Abstract
High-density lipoprotein (HDL) proteomic study has identified substantial changes associated with various disease states. In the current study, the HDL proteomes in patients with cerebral lacunar infarction (LACI) and control subjects were investigated. A total of 12 LACI patients without evident large vessel occlusions and 12 controls were enrolled in the study. The HDL fraction from each sample was isolated from the plasma by ultracentrifugation. The protemics of the HDL were investigated using nano liquid chromatography coupled to tandem mass spectrometry. There were 55 proteins identified as differentially expressed in the LACI and control groups. Among the 55 proteins, 33 were upregulated and 22 were downregulated in the patients with LACI. The identified proteins were associated with numerous molecular functions, including lipid and cholesterol transport, lipid metabolism, inflammatory response, the complement and coagulation pathway, metal ion metabolism, hemostasis and endopeptidase inhibitory activity. Serum amyloid A, apolipoprotein C (apoC-III) and apolipoprotein A-II (apoA-II) were selected to confirm the proteomics results via western blotting. HDL from the LACI patients exhibited an impaired ability to inhibit the binding of THP-1 cells to endothelial cells compared with the controls (P<0.01). ApoC-III-rich HDL also had a significantly reduced ability to inhibit the binding of THP-1 cells to endothelial cells (P<0.01). The expression of vascular cell adhesion molecule-1 protein by the endothelial cells exhibited a similar pattern of response to the different HDL samples. In conclusion, the present study demonstrates major modifications of the HDL proteome in patients with LACI. The ApoC-III enrichment of the HDL of patients with LACI may cause a reduction in the anti-inflammatory ability of HDL, which may contribute to the progression of the disease.
Keywords: high density lipoprotein, lacunar infarction, apolipoprotein C-III, anti-inflammatory
Introduction
High-density lipoprotein (HDL) particles are particles with a density of 1.063–1.210 g/ml that are composed of proteins and lipids (1). Compared with other lipoproteins, HDL contains a high level of protein. The protein/lipid ratio differs among HDL subpopulations. In the large and light HDL subfraction HDL2, the ratio is 1:2, and in small dense pre-HDL it is 10:1 (2). Apolipoprotein A-I (apoA-I) is the most abundant protein component (3) in HDL particles. ApoA-I constitutes ~70% of the protein content of HDL, followed by apoA-II, which constitutes 15–20% (4). The remaining proteins include apolipoprotein C (apoC-I, apoC-II and apoC-III), apolipoprotein E (apoE), apolipoprotein D (apoD), apolipoprotein M (apoM), apolipoprotein A-IV (apoA-IV) and other proteins that are involved in lipid metabolism, including lecithin:cholesterol acyl transferase and cholesteryl ester transfer protein. Proteomic studies (5–8) have identified ≥75 different proteins that are contained in HDL obtained by ultracentrifugation.
Regarding disease states, HDL proteomic studies have observed substantial changes in HDL in individuals with cardiovascular disease (8–11), diabetes mellitus (DM) (12), chronic kidney disease (13–15) and rheumatoid arthritis (16) in comparison with healthy individuals, while the plasma HDL-cholesterol (HDL-C) did not change markedly (8,9). Disorders such as atherosclerosis and type 2 DM cause a prominent chronic inflammatory state affecting endothelial cells, which induces a proteomic change of HDL with the subsequent impairment of their antiatherogenic, antioxidant and anti-inflammatory functions (17). HDL subpopulations are associated with stroke subtype; a previous study identified that smaller HDL-C particles were associated with a reduced risk of lacunar infarction (LACI) (18).
The biological mechanism underlying the beneficial role of small HDL particles in LACI is not well understood. It is considered that the anti-inflammatory effects of smaller HDL particles (19,20) inhibit angionecrosis or microatheroma formation in cerebral vessels, and thereby reduce the risk of LACI (21,22). Proteomics may be helpful in identifying the molecules associated with HDL that intervene in their inverse association with cerebrovascular disease, as the quantitative measurement of HDL-C level does not explain this satisfactorily (23). Proteomic analysis may drive a shift in the classical classification of HDL subfractions, from the previous physicochemical model towards a novel pattern based on their physiological function and pathophysiological roles (24,25). In the present study, the HDL proteomes in patients with LACI and controls were investigated.
Materials and methods
Ethics statement
The study was approved by the Ethics Committee of Peking University First Hospital (Beijing, China). Written informed consent was provided by all patients and controls.
Subjects and biochemical analysis
The study included 12 patients with LACI (7 males and 5 females, aged 45–69 years old) without any evident large vessel occlusions and 12 control subjects (7 males and 5 females, aged 48–62 years old). The patients and controls were recruited in the Neurology Ward of Peking University First Hospital from January 1, 2015 to March 31, 2015. Exclusion criteria were having infectious, inflammatory or autoimmune disorders, advanced kidney or liver failure, neoplastic disease, and a history of major surgery or trauma within the previous month. Patients with LACI were defined as having lacunar syndrome and signs, and brain neuroimaging evidence of an infarct of size ≤1.5 cm at a typical location (26,27). The patient and control groups were equivalent with regard to sex proportion and age range. Blood samples were drawn into EDTA-coated tubes following an 8-h fast. Fasting blood glucose and creatinine were determined using a Beckman CX5 Automated Analyzer. DM was defined either as records of fasting blood glucose >7.0 mmol/l, post-prandial blood glucose >11.1 mmol/l or used to on anti-diabetic treatments. Hyperlipidemia was defined as abnormally elevated levels of any or all lipids or lipoproteins in the blood and needed treatment with lipid-lowering therapy. The plasma was obtained by centrifugation at 200 × g for 10 min at 15°C, and was reserved at −80°C. All plasma samples underwent 2 freeze/thaw cycles. Measurements of the total cholesterol, HDL-C and triglycerides in the plasma were conducted by biochemical assays using a Beckman CX5 Automated Analyzer (Beckman Coulter, Inc., Brea, CA, USA). The quantification of apoA-II, serum amyloid A (SAA) and apoC-III in the individual HDL samples was conducted by an immunoblotting assay. A sample containing 10 µg total proteins was separated by 10% SDS-PAGE and blotted onto a nitrocellulose membrane. Anti-apoA-II (ab109897), anti-SAA (ab190802) and anti-apoC-III (ab76305) anti-bodies (all from Abcam, Cambridge, UK) were used as the primary antibodies. The primary antibodies were incubated at 4°C overnight. Horseradish peroxidase-conjugated goat anti-rabbit monoclonal antibody (cat. no. sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1:1,000) was used as the secondary antibody. The secondary antibody was incubated at 25°C for 1 h. Antibody binding was detected using a Super Signal West Pico kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol.
HDL isolation from plasma
HDL particles (density, 1.063–1.21 g/ml) in the plasma were separated by sequential density ultracentrifugation using potassium bromide (KBr) as described previously (28). Briefly, the plasma density was adjusted to 1.3 g/ml with KBr, and normal saline (1.006 g/ml) was layered over the adjusted plasma to form a discontinuous NaCl/KBr density gradient. The tubes loaded with sample and gradient were placed in the P40ST rotor of an ultracentrifuge (model CP70MX; Hitachi, Ltd., Tokyo, Japan) and were centrifuged at 350,000 × g for 3.5 h at 4°C. The HDL layer was collected. The protein concentration was measured in triplicate using a Micro BCA kit (Pierce; Thermo Fisher Scientific, Inc.). The purity of the HDL was evaluated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis using goat anti-apoA-I polyclonal antibody (ab64308; Abcam) and quantified through the measurement of apoA-I content by nephelometry (Dimension XPand; Siemens Healthineers, Erlangen, Germany).
Mass spectrometry (MS)
The specific gel band was excised and destained with 25 mM NH4HCO3 in 50% acetonitrile. Proteins were reduced by 10 mM dithiothreitol at 56°C for 30 min and alkylated using 50 mM iodoacetamide at 25°C for 30 min. After drying in 100% acetonitrile, the gel band was digested using sequencing grade trypsin (Promega Corporation, Madison, WI, USA) at 37°C overnight. The extracted peptides were suspended in 0.1% formic acid and subjected to nano liquid chromatography-MS/MS analysis. Peptides were eluted with a linear gradient from 5 to 40% of 100% acetonitrile and 0.1% formic acid at a flow rate of 300 nl/min using a self-made 100 µm × 10 cm reversed-phase C18 fused silica emitter. The data-dependent mass spectra were acquired with an LTQ Orbitrap Elite mass spectrometer equipped with a nano-electrospray ion source (Thermo Fisher Scientific, Inc.). Raw mass spectra files were processed with Proteome Discoverer 1.4 (Thermo Fisher Scientific, Inc.) and searched using the SEQUEST search engine (29) against the human uniprot database (version 2014_02; http://www.uniprot.org/). The precursor ion mass tolerance was set to 10 ppm, and the MS/MS tolerance was 0.02 Da. Relative quantification was based on the ratio of the areas under the reporter peaks. Protein-protein interaction networks of differentially expressed proteins were constructed using STRING 9.1 (http://string-db.org/). The name of each individual protein was given as a query to the STRING database and the corresponding PPI information was retrieved by enabling different prediction methods. The networks were made with a confidence cutoff of 0.4.
THP-1 cell adhesion assay and determination of adhesion molecules by western blot analysis
The method used was as described previously (30). THP-1 monocytic cells were labeled with 3 µg/ml 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester at 37°C for 30 min. The labeled cells were washed three times with phosphate-buffered saline (PBS), and were then resuspended in RPMI-1640 (R1640; Gibco, Paisley, UK) containing 0.1% bovine serum albumin (Gibco). The cell suspensions were overlaid (1.5×106 cells/ml, 500 µl/well) on confluent monolayers of human umbilical vein endothelial cells (HUVECs; CRL-1730; ATCC, Manassas, VA, USA) that had been grown in 12-well plates and treated with various types of HCL: HDL from the control group (HDLn), HDL from LACI patients (HDLLI) and HDL with an elevated level of apoC-III [HDL/apoC-III; obtained after incubation of HDL with apoC-III (100 µg/ml; TP306566; Origene, Beijing, China) for 2 h at 37°C]. PBS treatment was used as a negative control. Following incubation for 15 min at 37°C, nonadherent THP-1 monocytic cells were removed by washing five times with prewarmed RPMI-1640 containing 0.1% bovine serum albumin. The number of THP-1 monocytic cells on the HUVECs was counted in four views using fluorescence microscopy at ×100 magnification to determine the number of adhering cells.
Western blot analysis was conducted to investigate the expression of vascular cell adhesion molecule-1 (VCAM-1) by the endothelial cells. The cells were washed twice with cold PBS, and the cytoplasmic proteins were collected using Nucleoprotein Extraction kit (BSP009; Shenggong, Shanghai, China) following the manuals. The proteins were quantified using Protein Quantitative kit (DQ111-01; Transgen Biotech, Beijing, China) following the manuals. A sample containing 30 µg cytoplasmic proteins was separated by 10 or 15% SDS-PAGE and blotted onto a nitrocellulose membrane. Rabbit monoclonal antibody to VCAM-1 (ab134047; 1:1,000; Abcam) was used as the primary antibody and was incubated at 4°C overnight. Horseradish peroxidase-conjugated goat anti-rabbit monoclonal antibody (1:1,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) was used as the secondary antibody and was incubated at 25°C for 1 h. Antibody binding was detected using a Super Signal West Pico kit (Pierce; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation or number (%). Continuous data were analyzed using t-tests, while discrete data were analyzed using Chi-square tests. One-way ANOVA method was used for comparison of multiple groups. P<0.05 was considered to indicate a statistically significant difference. The SPSS 16.0 software package (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Gene Ontology (GO) analysis was conducted using DAVID bioinformatics resources (http://david.abcc.ncifcrf.gov) as described previously (31,32).
Results
Characteristics of the study population
The characteristics of the patients and controls are summarized in Table I. No difference in the proportions of sex, DM and hyperlipidemia was observed between the two groups. In addition, there was no difference in the plasma total cholesterol, triglyceride, HDL-C, fasting blood glucose and serum creatinine levels between the LACI and control subjects.
Table I.
Clinical characteristics of study subjects.
| Features | LACI patients (n=12) |
control (n=12) |
|---|---|---|
| Age (years) | 57±6 | 55±4 |
| Male, n (%) | 7 (58.33) | 7 (58.33) |
| DM, n (%) | 3 (25) | 3 (25) |
| Hyperlipidemia, n (%) | 6 (50) | 4 (33.33) |
| Total cholesterol (mg/dl) | 4.16±1.09 | 4.30±0.63 |
| Triglyceride (mg/dl) | 1.65±0.69 | 1.70±0.55 |
| HDL cholesterol (mmol/l) | 1.04±0.24 | 1.05±0.37 |
| Fasting blood glucose (mg/dl) | 5.59±1.41 | 6.08±1.71 |
| Creatinine (mg/dl) | 93.90±14.32 | 90.02±15.17 |
Data expressed as mean ± standard deviation or number (%). LACI, lacunar infarction; DM, diabetes mellitus; HDL, high-density lipoprotein.
HDL proteomics
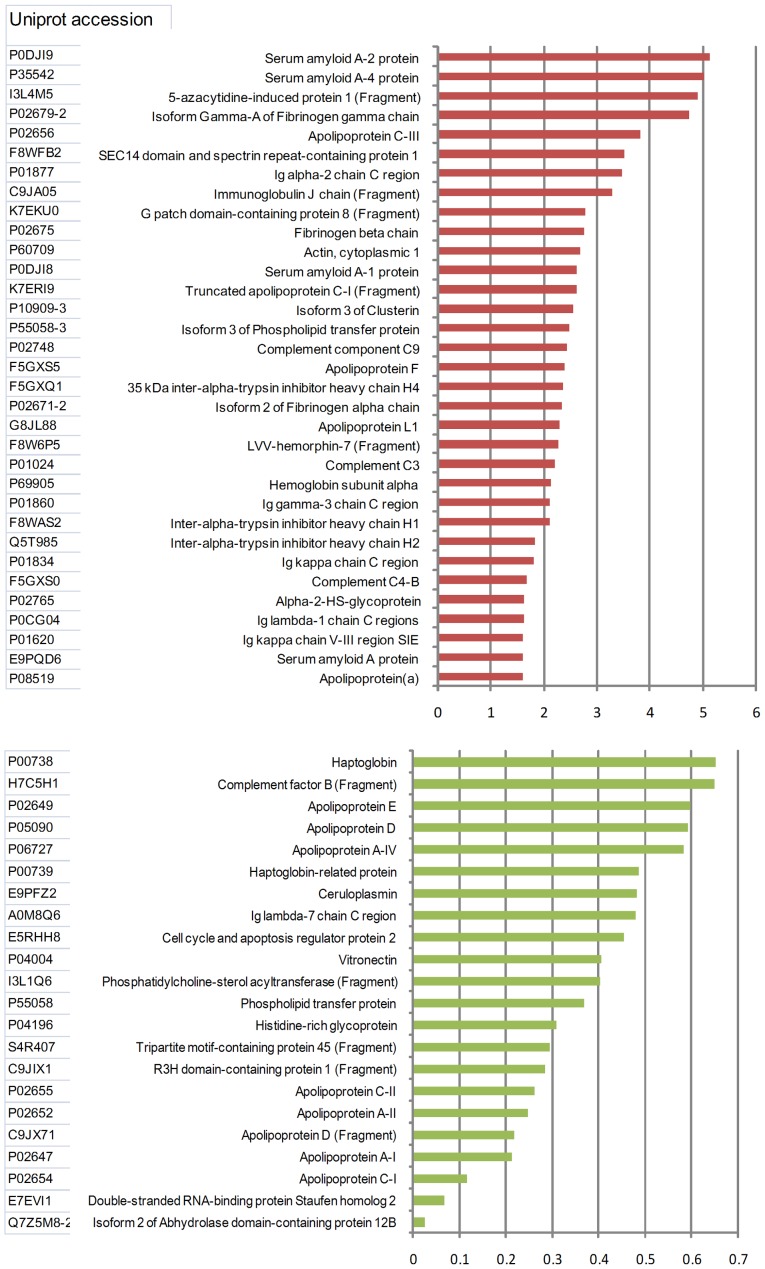
Proteins that had a differential expression of ≥1.5-fold or ≤0.67-fold relative in the LACI samples compared with the control samples were considered as differentially expressed. In total, 55 proteins were identified to be differentially expressed in the LACI and control groups (Fig. 1). Among these 55 proteins, 33 were upregulated and 22 were downregulated in the patients with LACI compared with the control subjects. The level of LACI's haptoglobin was less compared to 1/5 of the control subjects.
Figure 1.
Relative abundance of proteins identified by MS from the HDL of patients with LACI and healthy controls. Data are from 12 subjects with LACI and 12 controls. The relative abundance of the HDL-associated proteins was assessed as described in Materials and methods. MS, mass spectroscopy; HDL, high-density lipoprotein; LACI, lacunar infarction.
The GO classification system was used to classify the identified proteins into different clusters according to biological processes and molecular functions. Fig. 2 shows the GO categories of the identified proteins. The differentially identified proteins were associated with numerous molecular functions, including lipid and cholesterol metabolism, inflammatory response, the complement and coagulation pathway, hemostasis, metal ion metabolism and endopeptidase inhibitory activity. Proteins associated with lipid/cholesterol transport and metabolism exhibited the most significant changes.
Figure 2.
Gene Ontology category enrichments for the differentially expressed proteins in the high-density lipoprotein fraction. (A) Biological process and (B) molecular function of the differentially expressed proteins.
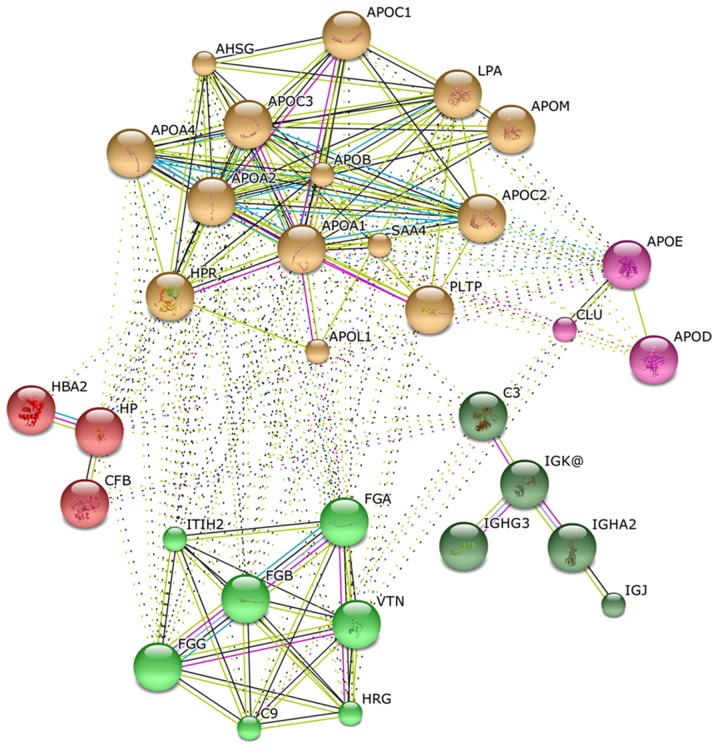
Fig. 3 shows an organic network that graphically depicts statistically significant correlations between identified proteins (nodes) as connecting lines (edges). Long/thin lines indicate weak correlations. Short/thick lines indicate strong correlations; tightly correlated proteins appear in the same color in the network. The differentially expressed proteins formed five different functional clusters, indicating an involvement in lipid metabolism, hemostasis, metal binding, hemoglobin metabolism and inflammatory response.
Figure 3.
Functional interaction network based on the String database and Gene Ontology classification. The green nodes correspond to proteins involved in hemostasis. The purple nodes correspond to proteins involved in metal binding. The yellow nodes correspond to proteins involved in lipid metabolism. The red nodes correspond to proteins involved in hemoglobin metabolism. The dark green nodes correspond to proteins involved in inflammatory response.
Biochemical confirmation of the differentially expressed proteins
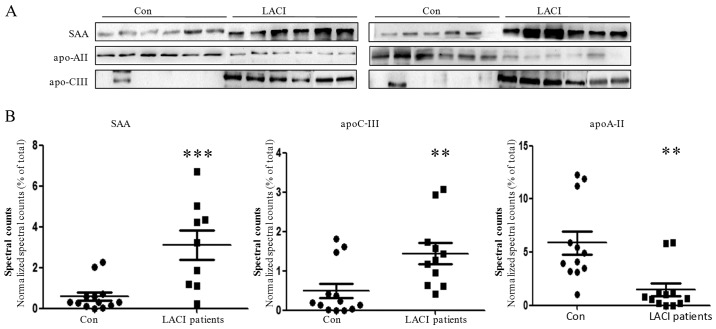
The expression levels of three proteins identified by MS analysis in the majority of the 12 LACI patients were evaluated by western blot assay to validate the difference observed. SAA (P<0.001) and apoC-III (P= 0.007) were significantly upregulated whereas apoA-II (P=0.002) was significantly downregulated in the LACI patient group compared with the controls in the proteomic analysis. The results of the western blot analysis were in agreement with the MS findings, with the majority of the patients having a decreased level of apoA-II and increased levels of apoC-III and SAA (Fig. 4).
Figure 4.
Validation of apoA-II, SAA and apoC-III levels in high-density lipoprotein isolated from control and LACI subjects by western blot analysis. (A) apoA-II, apoC-III and SAA were quantified by western blot analysis. (B) Mass spectometry results of apoA-II, apoC-III and SAA between the patients and controls. **P<0.01 and ***P<0.001 vs. the control by a two-tailed Student's t-test. apoA-II, apolipoprotein A-II; SAA, serum amyloid A; apoC-III, apolipoprotein C-III; LACI, lacunar infarction.
HDL of LACI patients has an increased apoC-III content that impairs its anti-inflammatory function
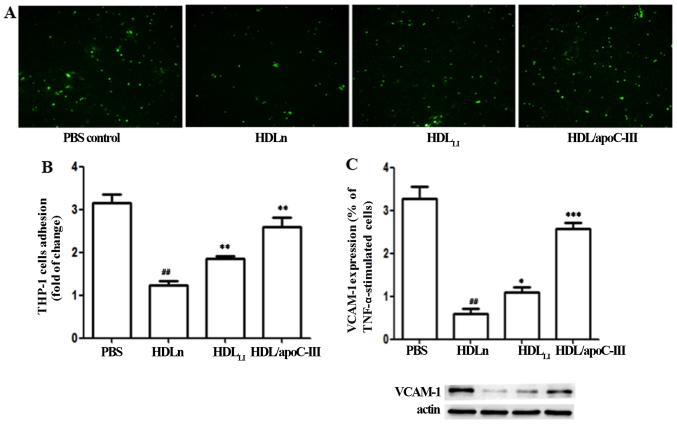
The treatment of HUVECs with HDLn reduced the binding of THP-1 cells to the HUVECs compared with the PBS-treated controls (Fig. 5). However, HDLLI had an impaired ability to inhibit the binding of THP-1 cells to HUVECs compared with HDLn (P<0.01). HDL/apoC-III also exhibited a significantly reduced ability to inhibit the binding of THP-1 cells to HUVECs compared with HDLn (P<0.01). The expression of VCAM-1 protein exhibited a response to the HDL treatments that paralleled the response of the binding ability. Treatment with HDL/apoC-III induced a significantly higher expression of VCAM-1 compared with HDLn (P<0.001). Treatment with HDLLI induced a significantly higher expression of VCAM-1 compared with HDLn (P<0.05).
Figure 5.
HDLLI and HDL/apoC-III have impaired ability to inhibit THP-1 cell adhesion. (A) THP-1 cell adhesion to endothelial cells (cells labeled with 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester). (B) Quantification of the THP-1 cell adhesion and (C) VCAM-1 expression of endothelial cells. *P<0.05, **P<0.01 and ***P<0.01 vs. the PBS control ##P<0.01 vs. the PBS control. HDLn, HDL from the control group; HDLLI, HDL from patients with lacunar infarction; HDL/apoC-III, HDL with an elevated content of apolipoproten C-III; VCAM-1, vascular cell adhesion molecule-1.
Discussion
There are ~50 proteins associated with the HDL fraction that have been identified by previous studies using MS methods (5,7,8,13,25,33). In the present study, 55 differentially expressed proteins were identified, which included the majority of the previously identified proteins (19,25–28,30,31). The proteins identified as being associated with HDL are involved in numerous functions, including lipid metabolism, inflammatory response, the complement and coagulation pathway, and endopeptidase inhibitory activity. Several apolipoproteins and enzymes associated with lipid metabolism were detected, including apoA-I, apoA-II, apoC-III, apoA-IV, apoC-I, apoC-II, apoD, apoJ, apoE, apoF, apoL-I, apoM, phospholipid transfer protein and phosphatidylcholine-sterol acyltransferase. Other proteins that are involved in the inflammation response and oxidative pathways were also identified, including serum amyloid A proteins and certain complement components.
There were 55 proteins that were identified to be differentially expressed between the patients and controls in the present study. Among these proteins, three proteins that were differentially expressed in the majority of the 12 LACI patients were further validated using a biochemical method. The results of western blot analysis validated the increase of apoC-III and SAA, as well as the reduction of apoA-II in the HDL fraction of LACI patients compared with the controls.
Another notable finding in the present study was that an increased level of apoC-III in the HDL of LACI patients was associated with an impaired anti-inflammatory function. ApoC-III is a small apolipoprotein that is synthesized mainly in the liver and circulates in the plasma in association with apoB-containing lipoproteins and HDL (35). The main physiological processes that apoC-III is involved in are inhibition of lipoprotein lipase and hepatic lipase, and inhibition of the hepatic uptake of triglyceride-rich particles (34). Furthermore, apoC-III activates β-integrin, protein kinase C β and downstream VCAM-1 in monocytes, which increases the adhesion of monocytes to vascular endothelial cells (35). In the present study, the HDL of LACI patients, with its upregulation of apoC-III, had a reduced ability to inhibit leukocyte binding to endothelial cells. Furthermore, ApoC-III-rich HDL did not inhibit monocyte adhesion to endothelial cells, while the HDL of control subjects decreased the adhesion, suggesting that apoC-III in HDL reduces the anti-inflammatory property of HDL. This phenomenon clearly implies that the apoC-III metabolism is changed in cerebrovacular disease.
In addition to a reverse cholesterol transport function, HDL has other vasculoprotective effects, including antioxidative and anti-inflammatory properties (4,36,37). For instance, HDL attenuates low-density lipoprotein (LDL) oxidation, a critical process in the onset and aggravation of atherosclerotic plaques (38). Metal ions, such as iron or copper, may promote lipid peroxidation in the process of LDL oxidation (39). The present study identified a number of proteins associated with metal ion metabolism. The level of haptoglobin was decreased in the HDL fraction of LACI patients compared with that of the control subjects. Haptoglobin is an acute phase protein. It exclusively binds to hemoglobin and releases it into the plasma during physiological and pathological hemolysis, thereby preventing iron- and heme-mediated oxidative side effects (40,41).
In conclusion, the present study revealed the proteomic changes of HDL in patients with LACI. There were 55 proteins that were identified to be quantitatively different between the patients with LACI and the control group, which were mainly associated with the processes of lipid metabolism, the inflammatory response, metal ion homeostasis, and the complement pathway. The expression levels of apoA-II, apoC-III and SAA were validated biochemically, and the results were consistent with the MS data. The ApoC-III enrichment of the HDL in patients with LACI may reduce the adhesion of THP-1 to endothelial cells, and thereby decrease the anti-inflammatory effect of HDL. Future studies with a larger number of subjects are required to determine whether the identified proteins are suitable and relevant risk biomarkers. Furthermore, the underlying mechanism of the apoC-III mediated HDL dysfunction requires elucidation.
References
- 1.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kontush A, Chapman MJ. Antiatherogenic small, dense HDL–guardian angel of the arterial wall. Nat Clin Pract Cardiovasc Med. 2006;3:144–153. doi: 10.1038/ncpcardio0500. [DOI] [PubMed] [Google Scholar]
- 3.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 4.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 5.Karlsson H, Leanderson P, Tagesson C, Lindahl M. Lipoproteomics II: Mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:1431–1445. doi: 10.1002/pmic.200401010. [DOI] [PubMed] [Google Scholar]
- 6.Heller M, Stalder D, Schlappritzi E, Hayn G, Matter U, Haeberli A. Mass spectrometry-based analytical tools for the molecular protein characterization of human plasma lipoproteins. Proteomics. 2005;5:2619–2630. doi: 10.1002/pmic.200401233. [DOI] [PubMed] [Google Scholar]
- 7.Rezaee F, Casetta B, Levels JH, Speijer D, Meijers JC. Proteomic analysis of high-density lipoprotein. Proteomics. 2006;6:721–730. doi: 10.1002/pmic.200500191. [DOI] [PubMed] [Google Scholar]
- 8.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan Y, Liu TR, Hu SW, Tian D, Li C, Zhong JK, Sun HG, Luo TT, Lai WY, Guo ZG. Acute coronary syndrome remodels the protein cargo and functions of high-density lipoprotein subfractions. PLoS One. 2014;9:e94264. doi: 10.1371/journal.pone.0094264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan LR, Wang DX, Liu H, Zhang XX, Zhao H, Hua L, Xu P, Li YS. A pro-atherogenic HDL profile in coronary heart disease patients: An iTRAQ labelling-based proteomic approach. PLoS One. 2014;9:e98368. doi: 10.1371/journal.pone.0098368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepedda AJ, Nieddu G, Zinellu E, De Muro P, Piredda F, Guarino A, Spirito R, Carta F, Turrini F, Formato M. Proteomic analysis of plasma-purified VLDL, LDL, and HDL fractions from atherosclerotic patients undergoing carotid endarterectomy: Identification of serum amyloid A as a potential marker. Oxid Med Cell Longev. 2013;385214 doi: 10.1155/2013/385214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ståhlman M, Fagerberg B, Adiels M, Ekroos K, Chapman JM, Kontush A, Borén J. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the HDL lipidome in type 2 diabetic subjects in the DIWA cohort: Impact on small HDL particles. Biochim Biophys Acta. 2013;1831:1609–1617. doi: 10.1016/j.bbalip.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22:1631–1641. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangé A, Goux A, Badiou S, Patrier L, Canaud B, Maudelonde T, Cristol JP, Solassol J. HDL proteome in hemodialysis patients: A quantitative nanoflow liquid chromatography-tandem mass spectrometry approach. PLoS One. 2012;7:e34107. doi: 10.1371/journal.pone.0034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopecky C, Genser B, Drechsler C, Krane V, Kaltenecker CC, Hengstschläger M, März W, Wanner C, Säemann MD, Weichhart T. Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin J Am Soc Nephrol. 2015;10:224–231. doi: 10.2215/CJN.06560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, Lee TD, Reddy ST. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: Association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012;64:1828–1837. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: Markers or mediators of cardiovascular disease. J Lipid Res. 2005;46:389–403. doi: 10.1194/jlr.R400017-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Chei CL, Yamagishi K, Kitamura A, Kiyama M, Imano H, Ohira T, Cui R, Tanigawa T, Sankai T, Ishikawa Y, et al. CIRCS Investigators: High-density lipoprotein subclasses and risk of stroke and its subtypes in Japanese population: The Circulatory Risk in Communities Study. Stroke. 2013;44:327–333. doi: 10.1161/STROKEAHA.112.674812. [DOI] [PubMed] [Google Scholar]
- 19.Ashby DT, Rye KA, Clay MA, Vadas MA, Gamble JR, Barter PJ. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1450–1455. doi: 10.1161/01.ATV.18.9.1450. [DOI] [PubMed] [Google Scholar]
- 20.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 21.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 22.Ogata J, Yamanishi H, Ishibashi-Ueda H. Review: Role of cerebral vessels in ischaemic injury of the brain. Neuropathol Appl Neurobiol. 2011;37:40–55. doi: 10.1111/j.1365-2990.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorci-Thomas MG, Thomas MJ. Why targeting HDL should work as a therapeutic tool, but has not. J Cardiovasc Pharmacol. 2013;62:239–246. doi: 10.1097/FJC.0b013e31829d48a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh JY, Chang CT, Huang MT, Chang CM, Chen CY, Shen MY, Liao HY, Wang GJ, Chen CH, Chen CJ, et al. Biochemical and functional characterization of charge-defined subfractions of high-density lipoprotein from normal adults. Anal Chem. 2013;85:11440–11448. doi: 10.1021/ac402516u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke. 2011;6:47–59. doi: 10.1111/j.1747-4949.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- 27.Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, Sugihara H, Morita Y, Hirose K, Okayama A, et al. Ischemic stroke subtypes in a Japanese population: Takashima Stroke Registry, 1988–2004. Stroke. 2010;41:1871–1876. doi: 10.1161/STROKEAHA.110.581033. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Ji L, Tong X, Pan B, Han JY, Huang Y, Chen YE, Pennathur S, Zhang Y, Zheng L. Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1. Am J Physiol Cell Physiol. 2011;301:C739–C748. doi: 10.1152/ajpcell.00055.2011. [DOI] [PubMed] [Google Scholar]
- 29.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 31.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9:5239–5249. doi: 10.1021/pr100520x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernelot Moens SJ, van Capelleveen JC, Stroes ESG. Inhibition of ApoCIII: The next CSK9? Curr Opin Lipidol. 2014;25:418–422. doi: 10.1097/MOL.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami A, Yoshida M. Apolipoprotein CIII links dyslipidemia with atherosclerosis. J Atheroscler Thromb. 2009;16:6–11. doi: 10.5551/jat.E607. [DOI] [PubMed] [Google Scholar]
- 36.Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: Beyond reverse cholesterol transport. Curr Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- 37.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007;18:427–434. doi: 10.1097/MOL.0b013e3282364a17. [DOI] [PubMed] [Google Scholar]
- 38.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann Bot (Lond) 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katoh N, Nakagawa H. Detection of haptoglobin in the high-density lipoprotein and the very high-density lipoprotein fractions from sera of calves with experimental pneumonia and cows with naturally occurring fatty liver. J Vet Med Sci. 1999;61:119–124. doi: 10.1292/jvms.61.119. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen MJ, Petersen SV, Jacobsen C, Oxvig C, Rees D, Møller HJ, Moestrup SK. Haptoglobin-related protein is a high-affinity hemoglobin-binding plasma protein. Blood. 2006;108:2846–2849. doi: 10.1182/blood-2006-05-022327. [DOI] [PubMed] [Google Scholar]