Abstract
Lychee seed is a traditional Chinese medicine and has many beneficial effects such as modulation of blood sugar and lipids, antioxidation, antivirus and antitumor. Studies have indicated that type II diabetes mellitus (T2DM) and Alzheimer's disease (AD) share common biological mechanisms including insulin resistance, impaired glucose metabolism, β-amyloid (Aβ) formation, oxidative stress and presence of advanced glycation end products (AGEs). The present study investigated the effects of lychee seed extract (LSE) on neuroprotection, cognitive function improvement and possible underlying mechanisms in a rat model of T2DM with cognitive impairment. We analyzed the chemical profile of LSE using a UHPLC-SPD chromatogram and evaluated its effect on the improvement of spatial learning and memory of rats by a Morris water maze. The levels of glucose, insulin, Aβ, AGEs, Tau protein and acetylcholinesterase in the blood and/or hippocampus of rats were determined by blood-glucose meter, radioimmunoassay, chemical chromatometry, enzyme-linked immunosorbent assay (ELISA) and immunohistochemical analysis, respectively. Results demonstrated that LSE consists of eight major and around 20 minor ingredients, and it remarkably prevents neuronal injury and improves cognitive functions in T2DM rats. The levels of glucose, insulin, Aβ, AGEs and Tau protein were significantly increased in the blood and/or hippocampus of T2DM rats, while LSE remarkably decreased their levels compared to vehicle treatment (P<0.01). The possible mechanisms may be associated with IR improvement and decreased formations of Aβ, AGEs and Tau protein in the hippocampus of T2DM rats. LSE may be developed as the agent for the treatment of T2DM and/or AD clinically.
Keywords: lychee seed extract, type II diabetes mellitus, Alzheimer's disease, cognitive impairment, neuronal injury, insulin resistance
Introduction
Lychee (Litchi in Chinese) is an edible fruit of the Sapindaceae family in China and Southeast Asia and it is botanically designated as Litchi chinensis Sonn (1,2). Lychee seed is the dry mature seed of a lychee and used as a traditional Chinese medicine named 'Li-zhi-he' in Chinese and was recorded by the Bencao Yanyi (Development of Herbal Medicine) and Bencao Gangmu (Compendium of Materia Medica) for regulating Qi, dispelling cold, alleviating pain and relieving polydipsia (3). Modern pharmacological studies have identified that some components of lychee seed have the effects of modulation of blood sugar by improving insulin resistance (IR) (4,5), lowering blood lipids (6), antioxidation (7), antivirus (8), anti-tumor (9) and preventing liver injury (10).
Type II diabetes mellitus (T2DM) is one of the most common chronic diseases in the world (11). T2DM is a metabolic disorder characterized by hyperglycemia and its development is due to pancreatic β cells failing to secrete sufficient amounts of insulin to meet metabolic demand (12,13). T2DM causes various serious complications of heart, eyes, nerves, liver and kidneys and is associated with decrements in cognitive function and changes in brain structure (14). IR in pancreatic β cells contributes to the pathogenesis of T2DM (13).
Alzheimer's disease (AD) is the major causative disease of dementia and it is characterized pathologically by the accumulation of senile plaques (SPs) and neurofibrillary tangles (NFTs) in the brain (15). Emerging evidence suggests that T2DM can contribute significantly to the onset and/or progression of AD either directly or as a cofactor (16). Numerous studies have evidenced that T2DM is a major risk factor in the pathology of AD, and that the two diseases share common biological mechanisms at the molecular level including IR, impaired glucose metabolism, β-amyloid (Aβ) formation, oxidative stress and presence of advanced glycation end products (AGEs) (16–19). IR is likely to be the basis for a variety of pathological changes in AD, and improvement of IR can delay the onset and progression of AD (20,21).
Currently, the drugs for AD treatment can only improve patients' cognition, however they do not slow the progression or cure the disease (22). Development of drugs for the treatment of AD have a very high failure rate due to lack of therapeutic efficacy or emergence of serious adverse effects; no new drug has been approved for AD treatment since 2003 (23). Therefore, discovery and development of novel anti-AD drugs is urgently needed. Previously, active ingredients from natural products and medicinal herbs for treatment of AD and/or diabetes mellitus have attracted substantial attention. Studies have shown that some natural products have neuroprotective effects (24,25). The aqueous extract from lychee seed improved the ability of learning and memory in mice (26). The saponins isolated and extracted from lychee seeds exhibited an anti-diabetic effect (27). Our preliminary unreported data also showed that saponins from lychee seeds significantly relieved cognitive dysfunction by improving IR in rats. In the present study, we analyzed the chemical profile of lychee seed extract (LSE) by ultrahigh performance liquid chromatography (UHPLC)-SPD. In order to study the effects of LSE on neuroprotection and cognitive function improvement, we also established a rat model of T2DM with cognitive impairment and investigated the ability of spatial learning and memory in rats by Morris water maze. In addition, the effects of LSE on neuropathology of the neurons were studied by pathohistological analysis. Furthermore, to explore the possible mechanisms associated with the effects of LSE on neuroprotection and cognitive function improvement, the levels of glucose, insulin, Aβ, acetylcholinesterase (AChE), AGEs, and Tau protein in blood and/or hippocampus were determined by blood-glucose meter, radioimmunoassay, chemical chromatometry, enzyme-linked immunosorbent assay (ELISA) and immunohistochemical analysis, respectively.
Materials and methods
Reagents
Donepezil hydrochloride tablets (Aricept, cat. no. H20070181) were purchased from Eisai China Inc. (Chengdu, China), and prepared to a mixed suspension at the concentration of 0.1 mg/ml as a positive control. Streptozotocin (STZ; cat. no. S0130) was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Aβ release analysis kit (cat. no. 200805) and serum insulin radioimmunoassay kit (cat. no. 200808) were purchased from the Chinese PLA General Hospital (Beijing, China). Tau protein assay kit (cat. no. BM3928) was purchased from Wuhan Boster Biological Technology, Ltd. (Hubei, China). AChE assay kit (cat. no. A024) was purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). AGEs assay kit (cat. no. CEB353Ge) was purchased from Wuhan USCN Business Co., Ltd. (Hubei, China). Morris water maze system (MT-2000) was purchased from Chengdu Techman Software Co., Ltd. (Sichuan, China).
Experimental animals
A total of 150 8–10-week old (body weight, 180–220 g) specific pathogen free grade male Sprague Dawley rats were purchased from the Experimental Animal Centre, Sichuan Provincial Academy of Medical Sciences (Chengdu, China; SCXK201302). All rats were housed in sterile plastic cages (up to 4 rats per cage) with free access to water and food ad libitum in a controlled room temperature (22±1°C), humidity (50–70%) and under a 12-h light/dark cycle. All animal experiments were performed strictly in accordance with institutional guidelines and followed an approved protocol (permit no. 250114) by the Committee on Use and Care of Animals of Southwest Medical University (Sichuan, China). There were 10–12 rats for each experimental group.
Collection, extraction and isolation of lychee seeds
Lychee seeds were purchased from a local market in Luzhou, China, and were authenticated by Professor Can Tang of School of Pharmacy, Southwest Medical University (Luzhou, China). A total mass of 1,000 g of air-dried lychee seeds were ground and soaked with 1,000 ml of 70% ethanol overnight, extracted by percolation with 8,000 ml of 70% ethanol at the speed of 5 ml/min/kg. Following this, the solvents were evaporated under vacuum, then the LSE was concentrated to dryness using a rotary vacuum evaporator and collected to yield a total mass of 185.2 g dried powder.
Analysis of chemical profile of LSE
The chemical profile of LSE was performed at 254 nm by an LCMS-8040 UHPLC system (Shimadzu Corp., Kyoto, Japan). Chromatographic analyses were performed at 45°C with an Inert Sustain C18 column (2.0 μm particle size, 50×2.1 mm; GL Sciences, Tokyo, Japan), using water: Formic acid (100:0.1, v/v) and methanol as the mobile phase A and B, respectively. The mobile phase was delivered at a rate of 0.25 ml/min with injection volume of 1 μl. The gradient separation process was as follows: 15–15% B at 0–2.0 min, 15–80% B at 2.0–5.0 min, 80–80% B at 5.0–7.0 min, 80–15% B at 7.0–7.5 min. The data were analyzed by LabSolutions software version 5.75 (Shimadzu Corp.).
Morris water maze test
The spatial learning and memory ability was investigated using the Morris water maze test as described previously (28,29). Briefly, the test was conducted in a round white pool (94 cm in diameter and 31 cm deep) filled with water (30 cm depth) with the temperature ~25°C. The escape platform was a 25 cm2 Plexiglas square, placed in the center of one quadrant of the pool, 15 cm from the pool's edge and submerged 1 cm beneath the water surface.
Hidden platform test: Each rat was trained in a circular pool, which randomly divided into four equal quadrants by a hidden platform with four trials for 120 sec per trial daily with 30 min intervals between the trials for 5 consecutive days. The time for the rat to seek the platform was record by an online image video tracking system and within 120 sec as the escape latency.
Spatial probe test: The platform was removed from the pool after the hidden platform test, each rat was left to one quadrant of the pool which was farthest from the primary platform. The number of rats crossing the platform, the time spent in the target quadrant and the percentage of time spent in the target quadrant were recorded by a tracking system.
Establishment of the rat model of T2DM with cognitive impairment
Rats were selected by Morris water maze test as the standard of 40–120 sec to find the platform, the rat was eliminated if it found the platform in <40 sec or more than 120 sec, as described previously (29,30). The selected rats were randomly divided into control group (n=12) and study group (n=100). The rats in the control group were fed with normal standard diet, while the rats in the study group were fed with high fat, high sugar and high protein diet (HFSPD, food ratio: carbohydrate 25%, protein 15.2%, fat (lard) 58.8%, and sodium cholate 1% (31,32). The body weights of rats were weighed every two weeks. Then, the rats in the study group were intraperitoneally (IP) injected with STZ (27 mg/kg) once and the rats in the control group were injected the same volume of citric acid buffer for 8 weeks. The blood glucose and serum insulin of the rats were measured 72 h following STZ injection and was calculated by Homeostasis Model Assessment (HOMA = fasting blood glucose × fasting insulin/22.5) for IR index to judge development of T2DM. The rats were then continuously fed the HFSP diet and weighed weekly for 4 more weeks. Finally, the rats were further examined by a Morris water maze test for cognitive impairment and other experiments.
Drug preparation and administration
The T2DM rats with cognitive impairment were randomly divided into five groups with 10 rats for each group and treated with normal saline (NS) 1 ml/kg (negative control), donepezil 0.42 mg/kg (positive control), LSE 0.7, 1.4 and 2.8 g/kg by intragastric (IG) administration once a day (daily) at 8 a.m. for 28 consecutive days. In addition, 10 normal rats on a normal diet were given equal volume of NS as additional control. The dose of donepezil was selected as previous described from literature (33).
Measurement of blood glucose
The blood samples of rats were collected from the tail veins, and blood glucose was measured by blood-glucose meter, as described previously (34). HOMA was calculated for IR index and the method of calculation is described above.
Determination of the levels of glucose, insulin, AChE, Aβ and AGEs in the blood and/or hippocampus of rats
A total of 10 rats for each group were randomly selected after Morris water maze test, then weighed and anesthetized with 1% pentobarbital sodium (0.4 ml/kg) by IP injection. Bloods were taken from the abdominal aorta for detection of the levels of insulin, AChE and Aβ. The tissues of brain were collected. The freshly dissected hippocampal tissues were isolated to prepare hippocampal tissue homogenates for detection of the contents of AChE, Aβ and AGEs. Insulin, AChE, Aβ and AGEs were measured by serum insulin radioimmunoassay kit, AChE assay kit, Aβ release analysis kit and AGEs assay kit, respectively.
Determination of the expression of Tau protein in hippocampal neurons of rats by immunohistochemical analysis
The expression of Tau protein in the hippocampus of rats was carried out with immunohistochemical staining and examined under microscope, as previous described (33). The positive rate of the expression of Tau protein was calculated by GD-10.0 image analysis system. A total of 10 rats were used for each group.
Histopathological examination of hippocampus neuron CA1 pyramidal cells of rats with hematoxylin and eosin (H&E) staining
The rats were anesthetized by IP injection of 1.0% pentobarbital and perfusion with 4% paraformaldehyde to fix the brains for 24 h after treatment. Then, the brains of rats were removed and the brain tissues were weighed, fixed in 10% neutral buffered formalin, dehydrated and embedded in paraffin for coronal microtome sections (~4–5 μm) with H&E staining for the study, as described previously (35). The sections were observed for pathohistological changes under light microscope.
Statistical analysis
All the data were presented as mean ± standard deviation (SD). Statistical differences of the data among the means of two or more groups were analyzed by Student's t-test and/or one-way univariate analysis of variance (ANOVA). A value of P<0.05 was considered to indicate a statistically significant difference, and a value of P<0.01 was considered to indicate a highly statistically significant difference.
Results
The chemical profile of LSE
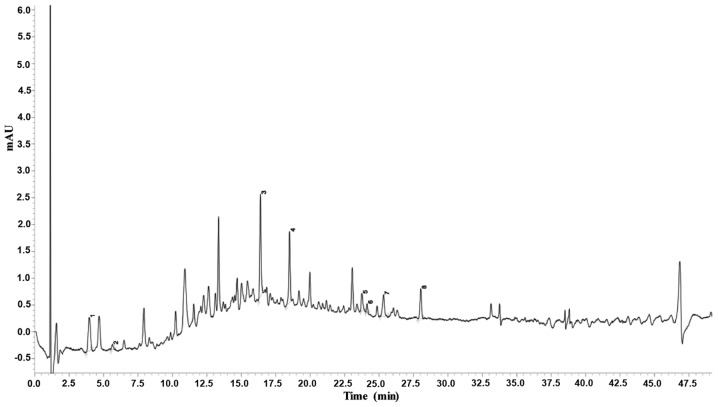
To determine the chemical profile of LSE isolated and extracted from lychee seed, we studied its chemical fingerprint at 254 nm by a UHPLC-SPD chromatogram and the data are presented in Fig. 1. The data display the main chemical compositions of LSE and indicate that it mainly consists of eight major (Fig. 1) and around 20 minor ingredients.
Figure 1.
Analysis of the chemical profile of LSE by a UHPLC-SPD-MS-MS chromatogram. The chemical fingerprint of LSE was analyzed at 254 nm by a Shimadzu LCMS-8040 UHPLC system, which comprised two LC-30AD pumps, a SIL-30AC autosampler with a CTO-30AC column oven, a DGU-20A5 degasser and a Shimadzu CBM-20A system controller. Chromatographic analyses were achieved at 45°C with an InertSustain C18 column using water-formic acid (100:0.1, v/v) and methanol as the mobile phase A and B, respectively. The delivered rate of mobile phase was 0.25 ml/min with the injection volume of 1 μl. For the gradient separation, the process was as follows: 15–15% B at 0–2.0 min, 15–80% B at 2.0–5.0 min, 80–80% B at 5.0–7.0 min, and 80–15% B at 7.0–7.5 min. The data were analyzed by LabSolutions software (version 5.75). The major ingredients of LSE are indicated as 1–8. The numbers indicate the following substances: 1, adenosine; 2, 5-hydroxymethyluridine; 3, 4-p-coumaroylquinic acid; 4, procyanidin B; 5, procyanidin A; 6, 5′-β-D-glucopyranosyloxy jasmonic acid; 7, 4-O-(trans-p-coumaroyl) quinic acid; and 8, procyanidin tetramer. LSE, lychee seed extract; UHPLC, ultrahigh performance liquid chromatography.
Establishment of a rat model of T2DM with cognitive impairment
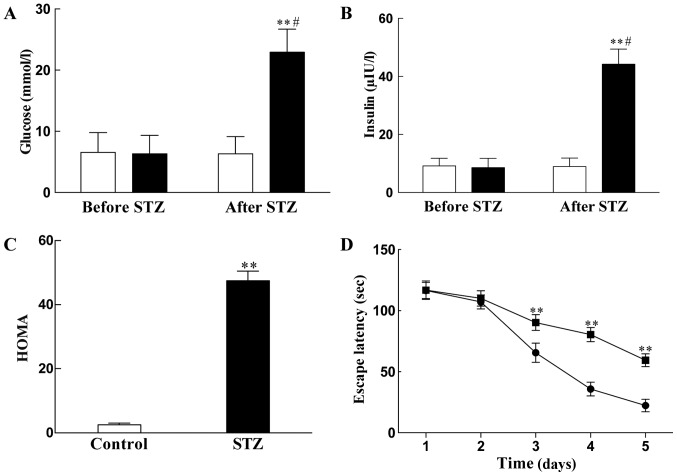
To establish the rat model of T2DM with cognitive impairment, the rats were initially fed with HFSP diet for 8 weeks and observed the general condition including food and water intakes, hair color, activities and body weight changes and compared to those of the rats with normal standard diet (control). These was no difference in general condition between the two groups, however, the body weights of the rats with HFSP diet were significantly increased (P<0.01) compared to those of control rats (Fig. 2A). Then, the rats were injected with a single dose of STZ (27 mg/kg, IP ×1) after 8 weeks on the HFSP diet. Interestingly, the food and water intakes, as well as urine volumes were gradually increased, while the body weights of the rats were decreased (P<0.01) following injection with STZ compared to the normal control rats (Fig. 2B), indicating the rats displayed the symptoms of T2DM.
Figure 2.
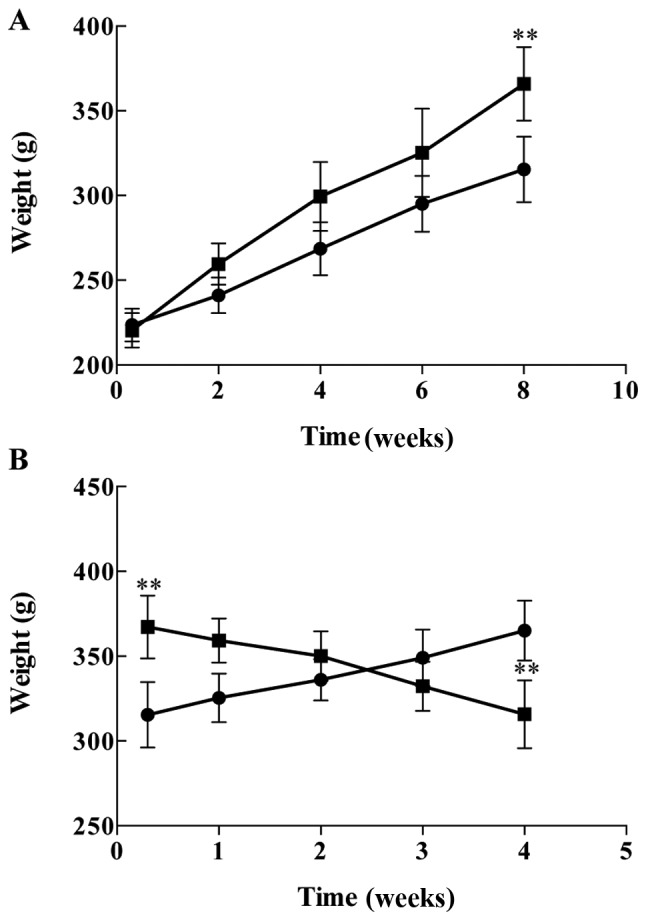
Changes in body weight of the rats on a regular standard diet (●) or high fat, high sugar and high protein (HFSP) diet (■) (A) before and (B) after STZ (27 mg/kg), single dose, by intraperitoneal injection. A total of 12 rats were used for regular standard diet (control) group and 120 rats for HFSP diet group. The results are expressed as mean ± standard deviation. **P<0.01 vs. control rats.
Next, the blood glucose and insulin levels were measured and HMOA was calculated in the T2DM rats and compared to those of the normal rats (control); the results are illustrated in Fig. 3. The data show that there were no significant differences of blood glucose (Fig. 3A), insulin (Fig. 3B) and HMOA (Fig. 3C) between the rats on the HFSP diet and the rats on regular standard diet (P>0.05) in the 8 weeks before STZ injection as 6.33±3.01 vs. 6.57±3.21 mmol/l, 8.57±3.18 vs. 9.14±2.66 μIU/l, and 2.1±0.43 vs. 2.67±0.38, respectively. However, the blood glucose, insulin and HMOA were increased significantly (P<0.01) following STZ injection in the rats on the HFSP diet compared to those of the rats on regular standard diet without STZ treatment, as 3.62-fold (22.93±3.74 vs. 6.34±2.81 mmol/l), 4.95-fold (44.23±5.21 vs. 8.94±2.95 μIU/l), and 17.89-fold (45.08±0.87 vs. 2.52±0.37) increases, respectively. These data indicated that the rat model of T2DM was successfully established (Fig. 3A–C).
Figure 3.
Effects of HFSP diet alone and in combination with STZ on (A) serum glucose (white bars, control rats; black bars, T2DM rats), (B) insulin (white bars, control rats; black bars, T2DM rats), (C) HOMA (white bars, control rats; black bars, T2DM rats) and (D) escape latency of the normal (control, ●; and T2DM, ■) rats. The rats were fed with HFSP diet for 8 weeks, then injected a single dose of STZ (27 mg/kg, intraperitoneal injection) and fed the HFSP diet for another 4 weeks. There were 10 rats used for each experimental group. The results are presented as mean ± standard deviation. **P<0.01 vs. STZ group; #P<0.01 vs. before HFSP diet. HFSP, high fat, high sugar and high protein; STZ, streptozocin; HOMA, homeostasis model assessment.
Finally, we evaluated the cognitive function of T2DM rats by a navigation test starting 1 h after STZ treatment during the first 5 days to see whether the cognition was impaired or not compared to the normal rats (control) and the results are presented in Fig. 3D. The escape latency of T2DM rats was slightly increased but no statistically significant differences were observed in the first and the second days after SZT treatment. However, the escape latency of T2DM rats was significantly higher than that of the normal rats on the days 3–5 (P<0.01). In the last navigation test (on day 5), the escape latency was 22.35±5.12 sec (Fig. 3A) for normal rats and 59.42±5.32 sec (Fig. 3B) for T2DM rats, respectively. Therefore, the number was 62.39% by the calculation formula of (B-A)/B%, suggesting that T2DM rats have obvious cognitive impairment according to the judgment standard of dementia criteria of (B-A)/B% >20% (36).
Effects of LSE on the cognitive function of the T2DM rats with cognitive impairment
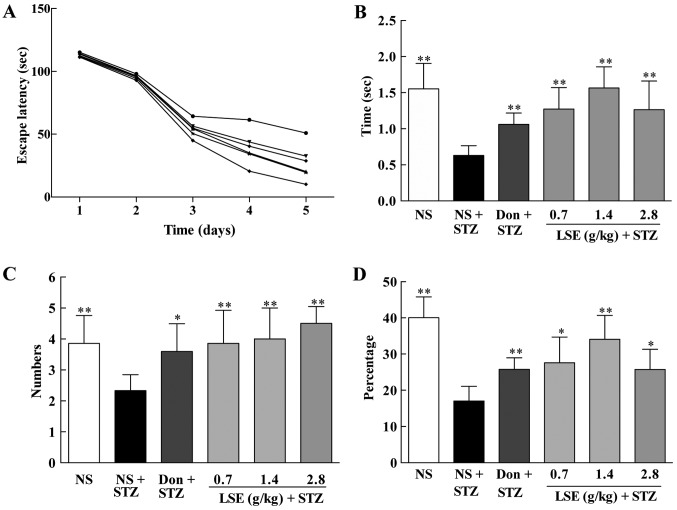
Following successfully establishing the rat model of T2DM with cognitive impairment, the effects of LSE on cognitive functions of the T2DM rats were investigated using Morris water maze test, a commonly used method for assessing cognitive functions, and compared to that of NS (as negative control) and donepezil (as positive control) treatments, as well as the normal rats treated with NS. The results are illustrated in Fig. 4. First, the Hidden platform test was performed in the normal rats and T2DM rats on the day 1–5 after STZ treatment to investigate the ability of learning and memory. As presented in Fig. 4A, the escape latency time of the T2DM rats was significantly increased compared to that of the normal rats (P<0.01). However, LSE at the doses of 0.7, 1.4 and 2.8 g/kg/day and donepezil at 0.42 mg/kg/day by intragastric administration for 28 consecutive days significantly shortened the escape latency compared to NS treatment (P<0.01) in the T2DM rats (Fig. 4A).
Figure 4.
Effects of LSE on the ability of spatial learning and memory in T2DM rats with cognitive impairment induced by high fat, high sugar and high protein diet and STZ (27 mg/kg, intraperitoneal ×1). The normal control rats were treated with NS (1 ml/kg/day), and T2DM rats were treated with NS (1 ml/kg/day), Don (0.42 mg/kg/day) or LSE (0.7, 1.4 and 2.8 g/kg/day ×28, IG). The cognitive function was assessed by Morris water maze test. (A) The escape latency of rats in days 1–5 after STZ injection. Normal control rats (■) treated with NS (1 ml/kg/day, IG ×28); T2DM rats (●) treated with NS (1 ml/kg/day, IG ×28); T2DM rats (▲) treated with donepezil (0.42 mg/kg/day, IG ×28); T2DM rats (▼) treated with LSE (0.7 g/kg/day, IG ×28); T2DM rats (◆) treated with LSE (1.4 g/kg/day, IG ×28); and T2DM rats (*) treated with LSE (2.8 g/kg/day, IG ×28). (B) The time that rat spent in target quadrant; (C) the number of rats crossing the platform; and (D) the percentage of time spent in the target quadrant. (B–D) The rats were assessed on day 5 after STZ treatment. There were 10 rats used for each experimental group and results are expressed as mean ± standard deviation. *P<0.05 vs. T2DM rats treated with NS; **P<0.01 vs. T2DM rats treated with NS. NS, normal saline; IG, intragastric; LSE, lychee seed extract; T2DM, type 2 diabetes mellitus; STZ, streptozotocin; Don, donezapil.
Next, the spatial probe test was performed for the rats. Similarly, the residence time (Fig. 4B), the numbers of crossing platform (Fig. 4C), and the percentage of time spent in the target quadrant (Fig. 4D) were significantly shortened in the T2DM rats (P<0.01) compared to those of the normal rats. LSE (0.7, 1.4 and 2.8 g/kg/day ×28 days) significantly increased the residence time (Fig. 4B), the numbers of those crossing the platform (Fig. 4C), and the the percentage of time spent in the target quadrant (Fig. 4D) compared to NS treatment (P<0.01) in the T2DM rats. In addition, donepezil (0.42 mg/kg/day ×28 days) has similarly effect on the spatial learning and memory of the T2DM rats as LSE at the dose of 2.8 g/kg/day.
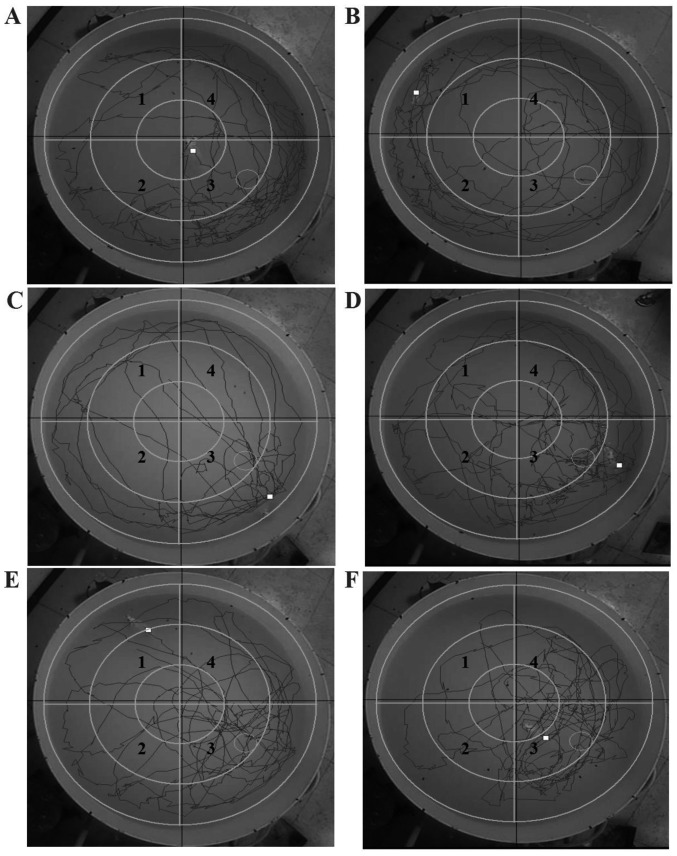
The detailed classical path of the latency to get to the platform for the normal rats treated with NS and the T2DM rats treated with NS, donepezil or LSE during the acquisition trials are shown in Fig. 5. The data indicate that LSE and donepezil can improve the ability of spatial learning and memory of the T2DM rats (Figs. 4 and 5).
Figure 5.
Effects of LSE on the ability of spatial learning and memory in type 2 diabetes mellitus rats with cognitive impairment induced by HFSP diet and (STZ, 27 mg/kg, intraperitoneal ×1). Detail running tracts: (A) Normal rats on regular diet (control) treated with NS (1 ml/kg/day, IG ×28); (B) rats on HFSP diet and treated with STZ and NS (1 ml/kg/day, IG ×28); (C) rats on HFSP diet and treated with STZ and donepezil (0.42 mg/kg/day, IG ×28); (D) rats on HFSP diet and treated with STZ and LSE (0.7 g/kg/day, IG ×28); (E) rats on HFSP diet and treated with STZ and LSE (1.4 g/kg/day, IG ×28); and (F) rats on HFSP diet and treated with STZ and LSE (2.8 g/kg/day, IG ×28). There were 10 rats used for each experimental group. 1, First quadrant; 2, beta quadrant; 3, third quadrant; 4, delta quadrant. LSE, lychee seed extract; HFSP, high fat, high sugar and high protein; STZ, streptozotocin; NS, normal saline; IG, intragastric.
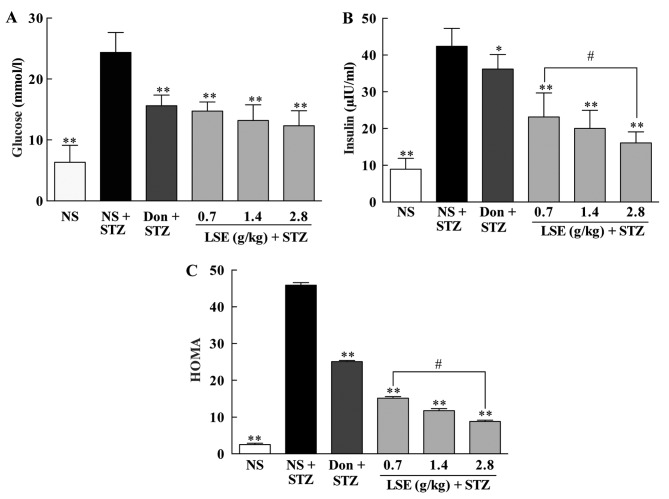
Effects of LSE on the blood glucose, insulin and HMOA in T2DM rats
The development of T2DM is due to pancreatic β cells failed to secrete sufficient insulin to meet metabolic demand to cause hyperglycemia (12). T2DM and AD share common biological mechanisms, including IR and impaired glucose metabolism (16). Therefore, we examined the effects of LSE on blood glucose, insulin and HMOA in the T2DM rats, and the results are shown in Fig. 6. The blood glucose (Fig. 6A), insulin (Fig. 6B) and HMOA (Fig. 6C) levels of the T2DM rats were significantly higher than those of the normal rats (P<0.01) as 24.35±3.30 vs. 6.34±2.81 mmol/l, 42.42±4.88 vs. 8.94±2.95 μIU/l and 45.90±0.72 vs. 2.52±0.37, respectively. There are 3.84-, 4.74-fold and 18-, 21-fold increases in blood glucose, insulin and HMOA in the T2DM rats compared to normal rats, respectively.
Figure 6.
Effects of LSE on the (A) blood glucose, (B) insulin and (C) HOMA in T2DM rats. The normal rats were treated with NS (1 ml/kg/day, IG ×28) and the T2DM rats induced by high fat, high sugar and high protein diet and STZ (27 mg/kg, intraperitoneal ×1) were treated with NS (1 ml/kg/day, IG ×28), Don (0.42 mg/kg/day, IG ×28) or LSE (0.7, 1.4 and 2.8 g/kg/day, IG ×28). There were 10 rats used for each experimental group and are expressed as the mean ± standard deviation. *P<0.05 vs. T2DM rats treated with NS; **P<0.01 vs. T2DM rats treated with NS; #P<0.01 vs. LSE 0.7 g/kg group. LSE, lychee seed extract; T2DM, type 2 diabetes mellitus; HOMA, homeostasis model assessment; NS, normal saline; STZ, streptozotocin; Don, donepezil; IG, intragastric.
However, LSE at the doses of 0.7, 1.4 and 2.8 g/kg/day significantly decreased the blood glucose (14.74±1.50, 13.19±2.57 and 12.33±2.46 mmol/l), insulin (23.09±6.54, 20.04±4.93 and 16.09±3.00 μIU/l), and HMOA (15.13±0.44, 11.75±0.56 and 8.82±0.33) in a dose-dependent manner (Fig. 6) compared to NS treatment in the T2DM rats (P<0.01), although the reductions did not reach the same level in normal rats. Interestingly, donepezil (4.2 mg/kg/day) also significantly decreased the blood glucose (15.60±1.77 μIU/l), insulin (36.19±3.95 μIU/l) and HMOA (25.09±0.31) in the T2DM rats compared to NS treatment (P<0.01), but it was less effective compared to those of LSE treatment even at the lowest dose of 0.7 g/kg/day (Fig. 6). These data indicate that LSE and donepezil are efficacy to decrease elevated blood glucose and insulin to overcome IR in T2DM rats, and that LSE is more effective than that of donepezil.
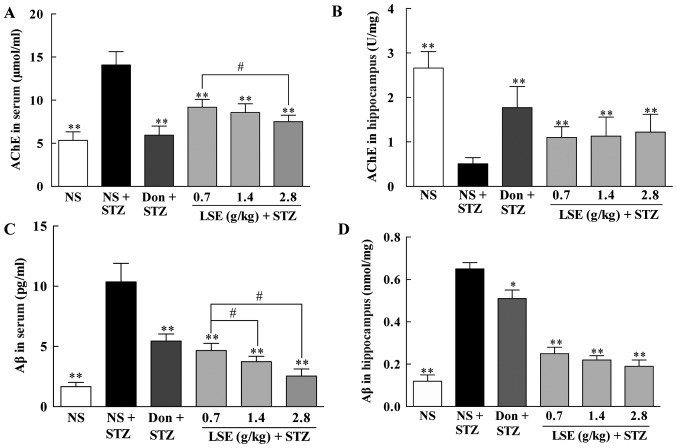
Effects of LSE on AChE and Aβ in the blood and hippocampus of T2DM rats
It has been found that AD is associated with reduction of cholinergic neurons activity (37). AChE inhibitors such as donepezil reduce the rate of acetylcholine degradation to increase its concentration in the brain to execute their anti-AD effect. Therefore, we evaluated the concentrations of AChE in the blood and in the hippocampus of T2DM rats and compared to that of the normal rats (Fig. 7A and B). Interestingly, the concentrations of AChE were significantly increased (P<0.01) in the blood (14.08±1.55 vs. 5.34±0.99 μmol/ml) but markedly decreased (P<0.01) in the hippocampus (0.51±0.14 vs. 2.66±0.37 U/mg) in the T2DM rats compared to that of normal control rats. However, LSE at the doses of 0.7, 1.4 and 2.8 g/kg/day significantly decreased (P<0.01) the concentrations of AChE in the blood (9.19±0.90, 8.57±1.00 and 7.51±0.75 μmol/ml, respectively), while increased (P<0.01) the concentrations of AChE in the hippocampus (1.10±0.24, 1.13±0.43 and 1.22±0.40 U/mg, respectively) compared to NS treatment in the T2DM rats (Fig. 7A and B). The AChE inhibitor donepezil also significantly decreased (P<0.01) the concentrations of AChE in the blood (5.94±1.04 μmol/ml) and increased (P <0.01) the concentrations of AChE in the hippocampus (1.77±0.47 U/mg).
Figure 7.
Effects of LSE on AChE (A and B) and Aβ (C and D) in the blood and the hippocampus of T2DM rats. The normal rats were treated with NS (1 ml/kg/day), and T2DM rats induced by high fat, high sugar and high protein diet and (STZ, 27 mg/kg, intraperitoneal ×1) were treated with NS (1 ml/kg/day), Don (0.42 mg/kg/day), or LSE (0.7, 1.4 and 2.8 g/kg/day, IG ×28 days). There were 10 rats used for each experimental group and are expressed as the mean ± standard deviation. *P<0.05 vs. T2DM rats treated with NS; **P<0.01 vs. T2DM rats treated with NS; #P<0.01 vs. LSE 0.7 g/kg group. LSE, lychee seed extract; AChE, acetylcholinesterase; NS, normal saline; STZ, streptozotocin; Don, donepezil; NS, normal saline.
One of the histopathological hallmarks of AD is deposition of Aβ plaques. Therefore, the effect of LSE on Aβ in the blood and hippocampus of T2DM rats was studied and compared to that of donepezil; the results are illustrated in Fig. 7C and D. The concentrations of Aβ are significantly increased (P<0.01) in the blood and in the hippocampus in T2DM rats compared to that of normal control rats (10.36±1.54 vs. 1.66±0.35 pg/ml in the blood and 0.65±0.03 vs. 0.12±0.03 mmol/mg in the hippocampus, respectively). Similarly, LSE at the doses of 0.7, 1.4 and 2.8 g/kg/day significantly decreased (P<0.01) the concentrations of Aβ in the blood (4.65±0.60, 3.73±0.45 and 2.54±0.59 pg/ml, respectively) and in the hippocampus (0.25±0.03, 0.22±0.02 and 0.19±0.03 mmol/mg, respectively) compared to NS treatment in T2DM rats (Fig. 7C and D). Donepezil also significantly decreased the concentrations of Aβ in the blood (5.45±0.58 pg/ml, P<0.01) and in the hippocampus (0.51±0.04 mmol/mg, P<0.05) but is less effective than that of LSE.
The data indicate that LSE and donepezil are efficacy to decrease AChE in the blood and increase AChE in the hippocampus as well as to reduce elevated Aβ in the blood and the hippocampus in T2DM rats. LSE is more effective in reduction of Aβ than that of donepezil while donepezil has a profound effect on AchE than that of LSE (Fig. 7).
Effects of LSE on reductions of AGEs and Tau protein in the hippocampus of T2DM rats
AGEs have been implicated in diabetes related complications and serve an important role in the pathogenesis of AD. Therefore, we studied the effect of LSE on AGEs in the hippocampus of T2DM rats and the results are presented in Fig. 8. AGEs in the hippocampus of T2DM rats are much higher (P<0.01) compared to that of the normal rats (675.48±29.41 vs. 285.90±41.22 pg/ml). However, LSE (0.7, 1.4 and 2.8 g/kg/day) and donepezil (4.2 mg/kg/day) partially but significantly decreased (P<0.01) AGEs in the hippocampus of T2DM rats compared to NS treatment, the concentrations of AGEs were reduced to 524.19±20.92, 486.77±32.90, 456.94±20.06 and 488.29±33.87 pg/ml, respectively.
Figure 8.
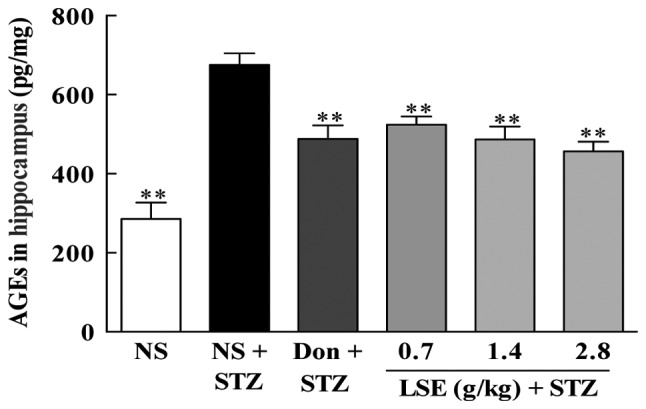
Effects of LSE on AGEs in the hippocampus of rats. The normal rats were treated with NS (1 ml/kg/day) and type 2 diabetes mellitus rats induced by high fat, high sugar and high protein diet and STZ (27 mg/kg, intraperitoneal ×1) were treated with NS (1 ml/kg/day), Don (0.42 mg/kg/day) or LSE (0.7, 1.4 and 2.8 g/kg/day) with intragastric administration daily for 28 days. There were 10 rats used for each experimental group and results are expressed as the mean ± standard deviation. **P<0.01 vs. T2DM rats treated with NS. LSE, lychee seed extract; AGEs, advanced glycation end products; NS, normal saline; STZ, streptozotocin; Don, donepezil.
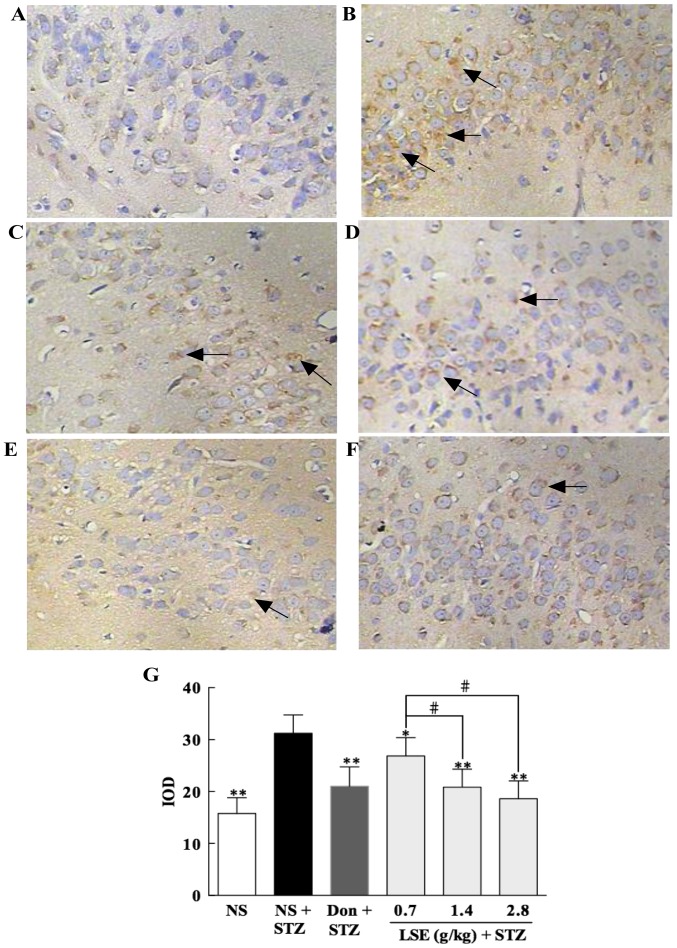
The intracellular accumulation of aggregated Tau protein is another histopathological hallmark of AD. Therefore, we investigated the effect of LSE on the expression of Tau protein in the hippocampus of T2DM rats with immunohistochemical staining under the microscope, and the representative photographs are illustrated in Fig. 9. The image of normal control rat treated with NS shows little brown particle deposition in the cytoplasm of neuronal cells (Fig. 9A); however, the image of the T2DM rat treated with NS indicates strong positive expression with the emergence of a large number of brown yellow granules (as denoted by arrows) in the cytoplasm of neuronal cells (Fig. 9B). Interestingly, the brown particle depositions in the cytoplasm of neuronal cells of the rats treated with donepezil (Fig. 9C); and LSE (0.7, 1.4 or 2.8 g/kg) (Fig. 9D–F) are significantly decreased compared to that of NS treatment, while the most profound reduction of brown particles in the cytoplasm of cells is observed with the rat treated with high dose of LSE (2.8 g/kg/day).
Figure 9.
Effect of LSE on the expression of Tau protein in the hippocampus of T2DM rats. The control rats were treated with NS (1 ml/kg/day, IG ×28), and the T2DM rats were treated with NS (0.2 ml/day, IG ×28), donepezil (0.42 mg/kg/day, IG ×28) or LSE (0.7, 1.4 and 2.8 g/kg/day, IG ×28). The brain tissues of rats were removed at 24 h after drug treatment (magnification, ×400). The representative histologic photographs are shown as: (A) Histological image of hippocampal neuronal cells of a control rat treated with NS, only little brown particle deposition in the cytoplasm of neuronal cells. (B) Hippocampal neuronal cells of a T2DM rat treated with NS, strong positive expression of Tau protein with the emergence of a large number of brown yellow granules (arrows) in the cytoplasm of neuronal cells. (C) Hippocampal neuronal cells of a T2DM rat treated with Don, brown particle depositions (arrows) in the cytoplasm of neuronal cells are significantly decreased compared to NS treatment. (D) Hippocampal neuronal cells of a T2DM rat treated with LSE (0.7 g/kg), brown particle depositions (arrows) in the cytoplasm of neuronal cells are significantly decreased compared to NS treatment. (E) Histologic picture of hippocampal neuronal cells of a T2DM rat treated with LSE (1.4 g/kg), brown particle depositions (arrow) in the cytoplasm of neuronal cells are significantly decreased compared with NS treatment. (F) Histologic picture of hippocampal neuronal cells of a T2DM rat treated with LSE (2.8 g/kg), brown particle depositions (arrow) in the cytoplasm of neuronal cells are also significantly decreased compared to NS treatment and the decrease is more obvious than those of the rats treated with donepezil and lower doses of LSE. (G) Quantitative results of bar graph for each group. Arrows indicate Tau protein as brown particle depositions. There were three rats for each group and results are presented as mean ± standard deviation. *P<0.05 vs. T2DM rats treated with NS; **P<0.01 vs. T2DM rats treated with NS; #P<0.01 vs. LSE 0.7 g/kg group. LSE, lychee seed extract; T2DM, type 2 diabetes mellitus; Don, donezapil; IOD, integrated option density; STZ, streptozotocin; NS, normal saline.
The summary results of the expression of Tau protein in the neuronal cells are presented in Fig. 9G. The data indicate that the positive expression of Tau protein in the neuronal cells in T2DM rats is significantly increased (P<0.01) compared to normal rats (control). Interestingly, the Tau protein in the neuronal cells is significantly decreased (P<0.05 or P<0.01) when the rats were treated with LSE or donepezil compared to that of NS treatment, and the most obvious decrease is observed with the group administered with LSE at a concentration of 2.8 g/kg. The results indicate that LSE and donepezil can effectively prevent the depositions of AGEs and Tau protein in the neuronal cells of the hippocampus of T2DM rats and high dose of LSE is more effective than that of donepezil.
Effect of LSE on neuronal injury protection in the CA1 area of hippocampus in T2DM rats by histopathological exanimation
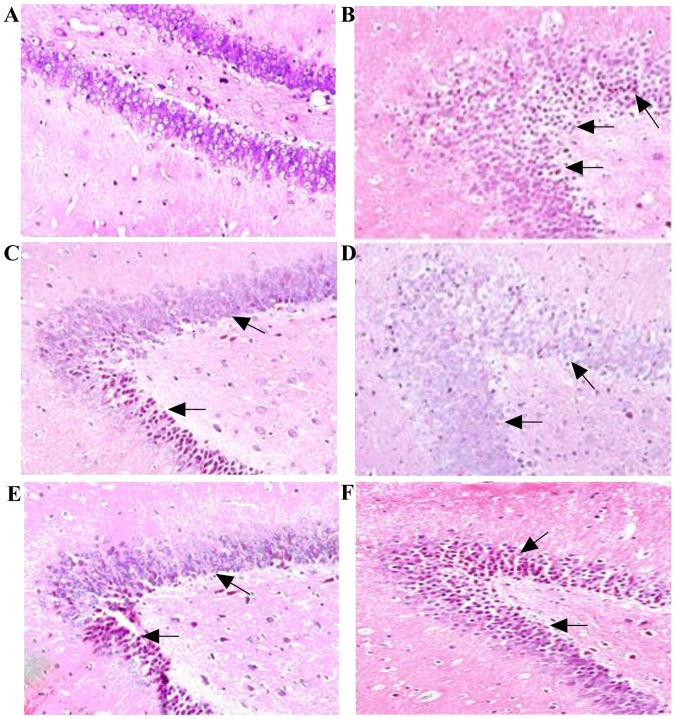
We further investigated the effect of LSE on neuronal injury protection and compared to that of donepezil in T2DM rats by histopathological examination and the results are illustrated in Fig. 10.
Figure 10.
Protective effect of LSE on the hippocampal neurons injury in T2DM rats. The representative photographs of the hippocampal neurons in the normal (control) and T2DM rats: The control rats were treated with NS (1 ml/kg/day) and T2DM rats were treated with NS (1 ml/kg/day), donepezil (0.42 mg/kg/day) or LSE (0.7, 1.4 and 2.8 g/kg/day) ×28 days with intragastric administration. The brain tissues of rats were removed at 24 h after NS or drug treatment. Conventional formalin fixed paraffin-embedded sections were stained with hematoxylin and eosin and observed under light microscope (magnification, ×200). The representative histologic photographs are shown as: (A) Histological image of hippocampal neurons of a control rat treated with NS, shows preserved normal histological features of neurons. (B) Hippocampal neurons of a T2DM rat treated with NS, shows severe damaged neurons. (C) Hippocampal neurons of a T2DM rat treated with donepezil, shows still damaged neurons but less severe than that of the T2DM rat treated with NS. (D) Hippocampal neurons of a T2DM rat treated with LSE 0.7 g/kg, shows near normal histological features of neurons. (E) Hippocampal neurons of a T2DM rat treated with LSE 1.4 g/kg, shows near normal histological features of neurons. (F) Hippocampal neurons of a T2DM rat treated with LSE 2.8 g/kg, shows normal histological features of neurons. Arrows indicate damaged hippocampal neurons with loose arrangement, cytoplasmic and nuclear condensation. There were three rats for each group. LSE, lychee seed extract; T2DM, type 2 diabetes mellitus; NS, normal saline.
The histological changes were observed in the sections of the neurons of the rats with H&E stain under a light microscope (magnification, ×200). The picture in Fig. 10A presents the characteristic of preserved normal histological features of the pyramidal cells in the CA1 area of hippocampus of the normal rat (control) treated with NS. The cells were closely arranged in order, the nucleus was clearly visible in large and round, and there was no obvious degeneration of neurons, such as nuclear condensation. However, as shows in Fig. 10B, the neuronal cells arranged in disorder and are sparse, cell morphology is irregular or spindle shaped and a large number of cells appeared shrieked volume and different layers of nuclear condensation in the T2DM rat treated with NS. However, the hippocampal neurons of the T2DM rats treated with donepezil are still damaged but much less severe than that of the T2DM rat treated with NS (Fig. 10C). Interestingly, the morphology of the neuronal cells is nearly (LSE, 0.7 and 1.4 g/kg) or completely normal (LSE, 2.8 g/kg) after LSE treatments, the cells arrange more closely and orderly with increased cell numbers and normal shape, a large and round nucleus, and a clear nucleolus (Fig. 10D–F).
The results indicated that LSE effectively protects the hippocampal neuronal cells injury in a dose-dependent manner in T2DM rats, and is more effective than donepezil.
Discussion
T2DM and AD are very common diseases. The rapid increases of T2DM and AD incidences in the elderly population have been observed. Evidence has proven that there is a close link between T2DM and AD, and that the two diseases share common biological mechanisms at a molecular level. T2DM can significantly contribute to the onset and/or progression of AD, and T2DM patients are at higher risk of AD (38). The common pathology for both T2DM and AD is IR, impaired glucose metabolism, Aβ formation, oxidative stress and presence of AGEs. IR may be the pathological basis of the link between T2DM and AD (39,40). IR promotes AD pathological changes of Aβ deposition and Tau protein phosphorylation and induces sustained high blood glucose to accelerate the formation of AGEs (41–44). Studies have indicated that abnormal structural components such as amyloid precursor (APP), apolipoprotein E (ApoE), very low density lipoprotein (VLDL), AD, Aβ and Tau protein are all related to the pathological changes of AD in the brain tissues (43–45). Aβ and Tau protein are the molecular basis for the formation of SPs and NFTs. IR indirectly enhances the activity of glycogen synthase kinase-3β (GSK-3β) and promotes the AD-like Tau hyperphosphorylation in the hippocampus of the mice and rats in animal models of insulin dysfunction (46). In addition, IR can regulate the activity of γ-secreting enzyme by GSK-3β (47), promote the synthesis of Aβ40 and Aβ42, inhibit the activity of insulin degrading enzyme and reduce the degradation of Aβ (48). Furthermore, insulin plays a pivotal role in regulation of energy metabolism, growth, survival and differentiation of neuronal cells via insulin signaling in the brain (49,50). Therefore, improving IR may be one of the important strategies for effective prevention and treatment of AD (51,52). Recent studies have demonstrated that improvement of IR may indeed delay the onset and/or progression of AD in the animals and patients (53,54). In the present studies, the blood glucose, insulin and HMOA are significantly increased in T2DM rats and LSE significantly decreases the elevated glucose, insulin and HMOA in a dose-dependent manner compared to NS treatment (P<0.01) in T2DM rats (Fig. 6). The data indicate that LSE can indeed improve IR in T2DM rats.
Currently, the drugs used for AD management can only improve cognition and behavior of AD patients, but cannot slow progression or cure the disease (22). In order to discover and develop more effective and less toxic novel anti-AD drugs, we established a rat model of T2DM with AD-like neurodegeneration and cognitive impairment by feeding the rats with HSFP diet for 8 weeks and then intraperitoneal injection of STZ (27 mg/kg) and continued HSFP diet for another 4 weeks. The rat model of T2DM was validated by high levels of blood glucose, insulin and HOMA as well as impaired spatial learning and memory of the rats (Fig. 3). The model has the characteristics and pathogenesis of both T2DM and AD. It is reliable, reproducible and easy to be produced and shows high resemblance as the T2DM and/or AD in the aging patients. Therefore, it is an excellent model for study of cognitive impairment and drug screening of T2DM and/or AD.
Natural products and medicinal herbs are important sources for drug discovery against various diseases including AD and T2DM (55). The use of drug substances derived from natural sources has a long tradition in medicine (56). In the present study, we evaluated the effect of LSE, which was isolated and extracted from lychee seeds on the anti-diabetic and anti-AD efficacy, and compared to donepezil, the standard drug for AD treatment. The main components of LSE were determined and it was found that is primarily consists of eight major ingredients and around 20 minor ingredients by a UHPLC-SPD chromatogram (Fig. 1). It has been reported that the chemical components of lychee seeds comprise volatile categories, saponins, flavonoids, organic, fatty, amino acids and sugar (7). The preliminary studies revealed that the saponins isolated and extracted from lychee seeds are the main effective ingredients for the anti-diabetic and anti-AD efficacy. In addition, it was demonstrated that LSE significantly improves cognitive function in T2DM rats with cognitive impairment for shortening the escape latency, increasing the number across the platform, platform quadrant dwell time and the percentage of time spent in the target quadrant compared to NS treatment (Figs. 4 and 5). Furthermore, LSE markedly alleviates neuronal injury in T2DM rats by morphological study (Fig. 10).
It has been reported that AD is associated with reduction of cholinergic activity of neurons (37). The dysfunction of cholinergic system is closely related to AD (21). Four of five FDA approved medications (donepezil, tacrine, rivastagmine and galantamine) for AD management are AChE inhibitors by reducing the rate of acetylcholine degradation, and thereby increase its concentration in the brain. AChE in the blood and the hippocampus of T2DM rats were investigated and the concentrations of AChE were significantly increased in the blood but significantly decreased in the hippocampus compared to that of normal control rats (Fig. 7A and B). The authors are unsure as to how to explain why the concentration of AChE is different in the blood and hippocampus in T2DM rats. Both LSE and donepezil significantly decreased the concentrations of AChE in the blood and increased the concentrations of AChE in the hippocampus compared to NS treatment (P<0.01) in T2DM rats (Fig. 7A and B).
Histopathological hallmarks of AD are deposition of Aβ plaques and formation of NFTs, which are primarily made up of aggregated Tau protein (57,58). The neurotoxic activities of Aβ include generation of reactive oxygen species (ROS), lipid peroxidation, calcium overload and eventually leading to neuronal death (59). The progressive accumulation of Tau protein leads to instability of the microtubular structure and results in loss of effective intracellular transport and neuronal death (60). AGEs also play an important role in the pathogenesis of both T2DM and AD. The present results showed that Aβ, Tau protein and AGEs in the hippocampus of T2DM rats were significantly increased compared to that of normal control rats (P<0.01). LSE can effectively prevent the depositions of Aβ, Tau protein and AGEs in the neuronal cells of hippocampus in T2DM rats and LSE is more effective than that of donepezil for reducing the formation of Aβ, Tau protein, and AGEs (Figs. 7D, 8 and 9). Therefore, LSE may be developed as the agent for the treatment of T2DM and/or AD. However, further studies are needed to find out and purify the active ingredient(s) from LSE and investigate the associated mechanistic action and molecular pathways of neuronal protection.
In conclusion, LSE mainly consists of eight major and around 20 minor ingredients, and significantly improves cognitive function such as the ability of spatial learning and memory and obviously protects from neuronal injury in T2DM rats with cognitive impairment through reducing blood glucose, improving IR and inhibiting the formation of Aβ, Tau protein and AGEs in the brain of rats. LSE is similar or even more effective for neuroprotection than that of donepezil, a classic drug for the treatment of AD. Therefore, LSE may be developed as the agent for the treatment of T2DM and/or AD clinically. However, further studies are required to find out the active contents of LSE and related long-term toxicity.
Acknowledgments
The present study was supported by grants from the Science and Technology Planning Project of Sichuan Province, China (grant nos. 2008SZ0050, 14JC0798 and LZ-LY-38), Science and Technology Program of Luzhou, Sichuan, China (grant nos. 14JC0056, 2015-S-43 and 2016LZXNYD-T03), Educational Commission of Sichuan Province, China (grant nos. 10ZA035, 15ZA0155 and 16ZA0187), Key Development Program of Southwest Medical University (grant nos. 2010ZD-010 and 2014QN-043) and the Distinguished Professor Research Startup Funding (S.C.) from Southwest Medical University (grant no. 2015-RCYJ0002). The authors would like to thank Professor Can Tang of the Department of Chinese Materia Medica, School of Pharmacy, Southwest Medical University (Luzhou, China) for authenticating the lychee seeds used in the studies.
Glossary
Abbreviations
- Aβ
β-amyloid
- AChE
acetylcholinesterase
- AD
Alzheimer's disease
- AGEs
advanced glycation end products
- APOE
apolipoprotein E
- APP
amyloid precursor
- ELISA
enzyme-linked immunosorbent assay
- GSK-3β
glycogen synthase kinase-3β
- H&E
hematoxylin and eosin
- HFSPD
high fat, high sugar, and high protein diet
- IG
intragastric
- IP
intraperitoneal
- IR
insulin resistance
- LSE
lychee seed extract
- NFTs
neurofibrillary tangles
- NS
normal saline
- ROS
reactive oxygen species
- SPs
senile plaques
- STZ
streptozotocin
- T2DM
type II diabetes mellitus
- UHPLC
ultrahigh performance liquid chromatography
- VLDL
very low density lipoprotein
References
- 1.Jiang YM, Yao L, Amon L, Li JR. Postharvest biology and technology of litchi fruit. J Food Agric Environ. 2003;1:76–81. [Google Scholar]
- 2.Yang B, Wang J, Zhao M, Liu Y, Wang W, Jiang Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr Res. 2006;341:634–638. doi: 10.1016/j.carres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Kilari EK, Putta S. Biological and phytopharmacological descriptions of Litchi chinensis. Pharmacogn Rev. 2016;10:60–65. doi: 10.4103/0973-7847.176548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao ZJ, Guo JW, Xu F. Effect of litchi saponin and litchi flavones on insulin resistance in HepG2 cells. J Pharm Pract. 2015;4:316–318. [Google Scholar]
- 5.Ye HM, Zhong CY, Lv JH. Improving effect of litchi seed saponins on learning and memory impairment in mice. Acad J Guangzhou Med Univ. 2015;2:10–14. [Google Scholar]
- 6.Zhang YM, Yuan H, Tian JX. Effects of saponin of litchi seed on gluconeogenesis and metabolism of blood lipid in mce. J Hangzhou Teach Coll. 2005;6:435–436. [Google Scholar]
- 7.Zhang J, Zhang C. Research progress on the antineoplastic pharmacological effects and mechanisms of Litchi seeds. Chin Med. 2015;6:20–26. doi: 10.4236/cm.2015.61003. [DOI] [Google Scholar]
- 8.Li W, Zhu YT, Huang ZY, He JJ, Pei J, Song JP. Experimental studies on anti-fluvirus effect of litchi seed in vivo. Chin J Ethnomed Ethnopharm. 2011;18:34–36. [Google Scholar]
- 9.Zhao M, Yang B, Wang J, Liu Y, Yu L, Jiang Y. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int Immunopharmacol. 2007;7:162–166. doi: 10.1016/j.intimp.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Xiao LY, Pan JQ, Rao WN. The research of protective effect of Litchi seed of experimental liver injury in mice. Chin J Tradit Chin Med Pharm. 2005;20:42–43. [Google Scholar]
- 11.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spellman CW. Pathophysiology of type 2 diabetes: Targeting islet cell dysfunction. J Am Osteopath Assoc. 2010;110(Suppl 2):S2–S7. [PubMed] [Google Scholar]
- 14.Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann NY Acad Sci. 2015;1353:60–71. doi: 10.1111/nyas.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura N. Diabetes mellitus induces Alzheimer's disease pathology: Histopathological evidence from animal models. Int J Mol Sci. 2016;17:503. doi: 10.3390/ijms17040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mushtaq G, Khan JA, Kamal MA. Biological mechanisms linking Alzheimer's disease and type-2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13:1192–1201. doi: 10.2174/1871527313666140917114537. [DOI] [PubMed] [Google Scholar]
- 17.Aliev G, Shahida K, Gan SH, Firoz C, Khan A, Abuzenadah AM, Kamal W, Kamal MA, Tan Y, Qu X, et al. Alzheimer disease and type 2 diabetes mellitus: The link to tyrosine hydroxylase and probable nutritional strategies. CNS Neurol Disord Drug Targets. 2014;13:467–477. doi: 10.2174/18715273113126660153. [DOI] [PubMed] [Google Scholar]
- 18.Riederer P, Bartl J, Laux G, Grünblatt E. Diabetes type II: A risk factor for depression-Parkinson-Alzheimer. Neurotox Res. 2011;19:253–265. doi: 10.1007/s12640-010-9203-1. [DOI] [PubMed] [Google Scholar]
- 19.Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63:2253–2261. doi: 10.2337/db14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer's disease. Trends Pharmacol Sci. 2002;23:288–293. doi: 10.1016/S0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 21.Mushtaq G, Greig NH, Khan JA, Kamal MA. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer's disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13:1432–1439. doi: 10.2174/1871527313666141023141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Jeong SK, Kim BC, Park KW, Dash A. Donepezil across the spectrum of Alzheimer's disease: Dose optimization and clinical relevance. Acta Neurol Scand. 2015;131:259–267. doi: 10.1111/ane.12386. [DOI] [PubMed] [Google Scholar]
- 23.Godyń J, Jończyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer's disease in clinical trials. Pharmacol Rep. 2016;68:127–138. doi: 10.1016/j.pharep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Lee HJ, Lee KW. Naturally occurring phytochemicals for the prevention of Alzheimer's disease. J Neurochem. 2010;112:1415–1430. doi: 10.1111/j.1471-4159.2009.06562.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang YJ, Liang BM. Determination of anti-diabete saponins from Litchi chinensis sonn. J Guangdong Pharm. 2004;14:13–15. [Google Scholar]
- 26.Spencer JP. Beyond antioxidants: The cellular and molecular interactions of flavonoids and how these underpin their actions on the brain. Proc Nutr Soc. 2010;69:244–260. doi: 10.1017/S0029665110000054. [DOI] [PubMed] [Google Scholar]
- 27.Ye HM, Zhong CY, Huang MX, Wang CY, Feng X, Chen XY, Lv JH. Effect of litchi seed aqueous extracts on learning and memory obstacles induced by D-galactose in mice and its mechanism. Zhong Yao Cai. 2013;36:438–440. In Chinese. [PubMed] [Google Scholar]
- 28.Barnhart CD, Yang D, Lein PJ. Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One. 2015;10:e0124521. doi: 10.1371/journal.pone.0124521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol. 2008;581:283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Ghiasi R, Ghadiri Soufi F, Somi MH, Mohaddes G, Mirzaie Bavil F, Naderi R, Alipour MR. Swim training improves HOMA-IR in type 2 diabetes induced by high fat diet and low dose of streptozotocin in male rats. Adv Pharm Bull. 2015;5:379–384. doi: 10.15171/apb.2015.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin- treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Liu C, Zhang XN. Comparison of the neuropsychological mechanisms of 2,6-diisopropylphenol and N-methyl-D-aspartate receptor antagonist against electroconvulsive therapy-induced learning and memory impairment in depressed rats. Mol Med Rep. 2015;12:3297–3308. doi: 10.3892/mmr.2015.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Luo Y, Feng A, Li T, Yang X, Nofech-Mozes R, Yu M, Wang C, Li Z, Yi F, et al. Ethanol induced impairment of glucose metabolism involves alterations of GABAergic signaling in pancreatic β-cells. Toxicology. 2014;326:44–52. doi: 10.1016/j.tox.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Ji O, Li JQ. The pharmacological effect of Puerarin extract on learning and memory ability of rats with Alzheimer disease. Pharm Clin Chin Mater Med. 2014;5:39–42. [Google Scholar]
- 37.Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer's disease. Drugs Today (Barc) 2003;39:75–83. doi: 10.1358/dot.2003.39.1.740206. [DOI] [PubMed] [Google Scholar]
- 38.Plum L, Belgardt BF, Brüning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease - is this type 3 diabetes. J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- 40.Narasimhan K, Govindasamy M, Gauthaman K, Kamal MA, Abuzenadeh AM, Al-Qahtani M, Kanagasabai R. Diabetes of the brain: Computational approaches and interventional strategies. CNS Neurol Disord Drug Targets. 2014;13:408–417. doi: 10.2174/18715273113126660156. [DOI] [PubMed] [Google Scholar]
- 41.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: Relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/WNL.50.1.164. [DOI] [PubMed] [Google Scholar]
- 42.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 43.Giacobini E, Gold G. Alzheimer disease therapy - moving from amyloid-β to tau. Nat Rev Neurol. 2013;9:677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 44.Ashraf GM, Greig NH, Khan TA, Hassan I, Tabrez S, Shakil S, Sheikh IA, Zaidi SK, Akram M, Jabir NR, et al. Protein misfolding and aggregation in Alzheimer's disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13:1280–1293. doi: 10.2174/1871527313666140917095514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deutsch SI, Rosse RB, Lakshman RM. Dysregulation of tau phosphorylation is a hypothesized point of convergence in the pathogenesis of alzheimer's disease, frontotemporal dementia and schizophrenia with therapeutic implications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1369–1380. doi: 10.1016/j.pnpbp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marszałek M. Diabetes type 2 and Alzheimer disease - one or two diseases? Mechanisms of association. Postepy Hig Med Dosw. 2013;67:653–671. doi: 10.5604/17322693.1059549. In Polish. [DOI] [PubMed] [Google Scholar]
- 48.El Khoury NB, Gratuze M, Papon MA, Bretteville A, Planel E. Insulin dysfunction and Tau pathology. Front Cell Neurosci. 2014;8:22. doi: 10.3389/fncel.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Wang R, Zhao Z, Ji Z, Xu S, Hölscher C, Sheng S. Coexistences of insulin signaling-related proteins and choline acetyltransferase in neurons. Brain Res. 2009;1249:237–243. doi: 10.1016/j.brainres.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 50.Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: An 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Tsui W, Rusinek H, Butler T, Mosconi L, Pirraglia E, Mozley D, Vallabhajosula S, Harada R, Furumoto S, et al. Cortical laminar binding of PET amyloid and tau tracers in Alzheimer disease. J Nucl Med. 2015;56:270–273. doi: 10.2967/jnumed.114.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, Khandelwal VK, Kemppainen S, Kaminska D, Mäkinen P, et al. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status. J Nutr Biochem. 2014;25:634–641. doi: 10.1016/j.jnutbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Hung AS, Liang Y, Chow TC, Tang HC, Wu SL, Wai MS, Yew DT. Mutated tau, amyloid and neuroinflammation in Alzheimer disease-A brief review. Prog Histochem Cytochem. 2016;51:1–8. doi: 10.1016/j.proghi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Anekonda TS, Reddy PH. Can herbs provide a new generation of drugs for treating Alzheimer's disease. Brain Res Brain Res Rev. 2005;50:361–376. doi: 10.1016/j.brainresrev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Farver D. The use of 'natural products' in clinical medicine. S D J Med. 1996;49:129–130. [PubMed] [Google Scholar]
- 57.Blass JP, Ko L, Wisniewski HM. Pathology of Alzheimer's disease. Psychiatr Clin North Am. 1991;14:397–420. [PubMed] [Google Scholar]
- 58.Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei W, Wang X, Kusiak JW. Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem. 2002;277:17649–17656. doi: 10.1074/jbc.M111704200. [DOI] [PubMed] [Google Scholar]
- 60.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]