, La X, Fan L et al. Immunosuppressive effects of mesenchymal stem cell transplantation in rat burn models. Int J Clin Exp Pathol 2015;8:5129–5136. [PMC free article] [PubMed] [Google Scholar]
- 18. Chacko SM, Ahmed S, Selvendiran K et al. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol 2010;299:C1562–C1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Xu Y, Zhao J et al. Conditioned medium from hypoxic bone marrow‐derived mesenchymal stem cells enhances wound healing in mice. PloS one 2014;9:e96161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JW, Lee JH, Lyoo YS et al. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol 2013;24:242–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egana JT, Fierro FA, Kruger S et al. Use of human mesenchymal cells to improve vascularization in a mouse model for scaffold‐based dermal regeneration. Tissue Eng Part A 2009;15:1191–1200. [DOI] [PubMed] [Google Scholar]
- 22. Weinstein‐Oppenheimer CR, Aceituno AR, Brown DI et al. The effect of an autologous cellular gel‐matrix integrated implant system on wound healing. J Transl Med 2010;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falanga V, Iwamoto S, Chartier M et al. Autologous bone marrow‐derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312. [DOI] [PubMed] [Google Scholar]
- 24. Mendez JJ, Ghaedi M, Sivarapatna A et al. Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials 2015;40:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith RK, Werling NJ, Dakin SG et al. Beneficial effects of autologous bone marrow‐derived mesenchymal stem cells in naturally occurring tendinopathy. PloS one 2013;8:e75697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Loon VJ, Scheffer CJ, Genn HJ et al. Clinical follow‐up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet Q 2014;34:92–97. [DOI] [PubMed] [Google Scholar]
- 27. Broeckx SY, Borena BM, Van Hecke L et al. Comparison of autologous versus allogeneic epithelial‐like stem cell treatment in an in vivo equine skin wound model. Cytotherapy 2015;17:1434–1446. [DOI] [PubMed] [Google Scholar]
- 28. Bussche L, Harman RM, Syracuse BA et al. Microencapsulated equine mesenchymal stromal cells promote cutaneous wound healing in vitro. Stem Cell Res Ther 2015;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spaas JH, Gomiero C, Broeckx SY et al. Wound‐healing markers after autologous and allogeneic epithelial‐like stem cell treatment. Cytotherapy 2016;18:562–569. [DOI] [PubMed] [Google Scholar]
- 30. Burk J, Ribitsch I, Gittel C et al. Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet J 2013;195:98–106. [DOI] [PubMed] [Google Scholar]
- 31. Iacono E, Brunori L, Pirrone A et al. Isolation, characterization and differentiation of mesenchymal stem cells from amniotic fluid, umbilical cord blood and Wharton's jelly in the horse. Reproduction 2012;143:455–468. [DOI] [PubMed] [Google Scholar]
- 32. Broeckx SY, Maes S, Martinello T et al. Equine epidermis: A source of epithelial‐like stem/progenitor cells with in vitro and in vivo regenerative capacities. Stem Cells Dev 2014;23:1134–1148. [DOI] [PubMed] [Google Scholar]
- 33. Aguiar C, Therrien J, Lemire P et al. Differentiation of equine induced pluripotent stem cells into a keratinocyte lineage. Equine Vet J 2016;48:338–345. [DOI] [PubMed] [Google Scholar]
- 34. Hackett CH, Greve L, Novakofski KD et al. Comparison of gene‐specific DNA methylation patterns in equine induced pluripotent stem cell lines with cells derived from equine adult and fetal tissues. Stem Cells Dev 2012;21:1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lepage SI, Nagy K, Sung HK et al. Generation, characterization, and multilineage potency of mesenchymal‐like progenitors derived from equine induced pluripotent stem cells. Stem Cells Dev 2016;25:80–89. [DOI] [PubMed] [Google Scholar]
- 36. Sharma R, Livesey MR, Wyllie DJ et al. Generation of functional neurons from feeder‐free, keratinocyte‐derived equine induced pluripotent stem cells. Stem Cells Dev 2014;23:1524–1534. [DOI] [PubMed] [Google Scholar]
- 37. Whitworth DJ, Ovchinnikov DA, Sun J et al. Generation and characterization of leukemia inhibitory factor‐dependent equine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev 2014;23:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carrade DD, Affolter VK, Outerbridge CA et al. Intradermal injections of equine allogeneic umbilical cord‐derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy 2011;13:1180–1192. [DOI] [PubMed] [Google Scholar]
- 39. Carrade DD, Lame MW, Kent MS et al. Comparative analysis of the immunomodulatory properties of equine adult‐derived mesenchymal stem cells(). Cell Med 2012;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schuh EM, Friedman MS, Carrade DD et al. Identification of variables that optimize isolation and culture of multipotent mesenchymal stem cells from equine umbilical‐cord blood. Am J Vet Res 2009;70:1526–1535. [DOI] [PubMed] [Google Scholar]
- 41. Hale BW, Goodrich LR, Frisbie DD et al. Effect of scaffold dilution on migration of mesenchymal stem cells from fibrin hydrogels. Am J Vet Res 2012;73:313–318. [DOI] [PubMed] [Google Scholar]
- 42. Mohanty N, Gulati BR, Kumar R et al. Phenotypical and functional characteristics of mesenchymal stem cells derived from equine umbilical cord blood. Cytotechnology 2016;68:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cornelisse CJ, Robinson NE, Berney CA et al. Thermographic study of in vivo modulation of vascular responses to phenylephrine and endothelin‐1 by dexamethasone in the horse. Equine Vet J 2006;38:119–126. [DOI] [PubMed] [Google Scholar]
- 44. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson WM, Nesti LJ, Tuan RS. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Translational Medicine 2012;1:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Badiavas AR, Badiavas EV. Potential benefits of allogeneic bone marrow mesenchymal stem cells for wound healing. Expert Opin Biol Ther 2011;11:1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borena BM, Martens A, Broeckx SY et al. Regenerative skin wound healing in mammals: State‐of‐the‐art on growth factor and stem cell based treatments. Cell Physiol Biochem 2015;36:1–23. [DOI] [PubMed] [Google Scholar]
- 48. Dash NR, Dash SN, Routray P et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Res 2009;12:359–366. [DOI] [PubMed] [Google Scholar]
- 49. Chung E, Rybalko VY, Hsieh PL et al. Fibrin‐based stem cell containing scaffold improves the dynamics of burn wound healing. Wound Repair Regen 2016;24:810–819. [DOI] [PubMed] [Google Scholar]
- 50. Fang S, Xu C, Zhang Y et al. Umbilical cord‐derived mesenchymal stem cell‐derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor‐beta/SMAD2 pathway during wound healing. Stem Cells Translational Medicine 2016;5:1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qi Y, Jiang D, Sindrilaru A et al. TSG‐6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full‐thickness skin wounds. J Invest Dermatol 2014;134:526–537. [DOI] [PubMed] [Google Scholar]
- 52. Zgheib C, Hodges M, Hu J et al. Mechanisms of mesenchymal stem cell correction of the impaired biomechanical properties of diabetic skin: The role of miR‐29a. Wound Repair Regen 2016;24:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sasaki M, Abe R, Fujita Y et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581–2587. [DOI] [PubMed] [Google Scholar]
- 54. Caputo WJ, Vaquero C, Monterosa A et al. A retrospective study of cryopreserved umbilical cord as an adjunctive therapy to promote the healing of chronic, complex foot ulcers with underlying osteomyelitis. Wound Repair Regen 2016;24:885–893. [DOI] [PubMed] [Google Scholar]
- 55. Ho SS, Murphy KC, Binder BY et al. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Translational Medicine 2016;5:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Futagami A, Ishizaki M, Fukuda Y et al. Wound healing involves induction of cyclooxygenase‐2 expression in rat skin. Lab Invest 2002;82:1503–1513. [DOI] [PubMed] [Google Scholar]
- 57. Simon AM, Manigrasso MB, O'Connor JP. Cyclo‐oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res 2002;17:963–976. [DOI] [PubMed] [Google Scholar]
- 58. Hamamoto T, Yabuki A, Yamato O et al. Immunohistochemical analysis of cyclooxygenase‐2 induction during wound healing in dog skin. Res Vet Sci 2009;87:349–354. [DOI] [PubMed] [Google Scholar]
- 59. Ishida Y, Kimura A, Nosaka M et al. Immunohistochemical analysis on cyclooxygenase‐2 for wound age determination. Int J Legal Med 2012;126:435–440. [DOI] [PubMed] [Google Scholar]
- 60. Bischofberger AS, Dart CM, Horadagoda N et al. Effect of Manuka honey gel on the transforming growth factor beta1 and beta3 concentrations, bacterial counts and histomorphology of contaminated full‐thickness skin wounds in equine distal limbs. Aust Vet J 2016;94:27–34. [DOI] [PubMed] [Google Scholar]
- 61. Theoret CL, Barber SM, Gordon JR. Temporal localization of immunoreactive transforming growth factor beta1 in normal equine skin and in full‐thickness dermal wounds. Vet Surg 2002;31:274–280. [DOI] [PubMed] [Google Scholar]
- 62. Haber M, Cao Z, Panjwani N et al. Effects of growth factors (EGF, PDGF‐BB and TGF‐beta 1) on cultured equine epithelial cells and keratocytes: Implications for wound healing. Vet Ophthalmol 2003;6:211–217. [DOI] [PubMed] [Google Scholar]
- 63. van den Boom R, Wilmink JM, O'Kane S et al. Transforming growth factor‐beta levels during second‐intention healing are related to the different course of wound contraction in horses and ponies. Wound Repair Regen 2002;10:188–194. [DOI] [PubMed] [Google Scholar]
- 64. Lichtman MK, Otero‐Vinas M, Falanga V. Transforming growth factor beta (TGF‐beta) isoforms in wound healing and fibrosis. Wound Repair Regen 2016;24:215–222. [DOI] [PubMed] [Google Scholar]
- 65. Carrade DD, Owens SD, Galuppo LD et al. Clinicopathologic findings following intra‐articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 2011;13:419–430. [DOI] [PubMed] [Google Scholar]
- 66. Chen L, Tredget EE, Liu C et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PloS one 2009;4:e7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pezzanite LM, Fortier LA, Antczak DF et al. Equine allogeneic bone marrow‐derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res Ther 2015;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Owens SD, Kol A, Walker NJ et al. Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in horses. Stem Cells Int 2016;2016:5830103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Williams LB, Co C, Koenig JB et al. Response to intravenous allogeneic equine cord blood‐derived mesenchymal stromal cells administered from chilled or frozen state in serum and protein‐free media. Front Vet Sci 2016;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Table 1


