Abstract
Introduction
The advent of new radiosurgical technology has enabled the treatment of patients with larger numbers of brain metastases. At what point the whole brain doses delivered in single fraction radiosurgical treatment begin to approximate a fraction of whole brain radiotherapy is unknown. Measuring the equivalent whole-brain dose for multiple lesions may yield useful clinical insights.
Methods
Twelve representative patients were chosen from our institutional database, having undergone radiosurgery for multiple lesions. All patients were treated using the Leksell Gamma Knife Perfexion radiosurgical platform. The entire brain was contoured and mean whole brain dose (MWBD) was calculated. For each plan, all enhancing tumors were outlined and total volume of tumors recorded.
Results
A total of 33 treatments were administered. Three patients underwent radiosurgery a single time, one patient on two occasions, four patients on three occasions, and four patients on four occasions. Median number of lesions treated was 8 (range 1-41). Dose ranged from 18-22 Gy per lesion. The median MWBD per treatment was 1.4 Gy (range 0.2-7.6 Gy). Median total tumor volume was 4.3 cm3 (range 0.048 cm3–21.72 cm3). No patients with less than 25 lesions treated had a MWBD greater than 4 Gy. Total tumor volume was also associated with greater MWBD, albeit with a greater number of outliers.
Conclusion
Both tumor number and volume were associated with increased MWBD. Patients with greater than 25 lesions treated per session or cumulative tumor volumes greater than 10 cm3 had MWBD’s greater than 4 Gy. Furthermore, tumor location and proximity to vital structures are equally important to consider, highlighting the need for individualized treatment planning.
Keywords: Multiple brain metastases, dosimetry, whole-brain radiation therapy, stereotactic radiosurgery, Gamma Knife, tumor volume
1 Introduction
The traditional paradigm for the treatment of a patient with brain metastases dictates that Stereotactic Radiosurgery (SRS) should be used to treat patients with 1-4 metastases, whereas patients with multiple metastases beyond that are best managed with whole brain radiation therapy (WBRT). This has been established through multiple randomized-controlled trials [1-3, 8], which found that the use of SRS alone in patients with 1-4 brain metastases, followed by frequent serial surveillance as well as potential salvage therapies, was a reasonable clinical strategy. Yet, new radiosurgical technology now allows for the treatment of multiple brain metastases with greater ease. As a result, more radiosurgery centers are treating patients with larger and larger numbers of brain metastases [7]. This has been generally supported by studies that have found survival was not affected by number of metastases, but rather by other factors such as age, Karnofsky Performance Status, etc [4, 9].
However, regardless of the clinical reasoning leading to the decision to treat or not treat with SRS, there likely is a point at which the number of brain metastases treated with stereotactic radiosurgery results in a significant dose of radiation to the whole brain. Yet, SRS treatment approaching whole-brain doses would likely present with fewer neurocognitive symptoms compared to WBRT as SRS occurs during a single session, while WBRT involves multiple sessions over many weeks. Advocates of SRS emphasize a comparative lack of neurocognitive decline compared to WBRT [3]. Though we believe that there may be a point at which the whole brain doses of radiosurgical treatment of many brain metastases will approach that of whole brain radiotherapy, radiosurgery may still be neurologically safer even when doses approach those seen in a single whole brain radiotherapy treatment. However, it is still important to investigate at what point SRS treatment approaches whole-brain doses to gain a better understanding of the physical dosimetric limits of radiosurgery in relation to WBRT, a modality whose limits and toxicities have been well studied. Therefore, we measured the dose to the whole brain delivered for 12 representative patients with multiple brain metastases treated at our institution. We hope that this study sheds light on the upper limits of dose that the brain can receive in single-session therapy when high numbers of brain metastases are treated with radiosurgery.
2 Methods
Twelve representative patients were chosen from our institutional database, having undergone radiosurgery for multiple lesions, sometimes on multiple occasions. Patients were arbitrarily chosen to provide a range of lesion numbers, target sizes, and doses. As this study does not investigate clinical outcomes and only examines the physical dose delivered to the brain, the patients were chosen for the physical number of lesions and the dose delivered. All patients were treated using the Leksell Gamma Knife Perfexion 10.1 radiosurgical platform, and all treatment plans were constructed using Leksell GammaPlan 10.1. The entire brain volume was contoured on the treatment planning MRI and mean whole brain dose (MWBD) was calculated. For each plan, all enhancing tumors were outlined and total volume of tumors within that single radiosurgery session was recorded.
3 Results
Three patients underwent radiosurgery once, one patient underwent two treatments, four patients underwent three treatments, and four patients underwent treatment on four occasions, for a total of 33 treatments. The several radiosurgical sessions were discrete treatment sessions that occurred on different days. Time courses for radiosurgical sessions from the first treatment to the last ranged from 3 months to 3 years. Our patient cohort consisted of 4 males and 8 females with ages ranging from 33 to 85. Cancer types treated included breast, bladder, prostate, melanoma, and non-small-cell lung cancer. 7 patients had a prior history of WBRT before SRS treatment for their metastatic disease. 10 out of 12 patients had been on previous or concurrent chemotherapy, immunotherapy, or other pharmacotherapy. The median number of lesions treated per session was 8 (range 1-41). The dose ranged from 18-22 Gy per lesion. The MWBD per treatment was 1.4 Gy (range 0.2-7.6 Gy) and median total tumor volume was 4.3 cm3 (range 0.048 cm3 – 21.72 cm3).
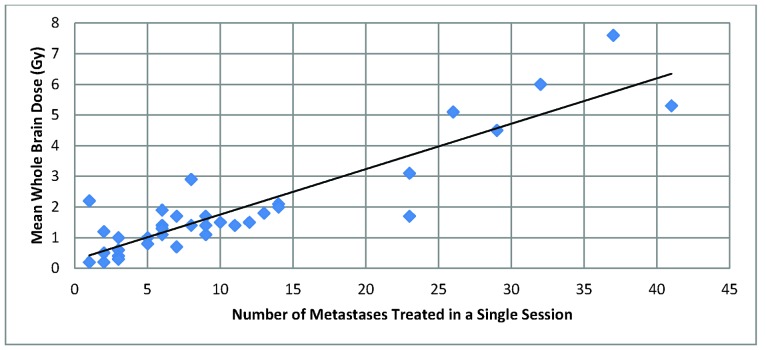
Overall, there was a positive correlation between number of metastases and MWBD. No patients with less than 20 lesions treated had a MWBD greater than 3 Gy, and no patients with less than 25 lesions treated had a MWBD greater than 4 Gy. The highest MWBD were seen for patients with 29, 41, 26, 32, and 37 lesions treated, with MWBD of 4.5, 5.3, 5.1, 6.0, and 7.6 Gy, respectively (See Figure 1).
Figure 1.
Number of metastases treated vs. Mean Whole Brain Dose. There is an overall positive trend between number of metastases treated and MWBD. No patients with less than 20 lesions treated had a MWBD greater than 3 Gy, and no patients with less than 25 lesions treated had a MWBD greater than 4 Gy.
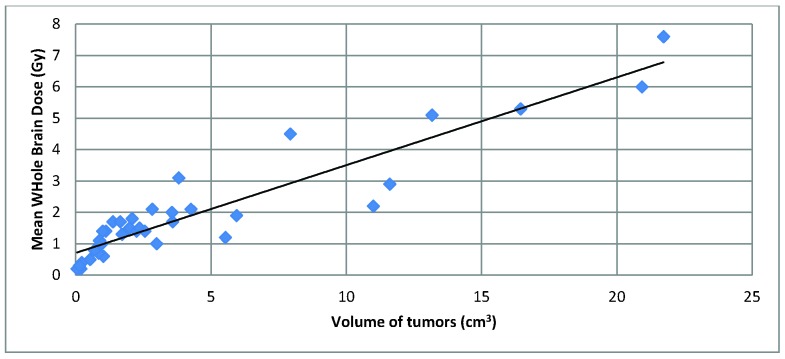
Total tumor volume was also associated with greater MWBD, although with some outliers (See Figure 2). One patient with a MWBD greater than 4 Gy had a lower overall tumor volume (7.9 cm3, all tumors treated to 20 Gy) compared to two patients with less than 3 Gy MWBD (11.6 cm3 treated to 18 Gy with a MWBD of 2.9 Gy, and 11.0 cm3 treated to 18 Gy with a MWBD of 2.2 Gy).
Figure 2.
Volume of tumors vs. Mean Whole Brain Dose. The volume of tumors is the cumulative tumor volume of a patient in a single radiosurgical treatment session. There is an overall positive trend between volume of tumors and MWBD, although with a few outliers.
4 Discussion
There appeared to be a positive correlation between number of metastases and MWBD, and between tumor volume and MWBD. The MWBD of 3 Gy was used for comparison as this is the “standard” dose typically given for one fraction of whole brain radiotherapy[4]. In particular, our data show that mean whole brain doses greater than 3 Gy are seen in patients with more than 20 brain metastases, and greater than 4 Gy per fraction if more than 25 brain lesions would exceed this level. Whole-brain doses greater than 4 Gy are also seen in patients whose cumulative tumor volume is greater than 10 cm3. In our opinion, these are reasonable potential upper limits in the number of brain lesions treated in a single session.
Our data correlating tumor volume with MWBD revealed an overall positive trend with a few outliers. Indeed, it is important to consider tumor volume in addition to lesion number, as treating 25 small tumors versus 25 larger lesions would lead to a higher MWBD. In contrast, a single large brain metastasis may instead be an indication for microsurgery as opposed to SRS.
The results of our study are consistent with the findings from other investigators studying cumulative WBRT dose toxicity of radiosurgery. Yamamoto et al. reported on 80 patients with 10 or more brain metastases (range 10-43) [10]. They found that the median cumulative dose to the whole brain was 4.71 (range: 2.16-8.51) Gy, which is consistent with our dose ranges. Yang et al. reported an in-silico study that identified 25 brain metastases as a possible theoretical maximum number of metastases that could be treated with radiosurgery in a single session, keeping the maximum point doses for each metastasis <40 Gy and the dose to 50% of the brain <5.0 Gy [11]. That these researchers have posted similar safety parameters as those mentioned in our study suggests that 25 lesions and a MWBD of 4 Gy can be both used as clinical benchmarks for theoretically safe administration of radiosurgery to the brain. This is also consistent with other studies that suggest, for whole brain radiation, treatment fractions greater than 3 Gy may increase the risk for neurotoxicity [5].
It is important to note that while 25 brain metastases may be a benchmark for the number of treatable lesions in a single session, it may be possible to safely treat more than 25 metastases for two reasons. First, we believe that the total cumulative dose delivered to the brain in one session is overall safe for normal tissue and may underrepresent the actual threshold of safety for radiation dose. This is because SRS is delivered in a single-session, while WBRT involves repeatedly exposing the brain to radiation over a course of 10-15 sessions. Therefore, the dosing guidelines established for WBRT single-session doses may not necessarily apply to SRS. In fact, it is possible that clinicians may be able to safely exceed the parameters of 25 metastases or 4 Gy in a single session without approaching the high levels of radiation seen in WBRT. Secondly, it may be possible to treat greater than 25 lesions with a MWBD lower than 4 Gy if a lower dose is prescribed to the tumors, which may be possible for more radiosensitive tumors, such as small cell lung tumors. Therefore, the radiosensitivity of the primary tumor may modify our recommendation. However, it still behooves providers utilizing SRS to be aware of the equivalent whole-brain dose when formulating their treatment plan.
The limitations of this study include the lack of a clinical correlate for safe WBRT doses and the true value of mean WBRT dose. Our study cannot specify at which point the WBRT dose begins to have any adverse neurocognitive impact. However, our study shows that generally the number of brain metastases is positively correlated with WBRT dose, and that after a certain number of metastases, the MWBD is equivalent to a single fraction of WBRT. Ideally, future studies would include a prospective trial to compare patients with less than 25 metastases to patients with greater than 25 metastases to assess for neurocognitive impairment and survival. Further limiting our analysis is the relatively small number of patients included compared to our prior analyses of patients with brain metastases [9]. However, we were attempting to provide a representative set of patients, rather than an exhaustive review of our database.
Limiting the interpretation of MWBD is the relatively inhomogeneous dose distribution of radiotherapy that occurs with SRS compared to whole brain radiotherapy. Focally high tumor dose is important for tumor control in SRS. Given the difference in radiation dose distribution, it is not clear whether a MWBD of 3 Gy or 4Gy is equivalent to a whole brain dose of radiotherapy of similar dose.Therefore, the location of local minimum and maximum dose may have a greater impact on neurotoxicity. However, the MWBD can serve as an initial benchmark for physicians and suggest a physical limit to radiosurgical technology in the relative sparing of normal brain tissue from radiation when treating multiple brain metastases.
5 Conclusion
Patients with greater than 25 lesions treated per session, using prescription doses of 18-22 Gy, had MWBD’s greater than 4 Gy. Though new radiosurgical technologies have allowed for the expedited treatment of patients with multiple metastases, there are physical limitations to the relative sparing of normal brain tissue from radiation as the number of lesions treated per session increases. Also, while tumor number and volume may serve as proxies for measuring whole-brain dose, the position of the individual tumors and their relation to normal brain structures may be even more important, underscoring the need for careful individual assessment and decision-making during treatment. Further study is needed to assess the neurocognitive sequellae of such treatment.
References
- 1. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363:1665-1672 [DOI] [PubMed] [Google Scholar]
- 2. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006; 295:2483-2491 [DOI] [PubMed] [Google Scholar]
- 3. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10:1037-1044 [DOI] [PubMed] [Google Scholar]
- 4. Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, Morris RE, Ammirati M, Andrews DW, Asher AL, Cobbs CS, Kondziolka D, Linskey ME, Loeffler JS, McDermott M, Mikkelsen T, Olson JJ, Paleologos NA, Ryken TC, Kalkanis SN. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010; 96:17-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist 2007; 12:884-898 [DOI] [PubMed] [Google Scholar]
- 6. Jairam V, Chiang VLS, Yu JB, Knisely JPS. Role of stereotactic radiosurgery in patients with more than four brain metastases. CNS Oncology 2013; 2:181-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knisely JP, Yamamoto M, Gross CP, Castrucci WA, Jokura H, Chiang VL. Radiosurgery alone for 5 or more brain metastases: expert opinion survey. J Neurosurg 2010; 113(Suppl):84-89 [DOI] [PubMed] [Google Scholar]
- 8. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999; 45:427-434 [DOI] [PubMed] [Google Scholar]
- 9. Raldow AC, Chiang VL, Knisely JP, Yu JB. Survival and Intracranial Control of Patients With 5 or More Brain Metastases Treated With Gamma Knife Stereotactic Radiosurgery. J Am Clin Oncol 2012; 14:14. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto M, Ide M, Nishio S, Urakawa Y. Gamma Knife radiosurgery for numerous brain metastases: is this a safe treatment? Int J Radiat Oncol Biol Phys 2002; 53:1279-1283 [DOI] [PubMed] [Google Scholar]
- 11. Yang CC, Ting J, X Wu, Markoe A. Dose volume histogram analysis of the gamma knife radiosurgery treating twenty-five metastatic intracranial tumors. Stereotact Funct Neurosurg 1998; 1(Suppl):41-49 [DOI] [PubMed] [Google Scholar]