Abstract
Background
Lung cancer is the leading cause of cancer-related death among Chinese Americans. A detailed examination of incidence trends by immigration status and histology may inform the etiology of lung cancer in this growing population.
Methods
California Cancer Registry data were enhanced with data on patient nativity. Lung cancer incidence rates for Chinese males and females were computed for the years 1990–2010, and rates by immigration status and histology were computed for 1990–2004. Trends were assessed with annual percentage change (APC) statistics (two-sided P values) based on linear regression.
Results
A total of 8,167 lung cancers were diagnosed among California Chinese from 1990 to 2010. Overall incidence increased nonstatistically among U.S.-born males (APC, 2.1; 95% CI, −4.9 to 9.7), but decreased significantly among foreign-born (APC, −1.7; 95% CI, −2.9 to −0.6). Statistically significant decreasing trends were observed for non–small cell lung cancer (NSCLC), specifically the squamous cell and large cell carcinoma subtypes among foreign-born males. Among females, incidence decreased nonsignificantly among U.S.-born (APC, −2.8; 95% CI, −9.1 to 4.0) but was stable among foreign-born (APC, −0.4; 95% CI, −1.7 to 1.0). A statistically significant decreasing trend was observed for squamous cell among foreign-born females.
Conclusions
These data provide critical evidence base to inform screening, research, and public health priorities in this growing population.
Impact
Given the low smoking prevalence among Chinese Americans, especially females, and few known lung cancer risk factors in U.S. never-smoker populations, additional research of etiologic genetic or biologic factors may elucidate knowledge regarding lung cancer diagnosed in never smokers.
Introduction
Numbering 4.0 million, Chinese Americans are the largest Asian population in the United States (1). Lung cancer is the most common cause of cancer-related death (2) and the second and fourth most common cancer diagnosed among U.S. Chinese males and females, respectively (3). We recently documented overall declines in lung cancer incidence among Chinese American males and females from 1990 to 2010, but trends in adenocarcinoma, the most common histologic subtype of lung cancer, were either increasing among males [annual percentage change (APC) of 1.3%; 95% confidence interval (CI), 0 to 2.5 from 1996 to 2010] or stable among females (APC, 0.3; 95% CI, −0.4 to 1.1) from 1990 to 2010); all other subtypes were declining over time (4). Adenocarcinoma is less strongly associated with tobacco smoke than small cell and squamous cell subtypes. In a meta-analysis of case–control studies from Europe and Canada, the odds ratio (OR) for adenocarcinoma comparing heavy smokers with never smokers was 21.9 (95% CI, 16.6–29.0) among males and 16.8 (9.2–30.6) among females, relative to 111.3 (69.8–177.5) for small cell lung cancer in males and 108.6 (50.7–232.8) for females, and 103.5 (74.8–143.2) for squamous cell lung cancer in males and 62.7 (31.5–124.6) for females (5). The extent to which these smoking-associated risks differ among Chinese Americans, however, has not been well documented.
Smoking patterns among Chinese Americans vary by acculturation in opposing directions between males and females—smoking increases with acculturation among females but decreases with acculturation among males (6). In addition to smoking, acculturation may be associated with other lung cancer risk factors, including exposure to second-hand smoke and indoor and outdoor air pollution, and previous history of lung diseases (7–18). In our prior analysis for diagnoses from 1998 to 2002, we found generally higher incidence rates of non–small cell lung cancer (NSCLC) among foreign-born Chinese males and females relative to U.S.-born, although within NSCLC histology groups, the higher incidence was statistically significant only for squamous cell among males (19); these associations may be due to higher prevalence of lung cancer risk factors among foreign-born than U.S.-born.
No studies, to our knowledge, have examined trends in the incidence of histologic subtypes of lung cancer among Asian Americans by immigrant status. These data are needed as they may be used to inform recent U.S. Preventive Task Force (USPTF) recommendations for low-dose spiral computed tomography (CT) lung cancer screening for heavy smokers and for surveillance of this common and fatal disease among the large and growing Chinese American population. These data can also be useful for informing lung cancer etiology, particularly as Chinese females are noted to have an exceptionally high burden of lung cancer in light of their low reported rates of smoking; examination of trends in histology-specific lung cancer by immigrant status can provide insights into the relative contributions of genetic and environmental factors. We used population-based California Cancer Registry data enhanced with information regarding patient nativity to examine cancer incidence trends among Chinese Americans. We characterized histology- and gender-specific lung cancer incidence trends among Chinese in California, the U.S. state with the largest Chinese population (1.35 million; 33.7% of the U.S. Chinese population) over a 21-year period (1990–2010) and by immigrant status over a 15-year period (1990–2004).
Materials and Methods
Cancer incidence data
We obtained data for all primary invasive lung cancer [International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site codes, C34.0–C34.9, excluding 9050–9055, 9140, 9590–9992] occurring among California Chinese American residents during the period between January 1, 1990, through December 31, 2010, from the California Cancer Registry [CCR, comprising four registries (San Francisco Bay Area, San Jose/Monterey, Los Angeles, and Greater California) within the NCI Surveillance, Epidemiology, End Results (SEER) program]. Information on patient race/ethnicity is primarily based on data collected in hospital medical records and defined by self-report from patients, assumptions from hospital personnel, or inference from other identification information of patients, including race/ethnicity, maiden name, surname, birthplace (20), and death records. The study cohort for incidence analysis included 8,167 invasive lung cancers diagnosed in patients whose race/ethnicity was classified as Chinese. Other patient characteristics were also obtained from CCR data, including age and year of diagnosis, birthplace, gender, residential address and stage at diagnosis, and histologic subtype [small cell carcinoma: 8041–8045, and 8246; squamous cell carcinoma: 8051, 8052, 8070–8078, 8083, and 8084; adenocarcinoma: 8050, 8140–8147, 8201, 8230, 8250–8255, 8260, 8263, 8290, 8310, 8320, 8323, 8220, 8350, 8441, 8460, 8470, 8471, 8480, 8481, 8490, 8500, 8503, 8507, 8550, and 8570–8576; large cell carcinoma: 8011–8015, 8082, and 8123, and NSCLC, not otherwise specified (NSCLC, NOS): 8010, 8020–8022, 8030– 8035, 8046, 8094, 8120, 8130, 8170, 8200, 8240–8249, 8340, 8430, 8525, 8551, 8560, 8562, 8580, 8940, 8972, and 8980].
As results from our prior studies showed that Asian patients in the CCR with unknown registry birthplace are more likely to be U.S.-born (21), random imputation of immigration status for cases with unknown birthplace would therefore lead to an underestimate of U.S.-born cases. To more accurately impute immigration status, we applied a statistical imputation method based on the age at receipt of social security numbers (SSN; ref. 22). By comparing the age of SSN issue with self-reported birthplace in previously interviewed cancer patients (n = 1,836) and based on maximization of the area under the receiver-operating characteristic curve and confirmation with logistic regression modeling, patients receiving a SSN before age 25 years were considered U.S.-born, and those who had received an SSN at or after age 25 years as foreign-born. This age cutoff point resulted in 84% sensitivity and 80% specificity for assigning foreign-born status across the Asian populations. For our study, registry-based birthplace data were available for 93% of the Chinese cases (72% from hospital records; 21% from death certificates). The missing birthplace information was imputed using the method described above for about 7% of cases, and randomly assigned based on the overall sample’s joint distribution of race/ethnicity, gender, age, and birthplace for the remaining <1% of cases for whom their SSNs were missing or invalid.
Patients’ residential address at diagnosis was geocoded and assigned to a census block group, which was linked to block group-level measures developed from census data. Neighbor-hood socioeconomic status (SES) measure is a composite index developed from principal component analysis, incorporating information on education, occupation, employment, household income, poverty, rent and house values from Census 1990 summary file (for cases diagnosed 1990–1995), Census 2000 Summary File (applied to cases diagnosed 1996–2005), and American Community Survey (ACS) 2007–2011 data (applied to cases diagnosed 2006–2010 as ACS replaced the decennial census long form in 2010; refs. 23, 24). Ethnic enclaves are neighborhoods that are ethnically distinct from its surrounding area and are characterized using a composite index based on four census indicator variables: percent recent immigrants, percent Asian/Pacific Islander (API) language-speaking households that were linguistically isolated, percentage of API language speakers with limited English proficiency, and percent API population (25). For cases diagnosed during the periods 1990–1995 and 1996–2005, this information was derived from the summary files of Census 1990 and 2000, respectively; for cases diagnosed in 2006–2010, we applied the Census 2000 measure as the component variables are lacking or unreliable in the ACS. Both neighborhood SES and ethnic enclave measures were classified into quintiles based on distributions across California.
Population data
Population data are obtained from U.S. Census data for 1990, 2000, and 2010. Race/ethnicity, age, and immigrant status are based on self-report—race/ethnicity and age from the Census short-form (1990, 2000, 2010) and immigrant status from the Census long-form (1990 and 2000). From census summary files, we obtained population counts by gender, race/ethnicity, and 5-year age group for California Chinese in censal years 1990, 2000, and 2010. For each intercensal year between 1990 and 2010, the population counts were imputed by linear interpolation methods. For Chinese population estimates by immigration status, data from the 5% (1 in 20) Integrated Public-Use Microdata Sample (IPUMS) of the 1990 and 2000 censuses were used to estimate the percentage of foreign-born persons, by age, for each gender and ethnic subgroup; population counts in censal years were imputed using the smooth spline function in the R statistical software package; and intercensal and postcensal year counts for 1990 to 2004 were computed by interpolation and extrapolation (26). Estimates were then adjusted to match total population estimates, by age and year, as provided by the California Department of Finance (for years 1988–1989) and the U.S. Census (for years 1990–2004). Because of lack of population estimate data from the 2010 Census for Chinese populations by immigration status, population denominator data were estimated for this study through 2004. Thus, cancer incidence rates by immigration status are available only through 2004.
Statistical analysis
Lung cancer histologic subtype-specific incidence rates and 95% confidence intervals (CI) were calculated as cases per 100,000 persons and age adjusted to the 2000 U.S. standard population using SEER Stat software (http://seer.cancer.gov/seer-stat/). Annual rates are shown graphically as trends (27), except for rates for U.S.-born by histology where 3-year averaged rates are shown. APC statistics (two-sided P values) using the weighted least squares method were used to characterize the magnitude and direction of trends (28).
Results
A total of 8,167 lung cancers were diagnosed among Chinese in California from 1990 to 2010; the majority, 7,265 (89%), were foreign-born, 79% of whom were born in China, followed by 7.4% in Taiwan, 5.1% in Hong Kong, and 2.8% in Vietnam. Among males (Table 1), foreign-born cases were older than U.S.-born cases (74.8% of foreign-born were age 65 or older relative to 69% of U.S.-born cases). Relatively more foreign-born than U.S.-born males were diagnosed in the more recent 2004–2010 time period. Both foreign-born and U.S.-born had a relatively high proportion of cases diagnosed as NSCLC NOS. A lower proportion of lung cancers among foreign-born males were diagnosed with localized stage (13.5%) relative to U.S.-born (17.1%); however, foreign-born had a higher proportion of unknown stage relative to U.S.-born (10.7% vs. 6.5%). A lower proportion of foreign-born than U.S.-born male cases lived in higher SES neighborhoods, while a higher proportion of foreign-born cases lived in higher enclave neighborhoods.
Table 1.
Characteristics of male Chinese American lung cancer cases by nativity, California, 1990–2010
| Characteristic | U.S.-born Chinese (N = 491) |
Foreign-born Chinese (N = 4,321) |
|---|---|---|
| Age at diagnosis, y | ||
| <50 | 6.9% | 4.7% |
| 50–59 | 12.4% | 11.1% |
| 60–64 | 11.6% | 9.3% |
| 65–69 | 12.8% | 14.3% |
| 70–74 | 19.3% | 19.0% |
| >75 | 36.9% | 41.5% |
| Year of diagnosis | ||
| 1990–1995 | 21.4% | 20.8% |
| 1996–2000 | 29.7% | 20.0% |
| 2001–2005 | 23.8% | 26.9% |
| 2006–2010 | 25.1% | 32.3% |
| Histology | ||
| Small cell carcinoma | 7.3% | 9.2% |
| NSCLC | 89.6% | 85.4% |
| Squamous | 12.2% | 17.1% |
| Adenocarcinoma | 46.6% | 38.7% |
| Large cell | 4.3% | 4.9% |
| NSCLC, not otherwise specified | 26.5% | 24.7% |
| Other lung cancer | 3.1% | 5.5% |
| Stage at diagnosis | ||
| Local | 17.1% | 13.5% |
| Regional | 17.7% | 19.2% |
| Distant | 58.7% | 56.6% |
| Unknown | 6.5% | 10.7% |
| Block group SESa | ||
| Quintile 1 (low SES) | 9.6% | 16.5% |
| Quintile 2 | 10.0% | 17.2% |
| Quintile 3 | 18.7% | 19.2% |
| Quintile 4 | 27.9% | 23.3% |
| Quintile 5 (high SES) | 33.8% | 23.9% |
| Block group ethnic enclavea | ||
| Quintile 1 (least ethnic) | 3.9% | 1.2% |
| Quintile 2 | 8.1% | 2.7% |
| Quintile 3 | 11.6% | 5.6% |
| Quintile 4 | 18.3% | 12.2% |
| Quintile 5 (most ethnic) | 54.0% | 73.4% |
| Unknown | 4.1% | 5.0% |
Statewide quintiles.
Among Chinese females (Table 2), age at diagnosis was similar to males, with a higher proportion of older foreign-born cases than U.S.-born cases (70.9% vs. 66.4% ages 65 or older). As with males, proportionally more foreign-born than U.S.-born females were diagnosed in the more recent 2004–2010 time period (33.1% vs. 29.9%). A lower proportion of foreign-born cases were diagnosed with localized stage (13.5% vs. 19.0% among U.S.-born) and a higher proportion of foreign-born were diagnosed with distant stage (60.0% vs. 52.6% among U.S.-born). As with Chinese male cases, proportionally more foreign-born female cases lived in lower SES neighborhoods and in more ethnic neighborhoods.
Table 2.
Characteristics of female Chinese American lung cancer cases by nativity, California, 1990–2010
| Characteristic | U.S.-born Chinese (N = 411) |
Foreign-born Chinese (N = 2,944) |
|---|---|---|
| Age at diagnosis, y | ||
| <50 | 9.3% | 7.6% |
| 50–59 | 14.8% | 12.9% |
| 60–64 | 9.5% | 8.6% |
| 65–69 | 14.6% | 12.8% |
| 70–74 | 14.8% | 15.3% |
| >75 | 37.0% | 42.8% |
| Year of diagnosis | ||
| 1990–1995 | 24.6% | 18.1% |
| 1996–2000 | 18.2% | 20.6% |
| 2001–2005 | 27.3% | 28.2% |
| 2006–2010 | 29.9% | 33.1% |
| Histology | ||
| Small cell carcinoma | 5.6% | 3.7% |
| NSCLC | 91.0% | 90.55% |
| Squamous | 8.5% | 5.9% |
| Adenocarcinoma | 55.5% | 58.7% |
| Large cell | 4.9% | 4.77% |
| NSCLC, not otherwise specified | 22.1% | 21.1% |
| Other lung cancer | 3.4% | 5.8% |
| Stage at diagnosis | ||
| Local | 19.0% | 13.5% |
| Regional | 21.4% | 16.7% |
| Distant | 52.6% | 60.0% |
| Unknown | 7.1% | 9.8% |
| Block group SESa | ||
| Quintile 1 (low SES) | 8.0% | 13.8% |
| Quintile 2 | 8.8% | 16.5% |
| Quintile 3 | 16.5% | 17.4% |
| Quintile 4 | 25.3% | 23.7% |
| Quintile 5 (high SES) | 41.4% | 28.5% |
| Block group ethnic enclavea | ||
| Quintile 1 (least ethnic) | 2.7% | 1.1% |
| Quintile 2 | 6.8% | 3.7% |
| Quintile 3 | 14.6% | 6.1% |
| Quintile 4 | 15.8% | 12.1% |
| Quintile 5 (most ethnic) | 54.0% | 71.8% |
| Unknown | 6.1% | 5.2% |
Statewide quintiles.
Among both Chinese males and females, overall lung cancer incidence rates declined significantly from 1990 to 2010 (−1.4% per year; 95% CI, −2.1 to −0.8 among males and −1.0% per year; 95% CI, −1.6 to −0.3 among females). Among males, statistically significant declines were observed for small cell (APC, −2.4; 95% CI, −3.8 to −0.9), NSCLC (APC, −1.6; 95% CI, −2.3 to −0.9), specifically, the squamous cell (APC, −4.7; 95% CI, −5.8 to −3.7), and large cell (APC, −8.6; 95% CI, −10.5 to −6.6) carcinoma to subtypes [Fig. 1, Table 3; Supplementary Tables S1 (males) and S2 (females)]. Among females, statistically significant declines were observed for NSCLC (APC, −0.8; 95% CI, −1.5 to −0.1), most notably the squamous cell carcinoma subtype (APC, −4.9; 95% CI, −6.5 to −3.1).
Figure 1.
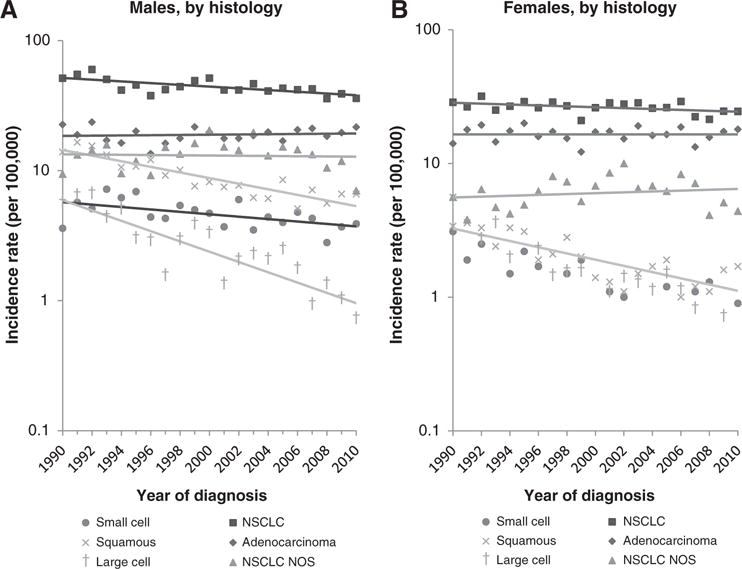
Lung cancer incidence trends among Chinese Americans, by sex and histology, California, 1990–2010. A, males, by histology. B, females, by histology.
Table 3.
Lung cancer incidence trends among Chinese Americans, by sex and histology, California, 1990–2010, APC (95% CI)
| Total | Small cell | NSCLC | Squamous | Adenocarcinoma | Large cell | NSCLC NOS | |
|---|---|---|---|---|---|---|---|
| Male | −1.4 (−2.1 to −0.8) | −2.4 (−3.8 to −0.9) | −1.6 (−2.3 to −0.9) | −4.7 (−5.8 to −3.7) | 0.3 (−0.5 to 1.2) | −8.6 (−10.5 to −6.6) | −0.8 (−2.9 to 1.3) |
| Female | −1.0 (−1.6 to −0.3) | Could not be estimated | −0.8 (−1.5 to −0.1) | −4.9 (−6.5 to −3.1) | 0 (−0.9 to 0.9) | Could not be estimated | −0.1 (−2.4 to 2.3) |
By immigrant status, among males, overall incidence increased nonstatistically from 1990 to 2004 among U.S.-born (APC, 2.1; 95% CI, −4.9 to 9.7), but decreased significantly among foreign-born [APC, −1.7; 95% CI, −2.9 to −0.6; Fig. 2; Table 4; Supplementary Tables S3 (foreign-born males) and S4 (U.S.-born males)]. No significant trends were observed for any histologic subtype among U.S.-born males, while statistically significant decreasing trends were observed for NSCLC (APC, −1.7; 95% CI, −3.1 to −0.3), specifically the squamous cell (APC, −6.8; 95% CI, −8.1 to −5.4), and large cell carcinoma subtypes (APC, −9.0; 95% CI, −12.8 to −5.0) among foreign-born males. Among females, incidence decreased non-statistically among U.S.-born (APC, −2.8; 95% CI, −9.1 to 4.0) but was stable among foreign-born [APC, 0.4; 95% CI, −1.7 to 1.0; Fig. 2; Table 4; Supplementary Tables S5 (foreign-born increasing trend for NSCLC NOS (APC, 3.9; 95% CI, 0.7 to 7.1) among foreign-born females.
Figure 2.
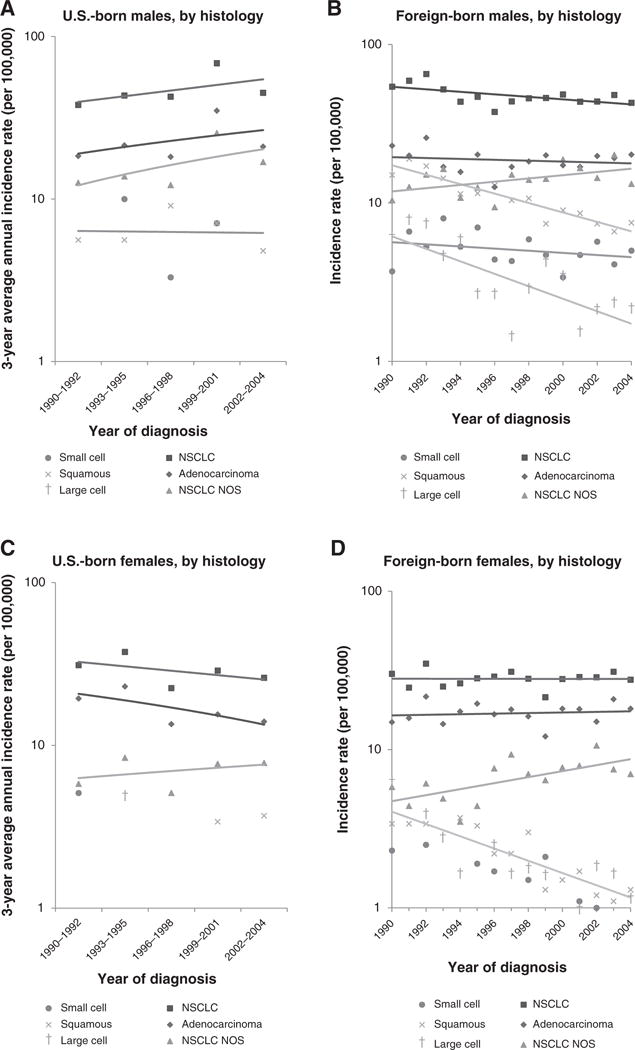
Lung cancer incidence trends among Chinese Americans, by sex, nativity, and histology, California, 1990–2004. A, U.S.-born males, by histology; B, foreign-born males, by histology; C, U.S.-born females, by histology; D, foreign-born females, by histology.
Table 4.
Lung cancer incidence trends among Chinese Americans, by sex, nativity, and histology, California, 1990–2004, APC (95% CI)
| Total | Small cell | NSCLC | Squamous | Adenocarcinoma | Large cell | NSCLC NOS | |
|---|---|---|---|---|---|---|---|
| USB male | 2.1 (−4.9 to 9.7) | Could not be estimated | 2.3 (−4.6 to 9.7) | −0.8 (−9.3 to 8.5) | 2.1 (−6.7 to 11.7) | Could not be estimated | 3.7 (−4.9 to 13.0) |
| FB male | −1.7 (−2.9 to −0.6) | −2.0 (−4.7 to 0.9) | −1.7 (−3.1 to −0.3) | −6.8 (−8.1 to −5.4) | −0.5 (−2.4 to 1.4) | −9.0 (−12.8 to −5.0) | 2.3 (−0.3 to 5.0) |
| USB female | −2.8 (−9.1 to 4.0) | Could not be estimated | −1.9 (−8.1 to 4.7) | Could not be estimated | −3.3 (−8.9 to 2.7) | Could not be estimated | 1.9 (−6.2 to 10.6) |
| FB female | −0.4 (−1.7 to 1.0) | Could not be estimated | 0 (−1.4 to 1.4) | −8.3 (−10.8 to −5.7) | 0.5 (−1.4 to 2.5) | Could not be estimated | 3.9 (0.7 to 7.1) |
Abbreviations: FB, foreign-born; USB, U.S. born.
Discussion
Our analysis of 8,167 lung cancers diagnosed among Chinese Americans in California found divergence in trends by sex and histologic subtype. For total lung cancers among California Chinese males, we observed declining trends among foreign-born but a suggestion of increasing trends among U.S.-born, while among California Chinese females, we found stable rates among foreign-born but a suggestion of decreasing trends among U.S.-born.
Among foreign-born males, the significant declines for most histologies indicate the success of lowered tobacco use and consequently the abatement of tobacco-associated lung cancers. However, among U.S.-born males, nonsignificantly increasing trends for adenocarcinoma and NSCLC NOS subtypes is a concern and warrant further study, particularly as tobacco intake has been documented to decrease with acculturation among Chinese males (6, 29). The significant decline in squamous cell lung cancer among foreign-born females correlates with the temporal decline in current smoking according to the California Health Interview Survey data from 5.6% current smokers in 2001 to 1% in 2010–2011 (29). Using national SEER data spanning over a 21-year period from 1990 to 2010, we recently documented an increase in the rate of lung adenocarcinoma from 1996 to 2010 (1.3% per year) among Chinese males and stable trends among Chinese females, in contrast to statistically significant declining trends of other lung cancer histologic subtypes (4); however, the increase in the rate of adenocarcinoma among males may be due to a concomitant decrease in the rate of unspecified histologic subtypes (30).
Among U.S.-born Chinese females, we found a nonsignificantly declining trend for adenocarcinoma, although the trend was unstable due to small numbers of U.S.-born cases, and a flat trend among foreign-born females. Proportionally more lung cancers were adenocarcinomas (nearly 60%) among Chinese females than males, and among U.S.-born than foreign-born females, while the reverse was true among males. Of the histologies described here, adenocarcinoma is least closely associated with tobacco intake (5); thus, this pattern suggests that risk factors other than smoking may be responsible for the observed lung cancer incidence trends and the majority of lung cancers among Chinese females. Epplein and colleagues found that Chinese American women had a 4-fold increase in risk of mainly adenocarcinoma and large cell carcinoma compared with that expected based on rates among non-Hispanic whites, after accounting for smoking status (31). Based primarily on data from Asia and some from Hawaii (32), the epidemiology of lung cancer appears to be distinctly unique among Asian females, with proportionally higher incidence of adenocarcinoma, and more than half of lung cancers occurring among never smokers. Some authors have reported a higher proportion of EGFR-TK mutations. In addition to the well-established association with second-hand tobacco smoke, studies in east Asia have also identified several other risk factors for lung cancer among female never smokers, including exposure to indoor cooking oil fumes especially in the presence of poor ventilation, prior lung infections, air pollution, dietary quality and reproductive history, and interaction with genetic factors, including genes in the acetylation and inflammation pathway (33–37). However, as the prevalence of some of these exposures, including indoor and outdoor air pollution, is considerably less common among Asian Americans in the United States, it is unknown the extent to which these risk factors together explain the lung cancer incidence patterns among Chinese American female never smokers. Without a clear understanding of the etiologic factors underlying lung cancer incidence among Chinese Americans males and females, we are at a loss for designing the appropriate intervention strategies to reduce their high burden of disease. In addition, a contemporary, focused study of potential risk factors in such a population may provide valuable insights into the understanding of lung cancer etiology among never smokers, which is likely a distinct disease entity from lung cancer among smokers (16).
The findings may be limited by misclassification of cancer registry data on race/ethnicity, which are primarily derived from medical records (38, 39); however, prior research shows good classification accuracy for Chinese Americans (40, 41). Incidence rates may also be underestimated due to exclusion of cases coded by cancer registrars as “Asian, not otherwise specified” from the numerator. Some of the incidence rates are unstable due to small numbers, especially among U.S.-born. The Chinese American population is very heterogeneous; however, the available data do not allow us to assess incidence by country of origin or length of residence in the United States. Because of the lack of population enumeration by birthplace from the 2010 Census, we were not able to derive population estimates beyond 2004. Our rates may also be affected by errors associated with the intercensal and especially postcensal annual population estimates (42). Finally, our results may not be generalizable to Chinese American populations outside of California.
Despite these potential limitations, this report based on data from the CCR covering more than one third of the U.S. Chinese American population provides the first-ever data on histology-specific lung cancer trends by immigration status, serving as a critical evidence base to inform screening, research, and public health priorities in this growing population. With the recent U.S. Preventive Task Force guidelines on low-dose CT lung cancer screening for heavy smokers, attention should now be given to encouraging screening among Chinese Americans, particularly given that this group has traditionally been among the slowest to adopt cancer screening recommendations (43). Given the low smoking prevalence among Chinese Americans, especially females, and few known lung cancer risk factors in U.S. never-smoker populations, additional research of etiologic genetic or biologic factors inherent to these populations may elucidate knowledge regarding lung cancer diagnosed in never smokers.
Supplementary Material
Acknowledgments
Grant Support
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. This study was supported by a grant from Genentech (awarded to S. Gomez) and with funds from the Stanford Cancer Institute (awarded to S. Glaser). Genentech, which develops products that target cancer entities described in this article, funded this study. The terms of this arrangement have been reviewed and approved by the Cancer Prevention Institute of California in accordance with its policy regarding objectivity in research.
S.L. Gomez reports receiving commercial research grant from Genentech. M. McCusker is Senior Real World Data Scientist at Genentech, Inc. and has ownership interest (including patents) in Roche. C.A. Clarke reports receiving commercial research grant from Genentech.
Footnotes
Authors’ Contributions
Conception and design: S.L. Gomez, M. McCusker, H.A. Wakelee, C.A. Clarke
Development of methodology: S.L. Gomez, M. McCusker, C.A. Clarke
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): S.L. Gomez, C.A. Clarke
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): S.L. Gomez, J. Yang, I. Cheng, H.A. Wakelee, M. Patel, C.A. Clarke
Writing, review, and/or revision of the manuscript: S.L. Gomez, J. Yang, S.-W. Lin, M. McCusker, A. Sandler, I. Cheng, H.A. Wakelee, M. Patel, C.A. Clarke
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S.L. Gomez, C.A. Clarke
Study supervision: S.L. Gomez, C.A. Clarke
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
Disclaimer
The ideas and opinions expressed in this article are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
References
- 1.U.S. Census Bureau. 2010 Census shows Asians are fastest-growing race group. 2012 Mar 21; [cited 2014 Sep 24]; Available from: https://www.census.gov/newsroom/releases/archives/2010_census/cb12-cn22.html.
- 2.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–56. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. J Natl Cancer Inst. 2013;105:1096–110. doi: 10.1093/jnci/djt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng I, Le GM, Noone AM, Gali K, Patel M, Haile RW, et al. Lung cancer incidence trends by histology type among Asian American, Native Hawaiian, and Pacific Islander populations in the United States, 1990–2010. Cancer Epidemiol Biomarkers Prev. 2014;23:2250–65. doi: 10.1158/1055-9965.EPI-14-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesch B, Kendzia B, Gustavsson P, Jockel KH, Johnen G, Pohlabeln H, et al. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int J Cancer. 2012;131:1210–9. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S, Rankin S, Stewart A, Oka R. Effects of acculturation on smoking behavior in Asian Americans: a meta-analysis. J Cardiovasc Nurs. 2008;23:67–73. doi: 10.1097/01.JCN.0000305057.96247.f2. [DOI] [PubMed] [Google Scholar]
- 7.Brennan P, Buffler PA, Reynolds P, Wu AH, Wichmann HE, Agudo A, et al. Secondhand smoke exposure in adulthood and risk of lung cancer among never smokers: a pooled analysis of two large studies. Int J Cancer. 2004;109:125–31. doi: 10.1002/ijc.11682. [DOI] [PubMed] [Google Scholar]
- 8.Fontham ET, Correa P, WuWilliams A, Reynolds P, Greenberg RS, Buffler PA, et al. Lung cancer in nonsmoking women: a multicenter case–control study. Cancer Epidemiol Biomarkers Prev. 1991;1:35–43. [PubMed] [Google Scholar]
- 9.Wu AH, Fontham ET, Reynolds P, Greenberg RS, Buffler P, Liff J, et al. Family history of cancer and risk of lung cancer among lifetime nonsmoking women in the United States. Am J Epidemiol. 1996;143:535–42. doi: 10.1093/oxfordjournals.aje.a008783. [DOI] [PubMed] [Google Scholar]
- 10.Mayne ST, Buenconsejo J, Janerich DT. Previous lung disease and risk of lung cancer among men and women nonsmokers. Am J Epidemiol. 1999;149:13–20. doi: 10.1093/oxfordjournals.aje.a009722. [DOI] [PubMed] [Google Scholar]
- 11.Luo RX, Wu B, Yi YN, Huang ZW, Lin RT. Indoor burning coal air pollution and lung cancer–a case–control study in Fuzhou, China. Lung Cancer. 1996;14:S113–9. doi: 10.1016/s0169-5002(96)90217-2. [DOI] [PubMed] [Google Scholar]
- 12.MacLennan R, Da Costa J, Day NE, Law CH, Ng YK, Shanmugaratnam K. Risk factors for lung cancer in Singapore Chinese, a population with high female incidence rates. Int J Cancer. 1977;20:854–60. doi: 10.1002/ijc.2910200606. [DOI] [PubMed] [Google Scholar]
- 13.Osann KE. Lung cancer in women: the importance of smoking, family history of cancer, and medical history of respiratory disease. Cancer Res. 1991;51:4893–7. [PubMed] [Google Scholar]
- 14.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–45. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seow A, Poh WT, Teh M, Eng P, Wang YT, Tan WC, et al. Fumes from meat cooking and lung cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2000;9:1215–21. [PubMed] [Google Scholar]
- 16.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 17.Vineis P, Hoek G, Krzyzanowski M, Vigna-Taglianti F, Veglia F, Airoldi L, et al. Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health. 2007;6:7. doi: 10.1186/1476-069X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu ZY, Blot WJ, Xiao HP, Wu A, Feng YP, Stone BJ, et al. Smoking, air pollution, and the high rates of lung cancer in Shenyang, China. J Natl Cancer Inst. 1989;81:1800–6. doi: 10.1093/jnci/81.23.1800. [DOI] [PubMed] [Google Scholar]
- 19.Raz DJ, Gomez SL, Chang ET, Kim JY, Keegan TH, Pham J, et al. Epidemiology of non-small cell lung cancer in Asian Americans: incidence patterns among six subgroups by nativity. J Thorac Oncol. 2008;3:1391–7. doi: 10.1097/JTO.0b013e31818ddff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NAACCR Asian/Pacific Islander Work Group. NAACCR Asian Pacific Islander identification algorithm [NAPIIA v1.2] Springfield, IL: Jul, 2008. http://www.naaccr.org/filesystem/pdf/NAPIIA_v1_2_08312009.pdf. Accessed 2010 Feb 19. [Google Scholar]
- 21.Gomez SL, Glaser SL, Kelsey JL, Lee MM. Bias in completeness of birthplace data for Asian groups in a population-based cancer registry (United States) Cancer Causes Control. 2004;15:243–53. doi: 10.1023/B:CACO.0000024244.91775.64. [DOI] [PubMed] [Google Scholar]
- 22.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan THM, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. American Journal of Public Health. 2010;100:861–9. doi: 10.2105/AJPH.2009.176651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Schupp CW, Harrati A, Clarke C, Keegan TH, Gomez SL. Developing an area-based socioeconomic measure from American Community Survey data. 2014 Contract No.: May 7, 2014. [Google Scholar]
- 24.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 25.Gomez SL, Glaser SL, McClure LA, Shema SJ, Kealey M, Keegan TH, et al. The California neighborhoods data system: a new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control. 2011;22:631–47. doi: 10.1007/s10552-011-9736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shryock H, Siegel J. The methods and materials of demography. Washington, DC: United States Bureau of the Census; 1973. [Google Scholar]
- 27.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–4. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. (correction: 2001;20:655) [DOI] [PubMed] [Google Scholar]
- 29.AskCHIS. data chis.ucla.edu [database on the Internet] 2009 Accessed 2012 Feb. [Google Scholar]
- 30.Yu M, Feuer EJ, Cronin KA, Caporaso NE. Use of multiple imputation to correct for bias in lung cancer incidence trends by histologic subtype. Cancer Epidemiol Biomarkers Prev. 2014;23:1546–58. doi: 10.1158/1055-9965.EPI-14-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epplein M, Schwartz SM, Potter JD, Weiss NS. Smoking-adjusted lung cancer incidence among Asian-Americans (United States) Cancer Causes Control. 2005;16:1085–90. doi: 10.1007/s10552-005-0330-6. [DOI] [PubMed] [Google Scholar]
- 32.Le Marchand L, Wilkens LR, Kolonel LN. Ethnic differences in the lung cancer risk associated with smoking. Cancer Epidemiol Biomarkers Prev. 1992;1:103–7. [PubMed] [Google Scholar]
- 33.Lim WY, Chen Y, Ali SM, Chuah KL, Eng P, Leong SS, et al. Polymorphisms in inflammatory pathway genes, host factors and lung cancer risk in Chinese female never-smokers. Carcinogenesis. 2011;32:522–9. doi: 10.1093/carcin/bgr006. [DOI] [PubMed] [Google Scholar]
- 34.Lim WY, Chen Y, Chuah KL, Eng P, Leong SS, Lim E, et al. Female reproductive factors, gene polymorphisms in the estrogen metabolism pathway, and risk of lung cancer in Chinese women. Am J Epidemiol. 2012;175:492–503. doi: 10.1093/aje/kwr332. [DOI] [PubMed] [Google Scholar]
- 35.Lim WY, Chuah KL, Eng P, Leong SS, Lim E, Lim TK, et al. Meat consumption and risk of lung cancer among never-smoking women. Nutr Cancer. 2011;63:850–9. doi: 10.1080/01635581.2011.589961. [DOI] [PubMed] [Google Scholar]
- 36.Seow A, Zhao B, Lee EJ, Poh WT, Teh M, Eng P, et al. Cytochrome P4501A2 (CYP1A2) activity and lung cancer risk: a preliminary study among Chinese women in Singapore. Carcinogenesis. 2001;22:673–7. doi: 10.1093/carcin/22.4.673. [DOI] [PubMed] [Google Scholar]
- 37.Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, et al. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10:1063–7. [PubMed] [Google Scholar]
- 38.Gomez SL, Le GM, West DW, Satariano WA, O’Connor L. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. Am J Public Health. 2003;93:1685–8. doi: 10.2105/ajph.93.10.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez SL, Lichtensztajn DY, Parikh P, Hasnain-Wynia R, Ponce N, Zingmond D. Hospital practices in the collection of patient race, ethnicity, and language data: a statewide survey, California, 2011. J Health Care Poor Underserved. 2014;25:1384–96. doi: 10.1353/hpu.2014.0126. [DOI] [PubMed] [Google Scholar]
- 40.Clegg L, Reichman M, Hankey B, Miller B, Lin Y, Johnson N, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–87. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 41.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry. Cancer Causes Control. 2006;17:771–81. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 42.Boscoe FP, Miller BA. Population estimation error and its impact on 1991– 1999 cancer rates. Professional Geographer. 2004;54:516–29. [Google Scholar]
- 43.Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


