Abstract
Previous outbreaks suggest that pregnant women with Ebola virus disease (EVD) are at increased risk for severe disease and death. Healthcare workers who treat pregnant women with EVD are at increased risk of body fluid exposure. Despite the absence of pregnant women with EVD in the United States, CDC activated the Maternal Health Team (MHT), a functional unit dedicated to emergency preparedness and response issues, on October 18, 2014. We describe major activities of the MHT. A high-priority MHT activity was to publish guiding principles early in the response. The MHT also prepared guidance documents, provided guidance and technical support for hospital preparedness, and addressed inquiries. We analyzed maternal health inquiries received through CDC-INFO, MHT, and CDC’s Medical Investigations Team from August 2014 to December 2015. Internal call logs used to capture, monitor, and track inquiries for the three data sources were merged. Inquiries not related to maternal health issues and duplicates were removed. Each inquiry was categorized by route (email/phone), inquirer type, and topic. In total, 201 inquiries were received from clinicians, public health professionals, and the public. The predominant topic was related to infection control for high-risk situations such as labor and delivery. During the Ebola response, most inquiries were received via email rather than telephone, a notable shift compared to the H1N1 emergency response. Lessons learned during the H1N1 and Ebola responses are currently informing CDC’s Zika Response, an unprecedented emergency response primarily focused on reproductive health issues.
Keywords: emergency response, Ebola, maternal health, pregnant women
Purpose
The largest Ebola virus epidemic in history occurred in West Africa from March 2014 to December 2015.1,2 More than 28,000 cases were reported; of those, more than 11,000 died. The overwhelming majority of cases and deaths occurred in Guinea, Liberia, and Sierra Leone. An additional 36 travel-related cases were reported in 6 other countries, including the United States.1 This challenging global public health emergency led to the largest emergency response in CDC’s history and included participation of ~4000 CDC staff members in both international and domestic efforts.2
The first case of Ebola in the United States was diagnosed in September 2014. In total, four patients were diagnosed with Ebola virus disease (EVD) in the United States, including two healthcare workers who cared for the first U.S. patient. An additional seven patients with EVD were transported from West Africa to the United States for care and treatment.2 The introduction of Ebola into the United States and subsequent transmission to healthcare workers served as a warning to the U.S. public health, medical, and hospital systems. As a result, CDC embarked on a vigorous emergency preparedness agenda within the United States.3 While the overall U.S. response included preparation for all types of Ebola patients, we will focus on those related to maternal health.
There is limited evidence regarding EVD in pregnancy because historically, Ebola outbreaks have occurred in resource-limited areas where information about pregnancy has not been systematically collected.4 Previous outbreaks suggest that pregnant women with EVD are more likely than the general population to have severe disease and die. Compared to women without EVD, they may be at higher risk for spontaneous abortion and hemorrhage, and their infants are not likely to survive. In addition, healthcare workers who treat pregnant women with EVD are at an increased risk of body fluid exposure when attending to women during the labor and delivery process.
Despite the absence of pregnant women with EVD in the United States, the CDC’s Division of Reproductive Health (DRH) mobilized the Maternal Health Team (MHT) on October 18, 2014, as part of the agency’s emergency response to address maternal health issues in the United States and internationally. In this article, we focus on the domestic response. We describe major activities of the MHT, including a timeline of events, inquiries received pertaining to maternal health, and publication of guidance documents and articles. We believe that sharing this experience can inform the routine inclusion of an MHT as a key component of any future emergency responses.
Description
The CDC Emergency Operations Center (EOC) was activated on July 9, 2014, and the WHO declared Ebola a public health emergency on August 7, 2014.2 The EOC is the onsite command center for coordinating emergency responses to domestic and international public health threats.5 Initially, the EOC managed clinical inquiries until the volume of inquiries exceeded staffing capacity and there was a need for special expertise, particularly as questions increased about the impact of EVD on pregnancy and delivery and the risk to healthcare providers attending to pregnant women. While the experts representing the MHT had provided technical expertise to the response since EOC activation, the MHT was formally activated on October 18, 2014. This is the second time that the MHT was activated as part of an emergency response; the initial activation was during the 2009–2010 Pandemic H1N1 Influenza response.6 The Ebola outbreak in West Africa officially ended in December 2015 when Guinea was declared Ebola free.1 On March 29, 2016, WHO declared that the Ebola outbreak in West Africa no longer constituted a public health emergency. Subsequently, the EOC activation for CDC’s Ebola response ended on March 31, 2016.2
The MHT in DRH is a functional unit that works on emergency preparedness issues related to reproductive health, and includes maternal child health experts from across the agency. It can quickly scale up and expand response capacity for events that disproportionately affect pregnant women and women with infants. This is referred to as MHT activation. During CDC’s Ebola Response, MHT subject matter experts (SMEs) continuously provided clinical and epidemiologic expertise to response-related scientific and epidemiologic activities. While the MHT officially deactivated on February 22, 2015, DRH scientists continued to respond to the needs of U.S. healthcare organizations through activities such as serving on the EOC Healthcare Domestic Infection Control Training Team, being deployed to a state health department to assist with Ebola inquiries, and assisting with Rapid Ebola Preparedness training for labor and delivery. Furthermore, while no women in the United States had Ebola during pregnancy, CDC Emergency Response Teams (CERT) have been deployed to assist U.S. local hospitals with deliveries of Ebola virus survivors.7
A high-priority MHT activity during CDC’s Ebola response was to publish guiding principles for pregnant women early in the response. These guiding principles assessed the following: whether pregnant women were more susceptible to infection with Ebola virus than the general population; if pregnant women with EVD were at an increased risk for severe illness and mortality compared to the general population; if EVD during pregnancy was associated with adverse pregnancy outcomes; and special considerations for maternal treatment and prophylaxis. Guiding principles were based on a critical review of what was currently known about EVD and its effects on pregnant women, and were disseminated through an article titled “What obstetrician-gynecologist should know about Ebola.”4
The MHT also prepared guidance documents, provided technical assistance for Rapid Ebola Preparedness visits to U.S. hospitals, and addressed inquiries related to Ebola and maternal health.8–10 The MHT developed several scientific products, including four peer-reviewed journal articles and a case report concerning a pregnant patient with Ebola in Sierra Leone (Table 1).
Table 1.
2014–2015 Scientific Products Developed by the Maternal Health Team
| Scientific products | Date of initial publication | Page views/Citationsa |
|---|---|---|
| Guidance documents | ||
| Recommendations for breastfeeding/infant feeding in the context of EVDb | September 17, 2014 | 31,067c |
| Guidance for screening and caring for pregnant women with Ebola in the United Statesb | November 1, 2014 | 21,623d |
| Articles | ||
| What obstetricians—gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention4 | September 9, 2014 | 48 |
| U.S. hospital preparedness for obstetric patients with possible Ebola10 | February 3, 2015 | 1 |
| EVD: focus on children14 | April 4, 2015 | 3 |
| Pregnancy, labor, and delivery after EVD and implications for infection control in obstetric services, United States7 | July 15, 2016 | 2 |
| Case report | ||
| A pregnant patient with EVD15 | September 17, 2015 | 5 |
Citations from Google Scholar (www.scholar.google.com) as of November 30, 2016.
Complete citations are in the reference list.
From September 17, 2015–September 13, 2016.
From November 1, 2015–September 13, 2016.
EVD, Ebola virus disease.
To inform future responses, we analyzed maternal health inquiries that were addressed by the MHT during the activation of the Ebola response (similar to the analyses of inquiries received during the Pandemic H1N1 response).6 Analyses of maternal health inquiries received during the response highlighted needs of the U.S. pregnant population and salient questions asked by medical and public health professionals and the general public.
Assessment
Inquiries to the CDC are received by CDC-INFO, the national contact center that provides science-based health information to the public, healthcare providers, and public health professionals either by phone or email. In an emergency response, some inquiries require input from SMEs. Consequently, CDC-INFO staff escalate these inquiries to the EOC for further assessment and review. Inquiries related to pregnant/postpartum women and infants are sent to the MHT for topic-specific expertise. Inquiries were also received via the CDC Medical Investigations Team. This team of clinicians was established to address questions from State Health Departments and healthcare providers regarding suspected cases of Ebola, including those returning from Ebola-affected countries. A phone line was also created to provide testing consultations when EVD was suspected and recommendations on screening for Ebola based on symptoms and epidemiologic risk factors. The Medical Investigations Team used CDC guidance and prepared responses to answer these inquiries. If there was no CDC-published guidance to answer the inquiry, then the inquiry was escalated to the EOC response team (e.g., MHT). The MHT provided technical assistance for escalated clinical inquiries for the duration of the CDC response.
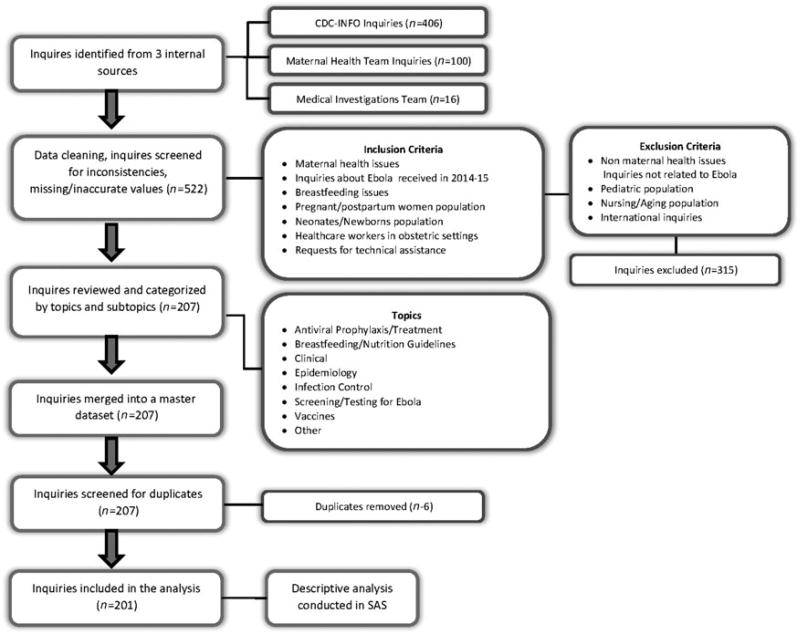
We analyzed inquiries on maternal health issues received through CDC-INFO, MHT, and the Medical Investigations Team from August 2014 to December 2015, when the outbreak was declared over in West Africa. Internal call logs used to capture, monitor, and track inquiries for the three data sources were merged. Duplicate inquiries and those not related to maternal health issues were removed. After reviewing 522 inquiries, 201 entries met our selection criteria for inclusion in our analysis (Fig. 1). We analyzed inquiries related to pregnant and postpartum women, neonates, and healthcare workers in obstetric settings.
FIG. 1.
Derivation of final samples of inquiries.
Each inquiry was categorized by route of inquiry, inquirer type, and topic area. The following major topics were used: antiviral prophylaxis/treatment, breastfeeding/nutrition guidelines, clinical, epidemiology, infection control, screening/testing for Ebola. and vaccine. Some topics were diverse enough that it was necessary to categorize into subtopics. A single inquiry could have more than one topic and subtopic. We also calculated the number of inquiries received by month.
Results
From August 2014 to December 2015, CDC-INFO received a total of 35,879 Ebola inquiries, and <1.0% (n = 108) were related to maternal health issues. Of the 201 inquiries received, 65% were received by email with the remainder by phone (35%). The majority of inquiries (85%) were received during 5 months of the MHT activation, October, 2014–February, 2015. Most of the inquiries came from clinicians (68%) and the general public (20%) (Table 2). Over half of the inquiries (54%) were received through CDC-INFO, and about a third (39%) were received directly by the MHT (Table 2).
Table 2.
Maternal Health Inquiries by Inquirer and Channel of Inquiry, August 2014–December 2015
| Inquirer | Channel of inquiry | |||
|---|---|---|---|---|
|
|
||||
| CDC- INFO |
Maternal heath team |
Medical investigations team |
Total | |
|
| ||||
| N | N | N | N (%) | |
| Clinician | 68 | 54 | 15 | 137 (68) |
| General public | 40 | 1 | 0 | 41 (20) |
| CDC internal | 0 | 17 | 0 | 17 (8) |
| Federal or state health partner | 0 | 3 | 0 | 3 (1) |
| Media | 0 | 3 | 0 | 3 (1) |
| Total N (%) | 108 (54) | 78 (39) | 15 (7) | 201 |
CDC-INFO, the national contact center that provides science-based health information by phone or email.
The 201 inquiries received covered 258 unique topics. Of the total topics covered, more than half related to infection control (57%) and nearly a third related to clinical topics (30%). Fewer inquiry topics were related to breastfeeding/nutrition guidelines (7%), epidemiology (3%), or screening/testing for Ebola (3%), only one inquiry was related to vaccines (<1%), and none were related to antiviral prophylaxis/treatment (Table 3).
Table 3.
Topics and Subtopics of Maternal Health Inquiries, August, 2014–December, 2015 (N= 258)
| Topics/subtopics | n | Percenta |
|---|---|---|
| Infection control | 147 | 57 |
| Obstetric care/labor and delivery risk factors | 75 | 29 |
| Pregnant and breastfeeding healthcare workers | 53 | 21 |
| Travel/transport guidelines | 20 | 8 |
| Infant guidelines | 19 | 7 |
| Isolation of mother and newborn | 10 | 4 |
| Clinical | 76 | 30 |
| Guidance/management of pregnant women with EVD | 54 | 21 |
| Risks/symptoms/transmission | 25 | 10 |
| Breastfeeding | 18 | 7 |
| Screening/testing for Ebola | 8 | 3 |
| Epidemiology | 8 | 3 |
| Research | 5 | 2 |
| Mortality | 1 | <1 |
| Surveillance systems | 2 | 1 |
| Vaccine | 1 | <1 |
Percentages may be >100 because >1 subtopic was included per topic.
The primary topic of inquiries through both CDC-INFO and the MHT was infection control (56% and 66%, respectively). About a third of the inquiries addressed by the Medical Investigations Team were on infection control (30.4%), whereas the majority (69.6%) received through this channel were clinical inquiries through the healthcare providers’ line.
As stated above, some topics were further divided into subtopics (Table 3). Among inquiries related to infection control (n = 147), most related to obstetric care risks (n = 75) and questions about risks to pregnant and breastfeeding healthcare workers (n = 53). In addition, most clinical inquiries (n = 76) related to guidance and management of pregnant women with EVD (n = 54) (Table 3).
Conclusion
The CDC MHT was formally activated in the domestic Ebola response on October 18, 2014. Due to a decrease in domestic inquiries, and an effort to refocus CDC Ebola response efforts in West Africa, the MHT was deactivated on February 22, 2015.
Substantially fewer inquiries were received during the Ebola response related to maternal health compared to the 2009 Pandemic H1N1 response.6 The reduction in inquiries is likely due to several factors, including the Ebola response being primarily internationally focused. In addition, this may be a result of increased preparedness efforts and rapid dissemination of guiding principles, resulting in less unanswered questions from healthcare providers and the public. A notable shift observed during the Ebola response was a reversal in the incoming channel for inquiries, with most received via email rather than telephone.6
The MHT faced many challenges throughout the Ebola response. This was the largest response in CDC’s history. As a result, many staff were deployed and unavailable to work specifically on maternal health issues. In addition, during an emergency, it is often difficult to get emergency responders and public health professionals to think about the unique needs of pregnant women and their infants. Little to no surveillance is conducted among this vulnerable population.11,12 This may be due to pregnant women making up just 1% of the general population and 5% of women of reproductive age at a given point in time in the United States, making it difficult to conduct population-based surveillance.
The MHT’s first activation for CDC’s Pandemic H1N1 response highlighted the needs of pregnant women during an influenza pandemic and the usefulness of disseminating health information via CDC’s website and social media.6 These lessons informed the MHT’s preparedness activities and response to the Ebola outbreak. Now, lessons learned from the Ebola response are currently informing CDC’s Zika virus response, an emergency response focusing on reproductive health. For CDC’s Zika response, the MHT joined colleagues from the National Center of Birth Defects and Developmental Disabilities and the National Center for Emerging and Zoonotic Infectious Diseases to create the Pregnancy and Birth Defects Task Force. This task force has been able to rapidly disseminate numerous scientific documents. For example, the MHT’s work on infection control in Ebola built the foundation for recommendations to prevent transmission of Zika virus in obstetric settings through implementation of standard precautions.13 With each emergency response and continued preparedness work, the MHT strives to advance the science around reproductive health and emergencies, and ultimately increase the health of women and infants.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.WHO Ebola Response Team. After Ebola in West Africa— Unpredictable risks, preventable epidemics. N Engl J Med. 2016;375:587–596. doi: 10.1056/NEJMsr1513109. [DOI] [PubMed] [Google Scholar]
- 2.Bell BP. Overview, control strategies, and lessons learned in the CDC response to the 2014–2016 Ebola epidemic. MMWR Suppl. 2016;65:4–11. doi: 10.15585/mmwr.su6503a2. [DOI] [PubMed] [Google Scholar]
- 3.Koonin LM, Jamieson DJ, Jernigan JA, et al. Systems for rapidly detecting and treating persons with ebola virus disease—United States. MMWR Morb Mortal Wkly Rep. 2015;64:222–225. [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What obstetrician-gynecologists should know about Ebola: A perspective from the Centers for Disease Control and Prevention. Obstet Gynecol. 2014;124:1005–1010. doi: 10.1097/AOG.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 5.Papagiotas SS, Frank M, Bruce S, Posid JM. From SARS to 2009 H1N1 influenza: The evolution of a public health incident management system at CDC. Public Health Rep. 2012;127:267–274. doi: 10.1177/003335491212700306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosby LG, Ellington SR, Forhan SE, et al. The Centers for Disease Control and Prevention’s maternal health response to 2009 H1N1 influenza. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S7–12. doi: 10.1016/j.ajog.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 7.Kamali A, Jamieson DJ, Kpaduwa J, et al. Pregnancy, labor, and delivery after Ebola virus disease and implications for infection control in obstetric services, United States. Emerg Infect Dis. 2016;22:1156–1161. doi: 10.3201/eid2207.160269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recommendations for Breastfeeding/Infant Feeding in the Context of Ebola. [Accessed September 10, 2016];2014 Available at: www.cdc.gov/vhf/ebola/hcp/recommendations-breastfeeding-infant-feeding-ebola.html.
- 9.Centers for Disease Control and Prevention. Screening and Caring for Pregnant Women with Ebola in the U.S. [Accessed September 10, 2016];2014 Available at: www.cdc.gov/vhf/ebola/healthcare-us/hospitals/pregnant-women.html.
- 10.Meaney-Delman D, Koonin LM, Jamieson DJ. US hospital preparedness for obstetrics patients with possible Ebola. Am J Obstet Gynecol. 2015;212:417–419. doi: 10.1016/j.ajog.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zotti ME, Williams AM, Robertson M, Horney J, Hsia J. Post-disaster reproductive health outcomes. Matern Child Health J. 2013;17:783–796. doi: 10.1007/s10995-012-1068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zotti ME, Williams AM, Wako E. Post-disaster health indicators for pregnant and postpartum women and infants. Matern Child Health J. 2015;19:1179–1188. doi: 10.1007/s10995-014-1643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson CK, Iwamoto M, Perkins KM, et al. Preventing transmission of Zika virus in labor and delivery settings through implementation of standard precautions—United States, 2016. MMWR. 2016;65:290–292. doi: 10.15585/mmwr.mm6511e3. [DOI] [PubMed] [Google Scholar]
- 14.Kourtis AP, Appelgren K, Chevalier MS, McElroy A. Ebola virus disease: Focus on children. Pediatr Infect Dis J. 2015;34:893–897. doi: 10.1097/INF.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oduyebo T, Pineda D, Lamin M, Leung A, Corbett C, Jamieson DJ. A pregnant patient with Ebola virus disease. Obstet Gynecol. 2015;126:1273–1275. doi: 10.1097/AOG.0000000000001092. [DOI] [PubMed] [Google Scholar]