Abstract
Cell therapy using endothelial progenitors holds promise for vascular repair in ischemic retinopathies. Using a well‐defined subpopulation of human cord blood‐derived endothelial progenitors known as endothelial colony‐forming cells (ECFCs), we have evaluated essential requirements for further development of this cell therapy targeting the ischemic retina, including dose response, delivery route, and toxicity. First, to evaluate therapeutic efficacy relating to cell dose, ECFCs were injected into the vitreous of mice with oxygen‐induced retinopathy. Using angiography and histology, we found that intravitreal delivery of low dose (1 × 103) ECFCs was as effective as higher cell doses (1 × 104, 1 × 105) in promoting vascular repair. Second, injection into the common carotid artery was tested as an alternative, systemic delivery route. Intracarotid ECFC delivery conferred therapeutic benefit which was comparable to intravitreal delivery using the same ECFC dose (1 × 105), although there were fewer human cells observed in the retinal vasculature following systemic delivery. Third, cell immunogenicity was evaluated by injecting ECFCs into the vitreous of healthy adult mice. Assessment of murine ocular tissues identified injected cells in the vitreous, while demonstrating integrity of the host retina. In addition, ECFCs did not invade into the retina, but remained in the vitreous, where they eventually underwent cell death within 3 days of delivery without evoking an inflammatory response. Human specific Alu sequences were not found in healthy mouse retinas after 3 days of ECFC delivery. These findings provide supportive preclinical evidence for the development of ECFCs as an efficacious cell product for ischemic retinopathies. stem cells translational medicine 2018;7:59–67
Keywords: Endothelial progenitors, Cell therapy, Endothelial colony‐forming cells, Stem cells, Ischemic retinopathy
Significance Statement.
The capacity of endothelial colony‐forming cells (ECFCs) to promote vascular repair and regeneration of the ischemic retina has potential for clinical translation; however, cell transplantation into the eye requires extensive preclinical testing. Our study provides supporting evidence to facilitate effective translation into clinics. We have demonstrated high purity of the ECFC cell product, minimal therapeutic dose, and efficacy using readouts that include in vivo angiography. We did not observe toxicity after ECFC intravitreal delivery into healthy adult eyes. Importantly, we also showed feasibility of intracarotid delivery for targeting the ipsilateral retina.
Introduction
Emerging evidence from preclinical investigations indicate that cell therapy could be a valid therapeutic option for eye disease such as age‐related macular degeneration, Stargardt's disease, and retinitis pigmentosa 1, 2. Ischemic retinopathies such as diabetic retinopathy, retinopathy of prematurity, and retinal vein occlusion are major causes of visual impairment and it has recently been suggested that the common, underlying vascular insufficiency of these diseases could also be treated using vasoregenerative cell therapy 3. Some difficulties associated with inducing therapeutic angiogenesis using proteins or gene therapy can be overcome with cell therapy 4. Various different cell types have been shown to promote revascularization of the ischemic retina such as CD34+ cells 5, 6, mesenchymal stromal cells 7, bone marrow Lin‐ hematopoietic stem cells 8, and myeloid angiogenic cells 9. However, it could be argued that in order to adequately regenerate damaged retinal vasculature, a bona fide endothelial progenitor is needed 10. Endothelial colony‐forming cells (ECFCs) are a distinct subpopulation of endothelial progenitors 11, characterized by their high proliferative potential and vasculogenic capacity 12. In preclinical studies, administration of ECFCs have demonstrated therapeutic efficacy by promoting vascular repair in ischemic tissues, including the myocardium 13, brain 14, hind limb 15, and kidney 16. ECFC cell therapy has also been shown to have impressive efficacy in murine models of retinal disease 17, 18, 19, 20, and there is a growing basis for using these cells in patients. Interestingly, there is debate about the mechanism of action for cell therapies. ECFCs exhibit an unequivocal endothelial phenotype, and therefore a cell replacement mechanism, whether as supportive to angiogenesis or de novo vasculogenesis has been reported 17. In addition, a paracrine mechanism of action has also been described 21, 22. Nevertheless, irrespective of the mechanism of action, there is consistent evidence for a therapeutic effect of ECFCs in revascularizing the ischemic retina 17, 19, 20, 23.
We have previously reported that intravitreal delivery of 1 × 105 ECFCs promotes vascular repair in a mouse model of ischemic retinopathy 17, 19, 23, although many obstacles remain to be addressed to optimize an ECFC cytotherapy strategy. In the present study, we sought to focus on some of these important bottlenecks for clinical translation such as the cell purity, immunophenotypic definition, the minimal therapeutic dose, alternative delivery systems, and cell toxicity when injected into a healthy adult eye.
Materials and Methods
Cell Isolation and Characterization
ECFCs were obtained from human umbilical cord blood with appropriate maternal consent and under full ethical approval in accordance with the Declaration of Helsinki. Cord blood samples are untraceable to donors. The mononuclear cell fraction was isolated by density gradient fractionation, resuspended in EGM‐2 (Lonza Ltd.) supplemented with 20% fetal bovine serum (HyClone), and plated in flasks precoated with rat tail collagen type I (BD Biosciences) and seeded at a density of 1 × 107 cells/ml. ECFC colonies were isolated from single umbilical cord blood samples without pooling different samples together and without considering the sex of the donor cord blood. ECFCs were characterized using standard flow cytometry immunophenotyping protocols using antibodies against CD31, CD105, CD14, and CD45 (eBioscience) and an Acoustic Focusing Cytometer (Attune NxT, Life Technologies). Further characterization was done with immunocytochemistry and confocal microscopy using antibodies against vimentin, CD105, and CD31 (Dako); β‐catenin (Cell Signaling Technology); and vWF (Abcam). In vitro three‐dimensional (3D) tube formation assays were performed by resuspending 1 × 105 ECFCs in 50 µl Growth Factor‐reduced Matrigel (BD Biosciences) and covered with previously described media. For all experiments, ECFCs at passage 9 were used. All cells used tested negative for mycoplasma by PCR (Sigma).
Oxygen‐Induced Retinopathy Mouse Model
Animal procedures were performed under U.K. Home Office licence in compliance with the Animals (Scientific Procedures) Act. C57Bl/6J mouse pups and their nursing dams (Harlan Laboratories) were exposed to hyperoxia (75% O2) in an oxygen chamber (Pro‐Ox 110, Chamber Controller, Biospherix, Redfield, NY) for 5 days from postnatal day P7 to P12 as previously described 24. Mice were randomly assigned to experimental groups and all were sacrificed at P17 by intraperitoneal injection of sodium pentobarbital. A control group were sacrificed at P12 to confirm reproducible vascular loss (n = 7).
Intravitreal Delivery of ECFCs: Dose‐Escalation Study
P13 mice were anaesthetized via intraperitoneal injection of xylazine (5 mg/kg, Bayer) and ketamine (100 mg/kg, Pfizer) and ECFCs were injected into the vitreous of the left eye at a dose of 1 × 103 (n = 7), 1 × 104 (n = 5) or 1 × 105 (n = 7) cells, resuspended in 1 µl, and using a 10 µl glass syringe with a 34G needle. The right eye received an equivalent 1 µl injection of vehicle (phenol red‐free Dulbecco's modified Eagle medium, DMEM). A subset of pups received a sham injection (empty 34G needle inserted and withdrawn) into the left eye and the right eye served as an uninjected control (n = 3). At P17, fluorescein angiographs were acquired using a confocal scanning laser ophthalmoscope (cSLO, Heidelberg Retina Angiograph 2, Heidelberg Engineering, Germany) prior to sacrifice, with the eyes enucleated for immunohistochemistry.
Systemic Delivery of ECFCs and Microspheres
In a separate cohort of mice, the left common carotid artery was exposed by blunt dissection under xylazine/ketamine anaesthesia at P13. 5,000 red‐orange (580/620) fluorescent 15 µm diameter polystyrene microspheres (FluoSpheres, Invitrogen, U.K.) were resuspended in a volume of 5 µl and injected into the left common carotid artery using a 10 µl glass syringe with a 34G needle. Pups were sacrificed 2 hours postinjection (P13) or at P14, P15, P16, or P17 (n = 1 for each time point) and the eyes were enucleated for immunohistochemistry. For the cell treatment groups, ECFCs (n = 7) were injected into the left common carotid artery, at a density of 1 × 105 cells suspended in 5 µl of DMEM. Another group received an intracarotid injection of DMEM only (n = 5). To directly compare systemic and intravitreal ECFC delivery, an additional group of mice (n = 8) received an intravitreal injection of 1 × 105 ECFCs in 1 µl DMEM vehicle into the left eye, with the right eye used as a vehicle‐injected control. At P17, all mice were sacrificed and the eyes were enucleated for immunohistochemistry.
Immunohistochemistry
Retinas were fixed in 4% paraformaldehyde for 1.5 hours, dissected, and incubated with a biotin‐conjugated isolectin B4 antibody (20 μg/ml, Sigma‐Aldrich, U.K.) and labeled with streptavidin AlexaFluor 488 (1/500, Invitrogen, U.K.). Retinas were imaged at ×4 magnification using an epifluorescent microscope (Nikon Eclipse E 400, Nikon) and experimental groups blinded for quantification of avascular, neovascular, and normovascular areas by manual delineation, using Image J software.
Intravitreal Delivery of ECFCs in Healthy, Adult Mice
In order to examine possible toxicity of ECFC therapy, irrespective of therapeutic efficacy, 1 × 105 ECFCs resuspended in 1 µl of DMEM were injected into the vitreous of the left eye of healthy 12‐week old male C57Bl/J6 mice under xylazine/ketamine anaesthesia. The right eye received an equivalent injection of DMEM. Mice were sacrificed at 2 hours, 12 hours, 24 hours, 3 days or 7 days postinjection (n = 5 each group). Eyes were enucleated and processed for histology or Alu‐PCR as previously described 25. Whole retinas were dissected from the eye to detect the presence of human DNA in each retina. The number of ECFCs in the retina was quantified using a standard curve constructed from a serial dilution of cells to relate human DNA concentration to the number of ECFCs. For histology, eyes were fixed in 4% paraformaldehyde for 4–6 hours, paraffin processed, and cut into 5 μm thick sections before H&E staining. Retinal sections were examined by light microscopy (Nikon Eclipse E 400, Nikon) to identify hematoxylin stained nuclei in the vitreous.
Statistical Analysis
All data analyses were performed blind to treatment group. Data presented as mean ± SD. Statistical analyses were undertaken using Prism 5 (GraphPad Software, San Diego, CA). One‐way ANOVA was used to compare avascular, neovascular and normovascular areas between groups with Bonferroni's post‐test. Statistical significance was set at p < .05.
Results
Characterization of ECFCs Purity, Potency, and Viability
ECFCs were isolated following protocols previously described 17, 26. ECFCs appeared as cobblestone‐shaped cell monolayers that stained positive for Vimentin and β‐catenin (Fig. 1A). In addition, the endothelial nature of ECFCs was confirmed by positivity to prototypical endothelial markers CD31, CD105, and von Willebrand Factor (Fig. 1A). ECFCs immunophenotype was characterized as a surrogate for purity using four markers by flow cytometry. ECFCs were consistently negative for hematopoietic markers CD14 and CD45; however, they highly expressed endothelial markers CD105 and CD31 (Fig. 1B). This, combined with extensive, previously published evidence, demonstrates that ECFCs are a highly pure population of endothelial cells (>99%) with no or minimal hematopoietic cell contamination (<1%). In addition, ECFCs were capable of forming tubes within 72 hours in an in vitro 3D Matrigel model (Fig. 1C). For cell delivery, ECFCs must pass through microneedles; therefore the effect of such physical stress on 1 × 105 ECFCs resuspended in 1 µl going through 34G and 36G needles was evaluated by assessing cell viability. Three methodologies (trypan blue exclusion, CASY electrical current exclusion, and calcein staining) indicated that ECFCs viability was comparable among cells that passed through the needles and controls (Supporting Information Fig. S1).
Figure 1.
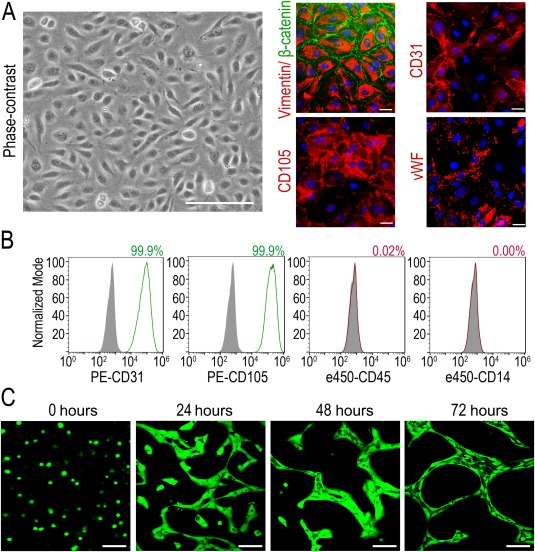
Phenotypical characterization of endothelial colony‐forming cells (ECFCs). (A): Representative phase‐contrast image of ECFCs. Scale bar = 200 µm, and immunocytochemistry for von Willebrand factor, CD31, CD105, vimentin, and β‐catenin. Scale bars = 25 µm. (B): Cell surface immunophenotyping of ECFCs by flow cytometry. Filled gray histograms show respective isotype controls and colored histograms show expression of endothelial markers (CD31, CD105, shown in green) and hematopoietic markers (CD45, CD14, shown in red). Percentage of positive cells for each marker is shown in the top right of each panel. (C): In vitro three‐dimensional Matrigel tubulogenesis assay showing tube‐like structures with lumens formed by ECFCs stained in green with Calcein. Scale bars = 100 µm.
Intravitreal Delivery of Low‐Dose ECFCs Demonstrates Comparable Therapeutic Efficacy to Higher Cell Doses in the Murine Oxygen‐Induced Retinopathy Model
Three different cell doses were tested by intravitreal injection into murine ischemic retinas, and the retinal vasculature was assessed by angiography and immunohistochemistry. Fluorescein angiograms showed that all ECFC doses reduced retinal avascular area when compared to sham and vehicle‐injected eyes (Fig. 2A). In agreement with this, flat‐mounted retinas stained with isolectin B4 to identify the vasculature showed similar results (Fig. 2B). Administration of 1 × 105 ECFCs significantly reduced avascular area to 11% ± 5% of total retinal area, compared to vehicle‐treated retinas (23% ± 4%, p < .001). Lower ECFC doses were similarly efficacious, where retinas treated with 1 × 104 and 1 × 103 ECFCs showed significantly reduced avascular areas of 15% ± 7% and 8% ± 7%, respectively (Fig. 2C). There was no statistically significant difference in avascular areas among the three ECFC doses tested, and the level of ECFC engraftment appears to decrease with lower doses. All ECFC‐treated retinas also demonstrated a significant reduction in the area of pathological neovascularization (Supporting Information Fig. S2). In summary, these experiments using the murine oxygen‐induced retinopathy (OIR) model indicate that ECFC minimal therapeutic dose can be scaled down to 1 × 103 ECFCs per microliter, per eye, without losing efficacy.
Figure 2.
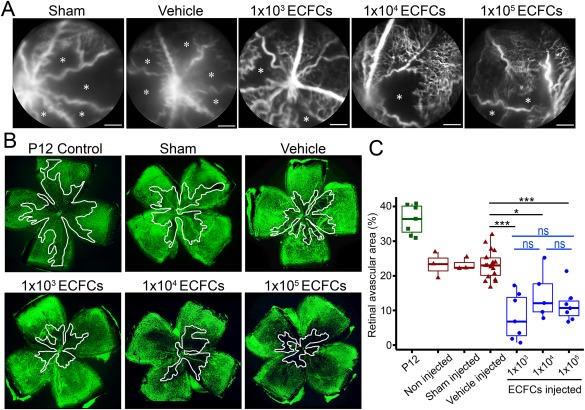
Intravitreal delivery of low dose ECFCs demonstrates therapeutic efficacy comparable to higher cell doses in the murine oxygen induced retinopathy model. (A): Representative fluorescein angiographs acquired in post‐natal day 17 (P17) mice demonstrating the retinal vasculature in vivo. Avascular areas are highlighted with an asterisk. Scale bars = 1 mm. (B): Representative flat‐mounted retinas (magnification ×4) from P17 mice stained with isolectin B4 (Alexa488) to identify the retinal vasculature. Avascular areas are delineated in white. (C): Quantification of avascular area expressed as a percentage of total retina area. Figure shows individual data points with mean, interquartiles, and range. *, p < .05; ***, p < .001, ns: not significant. One‐way ANOVA with Bonferroni's post‐test. Figure shows statistical significance compared to vehicle treated retinas. Abbreviations: ECFCs, endothelial colony‐forming cells; P12, post‐natal day 12.
Systemic Cell Delivery, via the Common Carotid Artery, Provides an Alternative Administration Route for ECFC‐Based Therapy
We have previously published that ECFCs cross the retinal inner limiting membrane (ILM) in neonatal mouse eyes following intravitreal injection 17, but since this route can sometimes be associated with complications 27 and may not be optimal for ECFC engraftment, we decided to use the common carotid artery as an alternative delivery route. Fluorescent microspheres were delivered into the left common carotid artery as a proof‐of‐principle study to demonstrate that beads delivered into the common carotid artery reach the retinal vasculature. Retinas examined 2 hours postinjection showed evidence of fluorescent microspheres within the vasculature (Fig. 3A). In addition, microspheres were only detected in the left retina, ipsilateral to the injection site, and were not detected in the contralateral right retina (Fig. 3A). Interestingly, fluorescent microspheres were not detected in the retinal vasculature in eyes sampled 24 hours postinjection, which indicated these microspheres reached the retinal vasculature but did not migrate into the retinal tissue. We then evaluated the potential for intracarotid delivery of 1 × 105 ECFCs in the OIR model. Flat‐mounted retinal tissues showed that avascular areas in ECFC‐injected animals were significantly reduced in ipsilateral retinas (left eye) but not in the contralateral retinas (right eye) (Fig. 3B, 3C). In addition, there was no significant difference in neovascularization among the experimental groups (Supporting Information Fig. S3A); however, only ipsilateral ECFC retinas demonstrated a significant increase in normovascular areas (Supporting Information Fig. S3B). Furthermore, we directly compared the therapeutic efficacy of ECFCs delivered systemically, with the same dose of ECFCs (1 × 105) administered locally by injection directly into the vitreous. The vasoreparative effects of both administration groups were comparable, with no significant difference in avascular, neovascular, and normovascular areas, demonstrating that both delivery routes have similar therapeutic benefit (Fig. 3D). ECFCs labeled with fluorescent Qdots (ThermoFisher) were identified incorporating into host retina irrespective of delivery route; however, there were fewer ECFCs in the systemic delivery than in the local intravitreal delivery (Supporting Information Fig. S4). Taken together, these data demonstrate that intracarotid cell delivery is a viable alternative to the intravitreal route for ECFCs.
Figure 3.
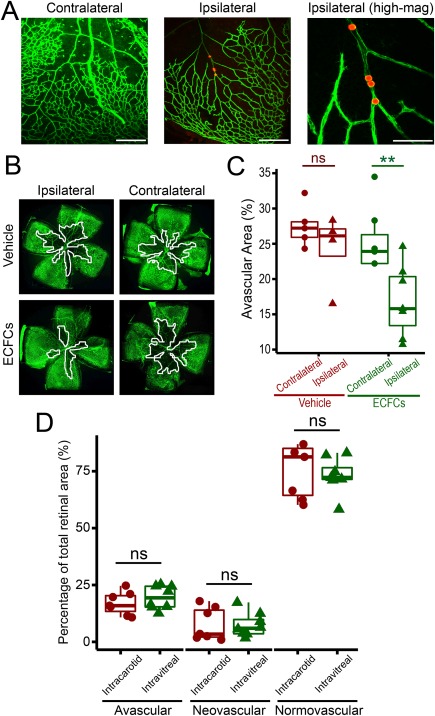
Systemic administration, via the common carotid artery, can be successfully used as an alternative route of delivery for ECFC‐cell based therapy. (A): High magnification photomicrographs of isolectin B4 (Alexa488) stained mouse retina flatmounts following intracarotid injection of orange‐red fluorescent microspheres. Left image shows retinal vasculature of the contralateral right eye on P13, where there is no evidence of microspheres 2 hours following systemic administration via the left common carotid artery (scale bar = 300 µm). Middle image shows microspheres in retinal vasculature of the ipsilateral left eye on P13, 2 hours after injection (scale bar = 300 µm). Image on right shows microspheres at higher magnification (scale bar = 100 µm). (B): Representative photomicrographs of isolectin B4 (Alexa488) stained retina flatmounts of both ipsilateral and contralateral eyes from P17 oxygen induced retinopathy mice following injection of either vehicle or 1 × 105 ECFCs into the left common carotid artery (magnification ×4). Avascular areas are outlined in white. (C): Quantification of avascular areas on retina flatmounts of vehicle and ECFC treated mice. Data presented as individual data points with mean, interquartiles, and range. **, p < .01, One‐way ANOVA with Bonferroni's post‐test. Figure shows statistical significance in ipsilateral left eyes compared to contralateral right eyes in ECFC treated mice. (D): Quantitative comparison of the therapeutic efficacy of intracarotid and intravitreal ECFC delivery routes. Ipsilateral retinas (left eye) from intracarotid ECFC treated mice were compared to retinas where ECFCs were delivered directly into the vitreous at the same cell dose (1 × 105 ECFCs). All retinas were examined at P17. Data presented as individual data points with mean, interquartiles, and range. Two sample t tests for intracarotid versus intravitreal, p > .05, ns: not significant. Abbreviations: ECFC, endothelial colony‐forming cell; P, postnatal day.
Human ECFCs Show No Adverse Effects Following Intravitreal Injection into Healthy Adult Mice
To investigate potential adverse effects of ECFCs delivered into a healthy eye, including immunogenicity, tumorigenicity, and toxicity, we delivered 1 × 105 ECFCs intravitreally into healthy adult mice. H&E sections showed that up to 12 hours following injection, ECFCs form an aggregate of cells clearly observed in the vitreous (Fig. 4A). From 24 hours to 7 days postinjection, the number of ECFCs in the vitreous progressively declined until only a few cells could be visualized. From 24 hours onward, most of ECFCs in the vitreous exhibit a pyknotic nucleus suggesting cell death. This is likely to be apoptosis because there was no evidence of a local inflammatory response. Alu‐PCR was used to quantify the number of human ECFCs present in the host retina. This methodology was validated in vitro with data demonstrating we can consistently correlate amount of DNA from a defined amount of cells with Ct values significantly lower than water controls (Fig. 4B). Heatmap for Alu‐PCR values peaked at 12 hours postinjection and gradually declined at 24 hours postinjection. From day 3 onward, no human DNA could be detected (Fig. 4C). Histological evaluation at different time points and up to 7 days after cell delivery showed that there was no immune cell infiltration, no tissue edema, no tumor formation, and no retinal detachment in ECFC‐injected retinas. Importantly, retinal tissue integrity was preserved and histology appeared normal (Fig. 4D). These results are evidence that human ECFCs did not induce an inflammatory response when injected into healthy mouse retina, and that ECFCs did not persist in healthy retina beyond 24 hours.
Figure 4.
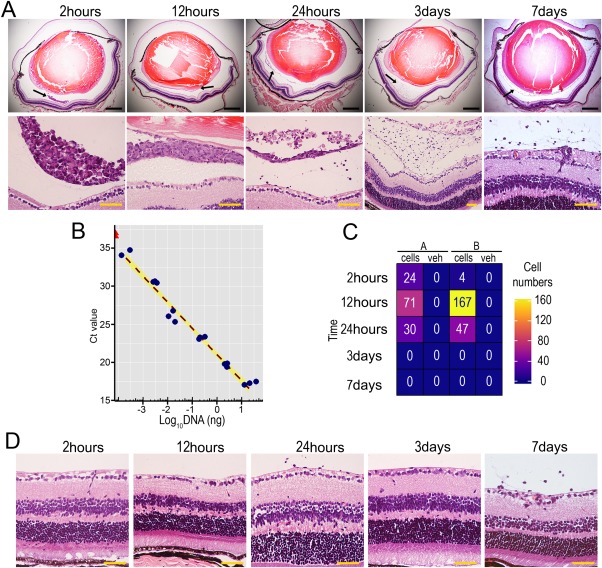
Endothelial colony forming cells (ECFCs) do not induce adverse effects in healthy, adult mouse eyes following intravitreal delivery. (A): Top panel shows representative H&E stained whole eye sections (×4) acquired 2 hours, 12 hours, 24 hours, 3 days, or 7 days following intravitreal injection of 1 × 105 ECFCs. Black scale bars: 500 µm. Black arrows denote the areas shown at higher magnification (×40) in the lower panel, where hematoxylin stained ECFCs are visible in the vitreous. Yellow scale bars: 50 µm. (B): Standard curve for Alu‐polymerase chain reaction (Alu‐PCR) analysis correlating amount of human DNA with PCR Ct value. Blue points are human DNA samples and red triangles are water samples. (C): Alu‐PCR for human ECFC tracking was used to detect human DNA in cell and vehicle injected retinas in two mice. The quantitative data are displayed in a heatmap with corresponding cell number indicated inside the tiles. (D): Mouse retinal tissue cross sections at different time points after cell delivery stained with H&E to assess for retinal integrity (×40 magnification). Yellow scale bars: 50 µm.
Discussion
This study provides preclinical evidence to facilitate the translation of an ECFC‐based cell therapy for the ischemic retina. Multiple critical steps in the development of cell therapy products have been described in international guidelines 28. Here, we focused on cell purity, escalation of cell dose related to efficacy, delivery route, and immunogenicity. Our results are supportive for further development of human cord blood‐derived ECFCs as a cell product, but at the same time, highlight that the developmental pathway for cell therapies is challenging. Among the next essential steps are the production of ECFCs in compliance with good manufacturing practice, evaluation of biodistribution, assessment of ECFC tumorigenicity, and the development of potency assays.
We showed evidence that ECFCs are not lysed when passed through 34G and 36G microneedles with internal diameters of 85 µm and 35 µm, respectively. The average diameter of early passage ECFCs in cell suspension is 12–18 µm. This highlights that ECFCs can be delivered with 36G microneedles while remaining intact.
We have demonstrated that local administration of 1 × 103 ECFCs promotes vascular repair of the murine ischemic retina with a therapeutic efficacy comparable to higher ECFC doses, that is, 1 × 105 ECFCs as previously reported 17. These data will assist for mouse‐to‐human cell dosage conversion using advanced allometric scaling and modeling. This finding further confirms feasibility of manufacture of adequate cell numbers, since in mice, the minimal therapeutic dose for an ischemic eye is approximately 500‐fold lower than the cell number required for an ischemic limb 29. This also has important implications for the cell product cost of manufacture and suggests that blood from a single umbilical cord will be able to generate enough ECFCs to treat tens of patients. Therefore, ECFCs could be banked as a frozen product for allogeneic cell therapies and cell cryopreservation technology for endothelial cells is progressing rapidly 30, 31, which is applicable to ECFCs. In addition, ECFCs may be primed prior to use by coculturing with mesenchymal stem/stromal cells to further improve vasculogenic potential and engraftment capacity 32. Our data also show that fluorescein retinal angiography is an effective tool to visualize areas of vascular loss in the OIR mouse model, and the images acquired in vivo are comparable to avascular areas observed on postmortem retinas. This has translational value because retinal angiography performed in the clinical setting is used as a primary read‐out in clinical trials. Based on our preclinical data, we recommend that optical coherence tomography angiography and fluorescein retinal angiography are considered as potential endpoints, if an ECFC cell therapy for ischemic retinopathies moves into clinical trials.
The current study demonstrates, for the first time, that ECFCs promote vascular repair when administered systemically, via the common carotid artery. Although intravitreal injections can enable a localized and concentrated cell delivery, the carotid artery delivery route may have advantages since it overcomes the need to cross the ILM, although ECFCs appear to readily cross this barrier in murine disease models 17, 19. In addition, the intracarotid delivery route also eliminates possible complications associated with intravitreal injections, such as endophthalmitis, retinal detachment, increased intraocular pressure, and ocular hemorrhage 27. Previously, intracarotid injections have been used to deliver cell therapy in rat stroke models 33, 34 and permits targeting of the ischemic retina while avoiding clearance via the venous system, where typically 70% of cells are found in the liver and spleen following intravenous administration 35. Interestingly, ECFCs only promoted vascular repair of the retina ipsilateral to the intracarotid injection site and had no therapeutic effect on the contralateral retina. This may result from ECFCs homing toward the ischemic retina which is closest in proximity to the injection site. The possibility of targeting only one retina is also of clinical relevance, since ischemic eye diseases such as retinal vein occlusion present usually as unilateral disease, although recurrence in fellow eye is observed in up to 5% cases within 3 years of initial diagnosis 36.
The mechanism of action for ECFCs in vascular repair is likely to be a combination of both direct cell replacement and paracrine effects. There is previous convincing evidence for these 17, 21, and our data, while demonstrating efficacy, cannot establish a sole major mechanism of action. Our results demonstrate ECFC engraftment into mouse ischemic retinal vasculature, albeit at low levels. This might relate to limitations of the experimental model used, such as the fact that we are injecting human cells into immunocompetent mice. Additionally, in the OIR model, a long‐lasting engraftment cannot be expected nor studied because endogenous mouse cells will spontaneously repair the ischemic retinal vasculature within 14–21 days 24. Therefore, there is need for further studies using models that allow assessment of engraftment and toxicity after 6–12 months of cell delivery. The ultimate proof for cell engraftment and immunogenicity will come from a first‐in‐human trial, which for allogeneic therapies from cord blood‐derived ECFCs will require some level of immunosuppression or alternatively an HLA‐matched cell transplant.
Since the OIR model uses neonatal mice whose retinas are not fully developed, we sought to evaluate any potential detrimental tissue responses to intravitreal injection of human ECFCs in healthy adult immunocompetent C57Bl/6J mice. We show evidence to indicate that ECFCs administered in the adult mouse eye did not trigger inflammation or neovascular formation, up to 7 days following intravitreal ECFC delivery. Progressively declining numbers of ECFCs were visible in the vitreous and importantly, ECFCs did not penetrate into the healthy retina presumably since there was no hypoxic stimulus for their chemotaxis. Indeed, vitreous localized ECFCs eventually underwent cell death without an inflammatory response in the retinal tissue. This adds to the safety profile of ECFCs as a cell therapy product, which was also reported by systemic tail vein delivery without formation of tumors among nine organs after 7 months follow‐up 37.
This preclinical study provides encouraging results that contribute to accumulating evidence for the validity of ECFCs as a cell therapy product for ischemic eye disease. Nevertheless, further studies are warranted to fulfil preclinical benchmarks required to prove safety and efficacy. This is extremely important to avoid preventable adverse effects such as the severe bilateral visual loss recently reported in three patients receiving intravitreal autologous “stem cell” injections 38. This again highlights the importance of studies at the preclinical stage, which are essential but usually overlooked due to the “eagerness” and various pressures to translate cell therapies into patients. We support the “fast‐track” development of novel cell therapies but always within national and international regulatory frameworks 28, 39.
Conclusion
ECFCs are a well‐defined and highly pure cell population with potential to be used as a cell therapy product for ischemic retinopathies. The ischemic retina is an attractive target because of easy access for both delivery and evaluation through angiography. In the mouse OIR model, we demonstrated that ECFC cell therapy can be successfully administered in a low dose, by local intravitreal injection or by systemic intracarotid delivery, with both promoting significant vascular repair of the ischemic retina.
Author Contributions
E.R.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; J.G.‐F. and C.O.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; L.‐D.A. and S.E.J.C.: collection and/or assembly of data, final approval of manuscript; A.W.S.: conception and design, final approval of manuscript; R.J.M.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Note Added in Proof
This article was published online on 22 November 2017. Minor edits have been made that do not affect data. This notice is included in the online and print versions to indicate that both have been corrected 28 December 2017.
Supporting information
Supporting Information
Acknowledgments
This research was supported by a grant from the Sir Jules Thorn Trust, the JDRF, the Leverhulme Trust, Fight for Sight, and the National Eye Research Centre.
References
- 1. Jones MK, Lu B, Girman S et al. Cell‐based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog Retin Eye Res 2017;58:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ehmann D, Shahlaee A, Ho AC. Cell therapy for retinal disease. Curr Opin Ophthalmol 2016;27:185–190. [DOI] [PubMed] [Google Scholar]
- 3. Park SS. Cell therapy applications for retinal vascular diseases: Diabetic retinopathy and retinal vein occlusion. Invest Ophthalmol Vis Sci 2016;57:ORSFj1–ORSFj10. [DOI] [PubMed] [Google Scholar]
- 4. O'Neill CL, O'Doherty MT, Wilson SE et al. Therapeutic revascularisation of ischaemic tissue: The opportunities and challenges for therapy using vascular stem/progenitor cells. Stem Cells Res Ther 2012;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caballero S, Sengupta N, Afzal A et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007;56:960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park SS, Caballero S, Bauer G et al. Long‐term effects of intravitreal injection of GMP‐grade bone‐marrow‐derived CD34+ cells in non‐SCID mice with acute ischemia‐reperfusion injury. Invest Ophthalmol Vis Sci 2012;53:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li N, Li XR, Yuan JQ. Effects of bone‐marrow mesenchymal stem cells implanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol 2009;247:503–514. [DOI] [PubMed] [Google Scholar]
- 8. Otani A, Dorrell MI, Kinder K et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow‐derived lineage‐negative hematopoetic stem cells. J Clin Invest 2004;114:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Medina RJ, O'Neill CL, O'Doherty TM et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin‐8. Mol Med 2011;17:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stitt AW, O'Neill CL, O'Doherty MT et al. Vascular stem cells and ischaemic retinopathies. Prog Retin Eye Res 2011;30:149–166. [DOI] [PubMed] [Google Scholar]
- 11. Medina RJ, O'Neill CL, Sweeney M et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 2010;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medina RJ, Barber CL, Sabatier F et al. Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Translational Medicine 2017;6:1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubois C, Liu X, Claus P et al. Differential effects of progenitor cell populations on left ventricular remodelling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol 2010;55:2232–2243. [DOI] [PubMed] [Google Scholar]
- 14. Ding J, Zhao Z, Wang C et al. Bioluminescence imaging of transplanted human endothelial colony‐forming cells in an ischemic mouse model. Brain Res 2016;1642:209–218. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz T, Leicht S, Radic T et al. Vascular incorporation of endothelial colony‐forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol 2012;32:e13–e21. [DOI] [PubMed] [Google Scholar]
- 16. Burger D, Viñas JL, Akbari S et al. Human endothelial colony‐forming cells protect against acute kidney injury: Role of exosomes. Am J Pathol 2015;185:2309–2323. [DOI] [PubMed] [Google Scholar]
- 17. Medina RJ, O'Neill CL, Humphreys MW et al. Outgrowth endothelial cells: Characterization and their potential for reversing ischemic retinopathy. Invest Ophthalmol Vis Sci 2010;51:5906–5913. [DOI] [PubMed] [Google Scholar]
- 18. Medina RJ, O'Neill CL, O'Doherty TM et al. Ex vivo expansion of human outgrowth endothelial cells leads to IL‐8‐mediated replicative senescence and impaired vasoreparative function. Stem Cells 2013;31:1657–1668. [DOI] [PubMed] [Google Scholar]
- 19. Cahoon JM, Rai RR, Carroll LS et al. Intravitreal AAV2.COMP‐Ang1 prevents neurovascular degeneration in a murine model of diabetic retinopathy. Diabetes 2015;64:4247–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakimoto S, Marchetti V, Aguilar E et al. CD44 expression in endothelial colony‐forming cells regulates neurovascular trophic effect. JCI Insight 2017;2:e89906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin RZ, Moreno‐Luna R, Li D et al. Human endothelial colony‐forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA 2014;111:10137–10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collett JA, Mehrotra P, Crone A et al. Endothelial colony forming cells ameliorate endothelial dysfunction via secreted factors following ischemia‐reperfusion injury. Am J Physiol Renal Physiol 2017;312:F897–F907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prasain N, Lee MR, Vemula S et al. Differentiation of human pluripotent stem cells to cells similar to cord‐blood endothelial colony‐forming cells. Nat Biotechnol 2014;32:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith LE, Wesolowski E, McLellan A et al. Oxygen‐induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–111. [PubMed] [Google Scholar]
- 25. Creane M, Howard L, O'Brien T et al. Biodistribution and retention of locally administered human mesenchymal stromal cells: Quantitative polymerase chain reaction‐based detection of human DNA in murine organs. Cytotherapy 2017;19:384–394. [DOI] [PubMed] [Google Scholar]
- 26. Prasain N, Meador JL, Yoder MC. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp 2012;62:3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghasemi Valafarjani K, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti‐VEGF agents: a review of literature. Eye 2013;27:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feigal EG, Tsokas K, Viswanathan S et al. Proceedings: International regulatory considerations on development pathways for cell therapies. Stem Cells Translational Medicine 2014;3:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SH, Lee JH, Han YS et al. Hypoxia accelerates vascular repair of endothelial colony‐forming cells on ischemic injury via STAT3‐BCL3 axis. Stem Cell Res Ther 2015;6:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sultani AB, Marquez‐Curtis LA, Elliot JA et al. Improved cryopreservation of human umbilical vein endothelial cells: A systematic approach. Sci Rep 2016;6:34393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng Y, Zhao G, Panhwar F et al. Vitreous cryopreservation of human umbilical vein endothelial cells with low concentration of cryoprotective agents for vascular tissue engineering. Tissue Eng Part C Methods 2016;22:964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shafiee A, Patel J, Wong HY et al. Priming of endothelial colony‐forming cells in a mesenchymal niche improves engraftment and vasculogenic potential by initiating mesenchymal transition orchestrated by NOTCH signalling. FASEB J 2017;31:610–624. [DOI] [PubMed] [Google Scholar]
- 33. Minnerup J, Seeger F, Kuhnert K et al. Intracarotid administration of human bone marrow mononuclear cells in rat photothrombotic ischemia. Exp Transl Stroke Med 2010;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitkari B, Kerkelä E, Nystedt J et al. Intra‐arterial infusion of human bone marrow‐derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol 2013;239:158–162. [DOI] [PubMed] [Google Scholar]
- 35. Aicher A, Brenner W, Zuhayra M et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labelling. Circulation 2003;107:2134–2139. [DOI] [PubMed] [Google Scholar]
- 36. Garcia‐Horton A, Al‐Ani F, Lazo‐Langner A. Retinal vein thrombosis: The internist's role in the etiologic and therapeutic management. Thromb Res 2016;148:118–124. [DOI] [PubMed] [Google Scholar]
- 37. Milbauer LC, Enestein JA, Roney M et al. Blood outgrowth endothelial cell migration and trapping in vivo: A window into gene therapy. Transl Res 2009;153:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuriyan AE, Albini TA, Townsend JH et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med 2017;376:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ilic N, Savic S, Siegel E et al. Examination of the regulatory frameworks applicable to biologic drugs (including stem cells and their progeny) in Europe, the U.S., and Australia: Part I–A method of manual documentary analysis. Stem Cells Translational Medicine 2012;1:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


