Abstract
Background
Adjuvant stereotactic radiosurgery (SRS) alone after surgical resection is increasingly being used to provide excellent local control while avoiding the side effects of whole brain radiation therapy (WBRT). We report our ten year experience using this treatment scheme.
Purpose/Objectives
To determine the rates and any correlates of local control, distant brain failure, and overall survival using SRS alone to the resection cavity.
Materials/Methods
We performed a retrospective analysis of 509 patients with brain metastasis who underwent Gamma Knife SRS at our institution between 2003 and 2013. Of this group 85 patients were identified that had resection of the metastasis and subsequent SRS to the cavity. Mean dose to the resection cavity was 17.3 Gy (range 14-20) to an average volume of 12cc (range 0.3-83cc). Multiple patient, tumor, and treatment specific factors were collected for analysis (see Table 1). Vital statistics were provided by our institution’s tumor registry. The primary endpoint of our analyses was recurrence free survival (RFS); defined as the duration in time between the date of SRS and any local or distant brain tumor recurrence.
Results
With a median follow up of 16.4 months, the overall local and distant brain failure at 12 months was 13% (95%CI 5%-21%) and 51% (95%CI 37%-64%) respectively. RPA was class 1 (5%), 2 (75%), and 3 (20%). The median overall survival (OS) was 20 months. The median RFS was 24 months with radiosensitive tumors: non small cell lung cancer (n=12), breast (n=16), gastrointestinal (n=7), small cell lung cancer (n=1), and other (n=9) compared to 5.6 months (p=0.006) in radioresistant tumors: melanoma (n=33), sarcoma (n=1), and renal cell carcinoma (n=6). Median OS for radioresistant and radiosensitive patients was 12 vs 25 months respectively (p=0.11). Additionally, there was a significant improved survival difference seen amongst those who had a gross total resection (GTR, n=46) compared to a sub total resection (n=39) with median OS of 27 vs 16 months (p=0.020) respectively. Radiographic changes suggestive of radiation necrosis were present in 6 patients, 2 of which were determined histiopathologicaly after surgical intervention. Due to the limited number of local recurrence events (n=10), there was insufficient power to analyze prognostic factors for local recurrence.
Conclusions
Our results compare favorably with multiple other institution experiences showing excellent local control with SRS to the resection cavity following resection. Radioresistant histologies were associated with a worse RFS. Patients undergoing GTR had a significantly longer OS than those with STR. At our institution we continue to offer patients SRS to the resection cavity for those with good performance status and limited brain metastases.
Keywords: Stereotactic Radiosurgery, GammaKnife, Resection Cavity, Brain Metastases
1. INTRODUCTION
Proper management of brain metastases plays a critical role in preserving quality of life, and in select cases improving survival.1,2,3,4 Whole brain radiation therapy (WBRT) has long been the standard of care. However, SRS is increasingly being utilized in order to avoid the neurocognitive side effects of WBRT.5 In select cases, surgical resection is used to achieve optimal outcomes, alleviate side effects from mass effect or obstruction, or to establish diagnosis. After resection alone local recurrence may be as high as 46%.6 Adjuvant WBRT has helped to decrease this high local recurrence (LR) rate down to approximately 10%.7 Adjuvant SRS has been employed at various institutions to help control the local site while at the same time avoiding the neurocognitive side effects of WBRT. Regardless of the radiosurgical modality, high local control rates of 70-94% have been achieved, with salvage WBRT being required in less than half of the patients for distant brain failures.8 Various patient stratification scoring systems have been employed to help determine which patients are ideal for the utilization of SRS. The graded prognostic assessment (GPA) is one such tool, which not only provides an estimation of a patient’s life expectancy, but also gauges how appropriate SRS may be. A current randomized controlled trial by the North Central Cancer Treatment Group (NCCTG) is attempting to answer the question of whether adjuvant WBRT or SRS is preferred for treatment of the resection cavity. We add to the growing body of knowledge in the adjuvant stereotactic management of brain metastases using GammaKnife (GK), coupled with a patterns of failure analysis. Additionally, we report on various patient, tumor, and treatment related factors, and the implication that these may have on both recurrence and survival.
2. METHODS
We performed a retrospective analysis of 509 patients with brain metastasis who underwent GK SRS at the Keck Medical Center of the University of Southern California (USC) between 2003 and 2013. Of this group 85 patients were identified that had resection of the metastasis with subsequent stereotactic radiosurgery (SRS) to the cavity.
2.1 GammaKnife Procedure
The procedure begins with the Leksell Gamma Knife head frame secured to the patient’s head under conscious sedation. Subsequently, the patient is taken for MRI of the brain with T1 pre and post gadolinium contrast and T2 sequences. Detailed T1 post contrast images are acquired with 2mm thin cuts. The stereotactic MR images are then used for treatment planning on the Leksell GammaPlan system. The resection cavity with approximately 0 2mm margin as well as any other brain metastases are contoured and defined as the target volume. In all cases the target volume was completely covered by the prescription dose. The dose was chosen based on cavity size as well as tumor diagnosis according to the various physician preferences. However, more recently we have standardized the prescribed dose by utilizing the dosing scheme found in the N107C clinical trial.9
2.2 Data Collection
Study data were collected and managed using REDCap electronic data capture tools hosted at USC.10 Individual patient MRIs were reviewed when available to determine the location of the recurrence in relation to the initial treated volume. Conformality index (CI) was defined as the prescription volume / treated volume. In determining resection cavity size, multiple MRI sequences were reviewed in order to make scalar measurements in all 3 dimensions. To determine date of death for patients the university cancer registry was accessed. Univariate and multivariate analyses were performed to determine factors associated with tumor recurrence and death. Factors that were looked at in the multivariate analysis included: sex, histology, extent of resection, number of brain metastases, and cardiovascular disease. Additional factors looked at in univariate analysis that were not significant included: patient age (<50, 50-59, and ≥60), ethnicity, performance status (KPS ≥70 vs lower), glucose and Hgb levels on the day of the procedure. This review was preapproved by the institutions review board.
2.3 Follow up
Standard follow up for patients included a repeat MRI brain with and without gadolinium contrast 4-6 weeks post SRS. Patients who developed additional brain metastases were discussed at our multidisciplinary SRS tumor board and treated accordingly.
2.4 Statistical Methods
The primary endpoint in the analyses was recurrence-free survival (RFS). RFS was defined as the duration in time between date of radiation (SRS date) and date of tumor recurrence shown on MRI scans either in the local area or a distant location of the brain. The first follow up MRI was obtained on average within 3 months. Patients who did not have recurrence of tumor in the brain were censored at the date of the last MRI showing free of tumor. Given the limited number of patients with local recurrence in our data, the primary analyses of RFS treated any recurrence of tumor in the brain (local or distant) as “failure”.
12 of the 85 patients (14%) did not have follow-up MRI scans after radiation, and as a result, whether they had tumor recurrence in the brain was unknown. In the primary analyses of RFS, we excluded these 12 patients. Secondary analyses using two other approaches to handle the patients with no follow-up MRI scans were performed to evaluate the influence of excluding these patients on the analyses results, including 1) taking them all as “failure” at the 3rd month after SRS, or 2) taking them as “failure” at their date of death and censoring those who were alive 1 month after radiation. Results from these secondary analyses (unreported) gave very similar conclusions regarding RFS as our primary analyses.
A secondary endpoint in the analyses was overall survival (OS), defined as the duration in time between date of radiation and date of death, with patients who were alive censored at the last follow-up date. Two patients did not have any follow-up data after radiation. They were censored one month after radiation.
RFS and OS probabilities were calculated using the Kaplan-Meier method with Greenwood standard errors. Analyses of RFS and OS in relation to patients and disease characteristics were based on the univariate and multivariable Cox regression models11. The variables that were associated with RFS or OS at p≤0.20 in univariate analyses were then included in a multivariate Cox regression model of RFS or OS, and a backward stepwise model selection method was used to select a final model of RFS or OS by successively dropping non-significant variables from the model and re-fitting reduced models until all remaining variables were statistically significant at 0.1512. All analyses were performed in STATA13.
3. RESULTS
A total of 85 patients were identified. For inclusion patients had to have a surgical resection of at least one brain metastasis followed by SRS to the remaining cavity. SRS was delivered within an average of 25 days post resection (range 1-97 days). Patients and disease characteristics are shown in Table 1. Median age was 57 years with a range between 29 and 81 years old. Majority of patients had Karnofsky performance status greater than 70. The most common metastasis histology type treated was melanoma (39%, Table 1). Of breast cancer metastases half were Her2Neu positive. In total 52 (64%) patients had additional metastatic sites, which included bone, liver, lung, and adrenal, of which metastasis to the lungs was the most common (47%). The average dimensions (+/-SD) of the resected tumor were 2.7cm (+/- 0.83), 2.9cm (+/- 0.87), and 2.6cm (+/- 0.70) in the transverse, anterior/posterior, and cranial/caudal dimensions respectively; with an average cavity volume of 12cc (SD+/- 12cc). The most common location of treated resection cavity was the frontal lobe. Cavities were treated with a mean dose of 17.3Gy, (range 14 to 20 Gy) to a mean treatment volume of 12cc. The dose was prescribed to the 50% isodose line in the vast majority of cases. The mean number of shots delivered was 9.4 with a conformality index of 1.68 (range 1.28 to 3.92) (Table 2). Conformality index was not significantly associated with LR or RFS.
Table 1.
Patient Characteristics
| Variables | Total (N=85) | % |
|---|---|---|
| Age at Time of SRS (in years) | ||
| Mean (range) | 57 (29,81) | |
| <50 | 21 | 25% |
| 50-59 | 30 | 35% |
| ≤60 | 34 | 40% |
| Sex | ||
| Female | 40 | 47% |
| Male | 45 | 53% |
| KPS: median (range) | 80(50,100) | |
| Race/Ethnicity | ||
| White | 51 | 68% |
| Black of African American | 5 | 7% |
| Hispanic | 13 | 17% |
| Asian | 6 | 8% |
| Unknown | 10 | |
| Primary Disease Site | ||
| Radio-Resistant | ||
| Melanoma | 33 | 39% |
| Sarcoma | 1 | 1% |
| Renal | 6 | 7% |
| Radio-sensitive | ||
| NSCLC | 12 | 14% |
| Breast | 16 | 19% |
| GI | 7 | 8% |
| SCLC | 1 | 1% |
| Other | 9 | 11% |
| Number of Metastases | ||
| Mean (range) | 1.7 (1,8) | |
| Single | 59 | 69% |
| Multiple | 26 | 31% |
| Extent of Resection | ||
| GTR | 46 | 54% |
| STR | 39 | 46% |
| RPA Class | ||
| 1 | 4 | 5% |
| 2 | 64 | 75% |
| 3 | 17 | 20% |
| Cardiovascular Disease | ||
| No | 74 | 87% |
| Yes | 11 | 13% |
Table 2.
SRS Details
| SRS Details | Min | Max | Mean | StDev |
|---|---|---|---|---|
| Dose (Gy) | 14 | 20 | 17.3 | 2.56 |
| Treatment Volume (cc) | 0.69 | 83 | 12 | 12 |
| Number of Shots | 1 | 26 | 9.4 | 4.9 |
| Conformality Index | 1.28 | 3.92 | 1.68 | 0.42 |
3.1 Patterns of Failure (Local and Distant Brain Recurrence)
Of these 85 patients, 12 did not have any follow-up MRI scans, and as a result, whether they had tumor recurrence in the brain was unknown, and 8 of the 12 have died. Of the 73 patients who had follow-up MRI scans, a total of 42 developed tumor recurrence in the brain, which included 3 patients who developed local recurrence without distant recurrence, 7 patients who had both local recurrence and distant recurrence, and 32 patients who had distant brain recurrence without local recurrence. 31 patients did not have recurrence as of their last MRI scan. Median duration between date of radiation and date of last MRI scan was 152 days (range: 21~1575 days). The cumulative incidences of local recurrence and distant brain recurrence can be found in Figure 1.
Figure 1.
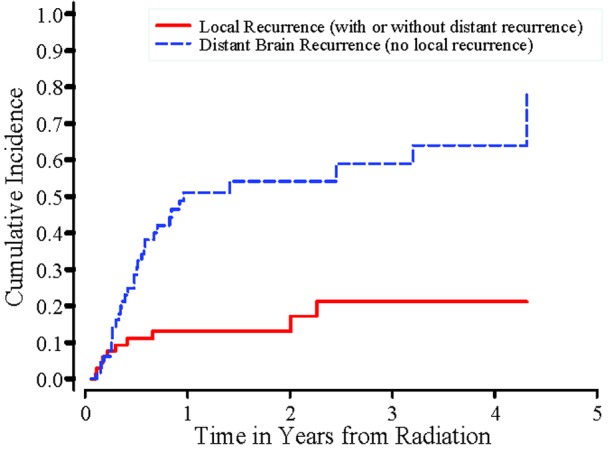
Cumulative Incidences of Local Recurrences and Distant Brain Recurrences
In the univariate analyses, a significant association was found between RFS and histology (radio-sensitive tumors vs. radio-resistant tumors) (Figure 2). Patients whose tumor was radio-sensitive had significantly better RFS than patients whose tumor was radio-resistant (p=0.006). There was also a statistically significant association between number of brain metastatic sites and RFS (p=0.049, Figure 3). Sex, extent of resection, cardiovascular disease and RPA class were not significantly associated with RFS. In the multivariable Cox regression analysis, after controlling for the other variables, there was a statistically significant difference in RFS between radio-sensitive and radio-resistant tumors (p=0.003). Extent of resection remained in the multivariate regression model of RFS, with a p value smaller than 0.15 (p=0.13). The remaining variables were not significantly associated with RFS in the multivariable analysis (p≤0.15).
Figure 2.
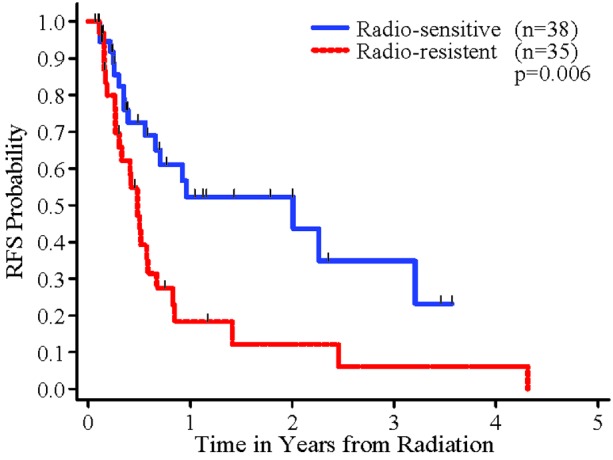
RFS for Radio-resistant and Radio-sensitive Tumors
Figure 3.
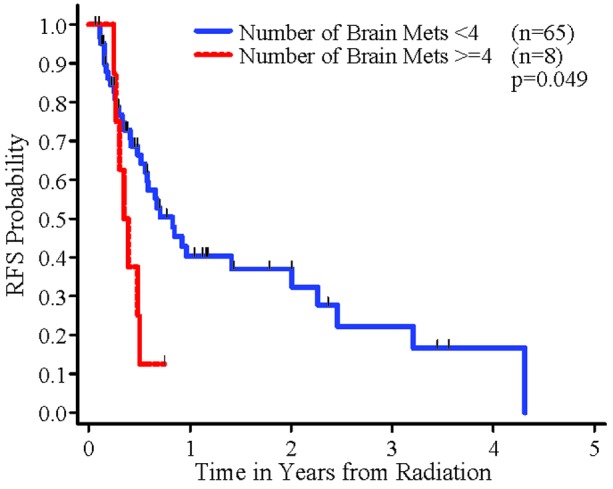
RFS by number of brain metastases
Analyses were also performed to examine whether there was an association between patients and disease characteristics and local recurrence. Due to the limited number of patients with local recurrence (n=10), those analyses did not have sufficient power and no significant association was found.
3.2 Overall Survival
Of the 85 patients, two patients did not have follow-up vital status data after radiation. Of the remaining patients, 52 died, and 31 were alive as of the last follow-up date with a median duration of follow-up of 498 days (range: 46~2311 days).
For OS, in the univariate analyses, radio-resistant tumors seemed to have worse OS than radio-sensitive tumors, but the difference did not reach the 0.05 significance level (p=0.12). Patients who got GTR had significantly better OS than patients who had STR (p=0.020, Figure 4). In addition, patients who had cardiovascular disease had significantly worse OS than patients who did not have cardiovascular disease (p=0.036). There was a marginally significant difference between number of brain metastatic sites and OS (p=0.091). Sex and RPA class were not significantly associated with OS. In the multivariable analysis, histology (radio sensitive vs. radio resistant tumors), extent of resection and cardiovascular disease were independently significantly associated with OS (p=0.032, 0.012, 0.019, respectively). The remaining variables were not significantly associated with OS in the multivariable analysis (p>0.15).
Figure 4.
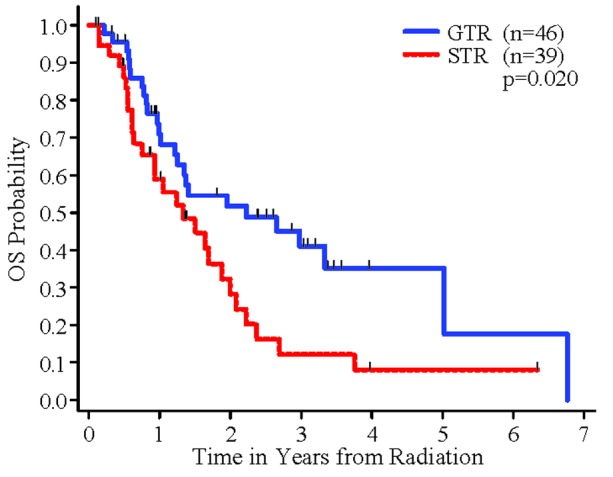
OS by Extent of Resection
Other variables that were examined in the RFS and OS analyses included age, KPS, preoperative maximum tumor dimension, HTN, diabetes, dyslipidemia, and CVD. We were interested in exploring any association between vasculopathy risk factors and an increased risk of failures because of hypoxia, or toxicities due to radiation necrosis or treatment effect. No evidence was found for an association between these variables and LR, RFS or OS.
3.3 Local Recurrence
Analyses were also performed to examine whether there was an association between patients and disease characteristics and local recurrence. Due to the limited number of patients with local recurrence (n=10), those analyses did not have sufficient power and no significant association was found. Of note, among the patients who had local recurrence four had a STR (66%).
3.4 Salvage Therapy
There were only three patients with an isolated local recurrence, one was treated with additional SRS and the other two with surgery. Among the 7 patients who had a local failure connected with distant failure, repeat SRS was utilized in 5, and WBRT in the other two. Isolated distant brain recurrence was found in 32 patients which was managed with additional SRS in 22 patients and WBRT in 7, one with surgery and two patients received no salvage treatment. Overall, salvage surgery was done in three patients (3.5%), and salvage WBRT in 9 patients (10.6%).
4. DISCUSSION
Our outcomes in treating the resection cavity with SRS add to the growing body of evidence that shows excellent control of the index lesion. In our series of 85 patients there was a crude local control rate of 88% observed. This agrees favorably with other institutions which have reported on local controls in the range of 70-94%.14,15,16 Despite the efficacy of this modality in treating the resection cavity, we experienced a single melanoma case that grew immediately after SRS without any interval of response. This demonstrates that at times the biology plays a predominant role in influencing outcomes.
4.1 Radiation Necrosis
One of the side effects of SRS includes radiation necrosis (RN). In a minority of patients, radiation related changes suggestive of RN may be seen.17 It is often difficult to distinguish RN from tumor progression (TP). Both may happen at the same time period, although TP may tend to occur earlier, and RN tends to occur later. Both can manifest with worsening appearance on MR imaging as well as clinical worsening. There are multiple techniques that are employed to help differentiate RN from TR including perfusion, permeability, cerebral blood volume, and MR spectroscopy, but none is definitive. To truly differentiate between the two clinical scenarios there must be histopathologic review of the area in question. There were 6 patients who had follow up imaging with radiographic findings suggestive of radiation necrosis. Two of which were symptomatic, who underwent surgery which provided pathologic confirmation of necrosis. (Table 3)
Table 3.
Patients with radiographic findings suggestive of RN. CI = conformality index
| Age at time of SRS | Prescription Volume | Method of Confirmation | CI | Dose | Extent of Resection | Radiographic Follow-Up | Date of SRS | Radiographic Details | Symptomatic |
|---|---|---|---|---|---|---|---|---|---|
| 47 | 10.48 | Radiographic | 1.58 | 18 | STR | 1/21/2014 | 11/27/2012 | low rCBV, enhancement | no |
| 75 | 17.5 | Radiographic | 1.6 | 15 | GTR | 1/28/2014 | 12/1/2011 | worsening flair, enhancement, low rCBV | no |
| 47 | 13 | Histologic | 1.56 | 16 | STR | 11/11/2010 | 1/12/2010 | enlarged cavity with fluid level, feathery peripheral enhancement ** | yes |
| 63 | 16.9 | Radiographic | 1.54 | 18 | GTR | 8/31/2010 | 7/22/2008 | peripheral enhancement, FLAIR changes | no |
| 62 | 12.5 | Radiographic | 1.76 | 15 | GTR | 5/6/2010 | 3/4/2008 | feathery rim enhancement | no |
| 65 | 10.3 | Histologic | 1.354 | 17 | GTR | 1/25/2014 | 10/8/2013 | stable blood volume, worsening edema, increased thickness of rim enhancement | yes |
As an index case, a 66 year old male diagnosed 4 years prior with melanoma developed a single brain metastasis in the left parietal lobe. He underwent craniotomy and gross total resection of the mass. On postoperative day 20 he underwent SRS to the resection cavity in order to improve local control. A dose of 17 Gy to the 50% isodose line was delivered with 11 shots to the cavity with a 2mm margin. The target volume was 10.3 mL with a prescription isodose volume of 14.0 mL, which yielded a conformality index of 1.35. The resection bed measured 2.3cm x 2.3 cm x 2.6 cm in the transverse (TR), anterior/posterior (AP), and cranial caudal (CC) dimensions respectively. At his 2 month follow up MRI there was noted to be increased T2 signal indicating worsening vasogenic edema. Also seen was increased thickening of the nodular enhancement along the resection cavity on post contrast MRI scan. Perfusion and permeability studies were performed showing findings more concerning for radiation necrosis or tumor effect rather than tumor progression. During this period of worsening radiographic appearance, he also had clinical deterioration with increased right sided weakness. He underwent maximal safe resection 15 weeks after GK SRS. Histopathalogic review revealed recurrent S-100 positive spindle cell neoplasm with 30% necrosis.
This case demonstrates the possible complexity that may arise following resection and SRS of brain metastases. There may be treatment related effects, RN, TP, or any combination of the three. Common imaging findings of RN include a feather like enhancement pattern with or without worsening edema in the setting of normal or decreased blood flow. Typical management of symptomatic radiographic worsening includes a trial of steroids and short interval follow up imaging. If symptoms and imaging improve then treatment related effects, or RN is presumed to be the etiology. However, if symptoms persist, then further medical management with bevacizumab and eventually surgery is recommended for both treatment and diagnosis.18
4.2 Gross Total Resection
An interesting finding was that a GTR before SRS to the resection cavity was associated with a statistically significant improvement in median overall survival from 16 to 27 months.
We looked at possible confounders, including age, KPS, preoperative maximum tumor dimension, HTN, diabetes, dyslipidemia, CVD. Of the 10 local recurrences that we experienced 6 had a STR and 4 had a GTR, which may explain the difference in survival. However, the number of local recurrences experienced were too few to show statistical significance. As in the treatment of other CNS tumors, the surgeon should attempt where possible a maximal safe resection. Our study gives further substance to this recommendation.
4.3 Number of Brain Metastases
A surrogate for anywhere in the brain failure was 4 or more brain metastases. Patients with this number of metastases had a statistically significant increased risk of developing new brain metastases on follow up. However, in our study there were only 8 patients treated that had 4 or more brain metastases. In a separate larger study looking at number of brain metastases at the time of SRS as a risk factor for recurrence, there was no significant difference in the rate of distant brain failure seen between <4 and 4 or more treated metastases.19
4.4 Prognostic Indices
With each case of brain metastases the physician must decide the optimal treatment approach. Patients with longer life expectancy and more favorable outcomes will benefit more from treatments that aim to control the disease with minimal long term side effects. At our institution we employ various prognostic indices to help with this decision. The diagnosis specific GPA further aids in selecting patients who are ideal candidates for aggressive local therapy.20
5. CONCLUSIONS
Our results compare favorably with multiple other institution experiences showing excellent local control with SRS to the resection cavity following resection. Radioresistant histologies were associated with a worse RFS. Patients undergoing GTR had a statistically significant increased OS than those with STR. At our institution we continue to offer patients SRS to the resection cavity for those with good performance status and limited brain metastases.
6. FUTURE DIRECTIONS
The N107C clinical trial which randomizes patients after resection to WBRT or SRS is ongoing, and it is hoped that it can be completed. This will provide us with prospective level 1 data to guide adjuvant therapy and management of the postoperative brain metastasis patient.
7. ACKNOWLEDGEMENTS
Special thanks for the guidance provided by Susan Groshen of the statistics department. Also, we thank the tumor registry staff of Norris Comprehensive Cancer Center for the access they provided to vital statistics.
REFERENCES
- 1. Andrews DW., Scott CB., Sperduto PW., Flanders AE., Gaspar LE., Schell MC., Werner-Wasik M., Demas W., Ryu J., Bahary JP., Souhami L., Rotman M., Mehta MP., Curran WJ., Jr. Whole brain radiation therapy with and without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet, 2004; 363: 1665-1672. [DOI] [PubMed] [Google Scholar]
- 2. Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys, 1994; 29: 711-717. [DOI] [PubMed] [Google Scholar]
- 3. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann Neurol, 1993; 33: 583-590. [DOI] [PubMed] [Google Scholar]
- 4. Patchell RA., Tibbs PA., Walsh JW., Dempsey RJ., Maruyama Y., Kryscio RJ., Markesbery WR., Macdonald JS., Young B. A randomized trial of surgery in the treatment of single metastasis to the brain. N Engl J Med, 1990; 322: 494-500. [DOI] [PubMed] [Google Scholar]
- 5. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomized controlled trial. Lancet Oncol, 2009; 10(11): 1037–1044. [DOI] [PubMed] [Google Scholar]
- 6. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA, 1998; 280: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 7. Kocher M., Soffietti R., Abacioglu U., Villà S., Fauchon F., Baumert BG., Fariselli L., Tzuk-Shina T., Kortmann RD., Carrie C., Ben Hassel M., Kouri M., Valeinis E., van den Berge D., Collette S., Collette L., Mueller RP. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol, 2011; 29: 134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rwigema JC., Wegner RE., Mintz AH., Paravati AJ., Burton SA., Ozhasoglu C., Heron DE. Stereotactic Radiosurgery to the Resection Cavity of Brain Metastases: A Retrospective Analysis and Literature Review. Stereot Funct Neuros, 2011; 89: 329-337. [DOI] [PubMed] [Google Scholar]
- 9. Brown P. (2014) A Phase III Trial of Post-Surgical Stereotactic Radiosurgery (SRS) Compared With Whole Brain Radiotherapy (WBRT) for Resected Metastatic Brain Disease: N107C. Retrieved from https://clinicaltrials.gov/ct2/show/NCT01372774?term=n107c&rank=1
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009; 42(2): 377-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cox DR., Oakes D. (1984). Analysisof survival data. London; New York: Chapman and Hall. [Google Scholar]
- 12. Hocking R. The analysis and selection of variables in linear regression. Biometrics. 1976; 32: 1-49 [Google Scholar]
- 13.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009 [Google Scholar]
- 14. Hartford AC., Paravati AJ., Spire WJ., Li Z., Jarvis LA., Fadul CE., Rhodes CH., Erkmen K., Friedman J., Gladstone DJ., Hug EB., Roberts DW., Simmons NE. Postoperative Stereotactic Radiosurgery Without Whole-Brain Radiation Therapy for Brain Metastases: Potential Role of Preoperative Tumor Size. Int J Radiat Oncol Biol Phys, 2013; 85(3): 650-655 [DOI] [PubMed] [Google Scholar]
- 15. Robbins JR., Ryu S, Kalkanis S., Cogan C., Rock J., Movsas B., Kim JH., Rosenblum M. Radiosurgery to the Surgical Cavity as Adjuvant Therapy for Resected Brain Metastasis. Neurosurgery, 2012; 71(5): 937-43 [DOI] [PubMed] [Google Scholar]
- 16. Atalar B1., Choi CY., Harsh G., 4th, Chang SD., Gibbs IC., Adler JR., Soltys SG. Cavity Volume Dynamics After Resection of Brain Metastases and Timing of Post Resection Cavity Stereotactic Radiosurgery. Neurourgery, 2013;,72(2), 180-185. [DOI] [PubMed] [Google Scholar]
- 17. Hoefnagels FWA., Lagerwaard FJ., Sanchez E., Haasbeek CJA., Knol DL., Slotman BJ., Vandertop WP. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol, 2009; 256: 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stockham AL., Ahluwalia M., Reddy CA., Suh JH., Kumar A., Vogelbaum MA., Barnett GH., Murphy ES., Chao ST. Results of a questionnaire regarding practice patterns for the diagnosis and treatment of intracranial radiation necrosis after SRS. J Neuro-Oncol, 2013; 115(3): 469-475. [DOI] [PubMed] [Google Scholar]
- 19. Likhacheva A., Pinnix C., Parikh N., Allen P., McAleer M., Chiu M., Sulman E., Mahajan A., Guha-Thakurta N., Prabhu S., Cahill D., Luo D., Shiu A., Brown P., Chang E. Predictors of Survival in Contemporary Practice After Initial Radiosurgery for Brain Metastases. Int J Radiat Oncol Biol Phys, 2013; 85(3): 656-661. [DOI] [PubMed] [Google Scholar]
- 20. Sperduto PW., Chao ST., Sneed PK., Luo X., Suh J., Roberge D., Bhatt A., Jensen AW., Brown PD., Shih H., Kirkpatrick J., Schwer A., Gaspar LE., Fiveash JB., Chiang V., Knisely J., Sperduto CM., Mehta M. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4259 patients. Int J Radiat Oncol Biol Phys, 2010; 77: 655-661. [DOI] [PubMed] [Google Scholar]


