Abstract
Objectives
Up to 25% of patients with stage I non-small cell lung cancer (NSCLC) are considered high-risk for surgery, due to severe medical comorbidity and/or poor pulmonary reserve. Many of these patients are treated with stereotactic body radiotherapy (SBRT). Prognosis in this subgroup of patients is difficult to determine. We investigated the association of impaired heart rate recovery (HRR) with survival in patients who received SBRT for treatment of early-stage lung cancer.
Methods
We collected data from consecutive patients who, between October 2009 and December 2012, received SBRT for treatment of lung cancer at the Cleveland Clinic, and had 6-minute walk test (6MWT) followed by HRR evaluation performed within six months of initiation of treatment. Impaired HRR was defined as a ≤ 12 beat decrease within the first minute following the 6MWT. Survival analyses were performed using Kaplan-Meier estimates and Cox proportional hazard ratios.
Results
Forty nine patients who received SBRT for treatment of early-stage lung cancer had HRR data available. Thirty two (65%) patients had impaired HRR following the 6MWT. In univariable and multivariable Cox regression analyses, impaired HRR was associated with poorer survival (HR: 11.0, 95% CI: 1.42 – 84.4, p = 0.004, and HR: 15.8, 95% CI: 1.96 – 128.0, p = 0.010, respectively). The 2-year overall survival rates were 52.6% for those with impaired HRR, and 94.1% for those with normal HRR.
Conclusion
Impaired HRR was associated with poorer survival in patients who received SBRT for treatment of early-stage lung cancer. HRR following the 6MWT can be one of the factors considered in patient selection for treatment with SBRT, along with other medical comorbidities.
Keywords: Stereotactic body stereotactic body radiotherapy, early-stage lung cancer, heart rate recovery, prognosis
1. INTRODUCTION
Surgery is the best curative treatment option for patients with early-stage lung cancer [1]. However, up to 25% of patients with stage I non-small cell lung cancer (NSCLC) are considered high-risk for lobectomy due to severe medical comorbidity and/or poor pulmonary reserve [2]. Stereotactic body radiotherapy (SBRT) is an effective alternative [3]. It involves a highly precise and accurate delivery of conformal radiation to small-volume targets and offers more aggressive dose intensification than can be safely achieved with conventional fractionated radiation therapy [4]. SBRT is the recommended treatment for patients who cannot tolerate lobectomy or segmentectomy for treatment of stage I NSCLC [2]. Despite treatment, the prognosis of patients receiving SBRT is poorer than those receiving surgery for early-stage lung cancer, primarily attributed to co-existing medical conditions. The 5-year overall survival (OS) rate is 25-45%, compared to 65-80% for those receiving lobectomies for stage I NSCLC [2].
While the introduction of SBRT is associated with a decrease in the number of stage I NSCLC patients who receive no treatment and an improvement in OS in the population of patients receiving radiotherapy [5], the high rate of underlying comorbidities in this population is the prime driver for non-cancer mortality. Better stratification of the competing risks in this patient population could improve patient selection and allocation of resources, however is quite challenging on an individual patient basis. Previous investigations examining pulmonary function test criteria alone have not demonstrated a particular cut-off value to guide patient selection [6-8], with the patients with the worst pulmonary function actually having improved survival in the Indiana University and Cleveland Clinic series. This is likely a reflection on the complex nature of the comorbidities in this select patient population, in which patients without significant pulmonary comorbidities likely have been deemed medically inoperable for other causes.
Heart rate recovery (HRR) measures autonomic activity, and is traditionally thought to reflect one’s physical fitness. It is obtained following a period of sustained activity, in this case the six-minute walk test (6MWT). In this present study, we investigated the association of HRR with survival. We hypothesized that a functional measurement such as HRR may be a better predictor of patient function than a less physiological measured variable such as pulmonary function testing alone, and that HRR may be a better tool to assist in pre-treatment patient selection and stratification.
2 METHODS
This study was approved by the Cleveland Clinic Institutional Review Board. We retrospectively reviewed consecutive patients who, between October 2009 and December 2012, received SBRT for treatment of early-stage lung cancer at the Cleveland Clinic, and had HRR evaluation following 6MWTs within 6 months of SBRT initiation. Data collected included variables previously shown to be associated with survival in patients with lung cancer: age [9], gender, smoking status [10], Karnofsky performance status (KPS) score [11], Charlson comorbidity index (CCI) [12], histologic subtype [13], and clinical stage. Tumor and treatment-related data included tumor size, location, positron emission tomography standardized uptake value (PET-SUV), and total radiation dose and number of fractions delivered. The primary outcome was OS.
Pretreatment pulmonary evaluation was performed according to the American College of Chest Physicians guideline for the physiologic evaluation of the lung cancer patient being considered for surgical resection [14], beginning with forced expiratory volume in 1 second (FEV1) and diffusion capacity of carbon monoxide (DLCO). In our practice, 6MWTs were frequently used in place of shuttle walk tests and stair climb test for patients being considered for curative treatment. All tests were done according to standards suggested by the American Thoracic Society Pulmonary Function Standards Committee [15], modified to include HRR evaluation. 6MWTs began after patients have been seated for 10 minutes. Pulse oximeters (Ohmeda Biox 3740 or Ohmeda 3900, Datex-Ohmeda Inc., Laurel, MD) with finger probes were used to obtain heart rates. HRR was defined as the difference between the heart rate at the end of the 6MWT and 1 minute after its completion, with the patient in the seated position. Impaired HRR was defined as a ≤ 12 beat decrease in heart rate as suggested by previous literature [16]. Other measurements obtained from the 6MWT included blood pressure (BP), distance walked during the 6MWT (6MWD), oxygen saturation, and Borg dyspnea score (BDS).
The decision to offer SBRT for early-stage lung cancer treatment was made by a multidisciplinary tumor board following appropriate clinical assessment. SBRT was performed according to the American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guidelines [17]. Patients were treated on a Novalis (BrainLAB, Feldkirchen, Germany) platform with the ExacTrac (BrainLAB) stereotactic body system for image guidance at the time of delivery. SBRT was delivered in doses 30-60 Gy, in 1-5 fractions. Doses were selected by the treating radiation oncologist using a risk-adapted or a trial-based approach. Survival time was defined as the difference (in months) between the date of treatment initiation and either the date of death or date last-known alive, documented in notes from clinic visits or through telephone contact.
2.1 Statistical analysis
Descriptive statistics for all continuous variables were summarized as means and standard deviations, and for categorical variables, counts and percentages. Student t-tests and Pearson’s chi-squared tests were used to assess potential differences in prognostic variables between the study sample and other patients receiving SBRT within the same time period. The Kaplan-Meier method was used to estimate OS. Survival analyses were performed using univariable and multivariable Cox proportional hazards regressions. Variables were considered for multivariable analyses if they have a p value of < 0.1 in univariable analyses. Correlation analyses were performed on all variables included in the multivariable analyses. Independent variables were retained in the model if their AICs were ≤ 0.05.
All analyses were two-tailed. Statistical significance was defined as p < 0.05. Hazard ratios (HRs) and 95% confidence intervals (CIs) were used whenever appropriate. SAS 9.3 (SAS Institute, Cary, NC), R software, and JMP Pro (SAS Institute, Cary, NC) were used for analyses.
3 RESULTS
A total of 438 patients were treated with SBRT between October 2009 and December 2012. Forty nine patients underwent SBRT for treatment of early-stage lung cancer and had 6MWTs and HRR evaluation prior to treatment; compared to the other 389 patients who received SBRT in the same time frame, there was no difference in age, gender, race, tobacco exposure, CCI, KPS, tumor size, tumor PET-SUV, histology, and clinical staging (E-Table 1).
E-Table 1.
Comparison of prognostic variables*
| Clinical variable | Study cohort | Comparative cohort | p-value |
|---|---|---|---|
| Age | 70.3 (8.7) | 73.8 (9.7) | 0.99 |
| Female, n (%) | 24 (49.0) | 218 (56.0) | 0.35 |
| Race, n (%) | 0.77 | ||
| African American | 9 (18.4) | 44 (12.8) | |
| Caucasian | 39 (79.6) | 305 (84.3) | |
| Pack-years | 50.0 (28.7) | 56.3 (39.8) | 0.23 |
| CCI, n (%) | 0.69 | ||
| 0-2 | 26 (60.5) | 212 (54.3) | |
| 3-6 | 17 (39.5) | 177 (45.4) | |
| KPS | 81.2 (9.3) | 78.1 (10.0) | 0.06 |
| Tumor size, cm | 2.8 (1.4) | 2.5 (1.3) | 0.12 |
| SUV | 10.4 (7.3) | 9.5 (6.8) | 0.17 |
| FEV1, % predicted | 59.8 (20.0) | 65.7 (56.8) | 0.90 |
| DLCO, % predicted | 44.5 (18.3) | 55.1 (19.8) | 1.00 |
| Histology, n (%) | 0.27 | ||
| Adenocarcinoma | 17 (34.7) | 96 (25.1) | |
| Squamous cell carcinoma | 13 (26.5) | 111 (29.0) | |
| Small cell carcinoma | 2 (4.1) | 7 (1.8) | |
| Non-diagnostic/never-biopsied | 17 (34.7) | 131 (34.2) | |
| Clinical stage | 0.62 | ||
| Ia | 37 (75.5) | 268 (68.9) | |
| Ib | 8 (16.3) | 90 (23.1) | |
| IIa | 4 (8.2) | 21 (5.4) |
6MWD = 6-minute walk distance; 6MWT = 6-minute walk test; BDS = Borg dyspnea score; BMI = body-mass index; CCI = Charlson comorbidity index; DBP = diastolic blood pressure; DLCO = diffusion capacity of carbon monoxide; FEV1 = forced expiratory volume within 1 second; HR = heart rate; HRR = heart rate recovery; KPS = Karnofsky performance status; LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe; SBP = systolic blood pressure; SUV = standardized uptake value
The 49 patients in the study cohort were compared to 389 other patients receiving SBRT within the same time period for early-stage lung cancer.
Values are expressed as means and standards of deviations, unless specified
Among the 49 patients, 48 (98%) patients were medically inoperable due to pulmonary (34 patients, 69%), cardiac (8 patients, 16%), or other co-morbidities (6 patients, 12%). One patient was medically operable but refused surgical treatment (2%). Patients had a mean age of 70 years, the majority of whom were current or former smokers (98%), with CCIs 0-2 (61%), and clinical stage I disease (92%); 4 patients (8%) had T2b tumors (Table 1). The mean tumor size was 2.8 cm. The majority of patients were treated with 50 Gy in 5 fractions or 60 Gy in 3 fractions (74%).
Table 1.
Patient demographics
| Patient characteristics | Value |
|---|---|
| Age | 70.3 (8.7) |
| Gender, n (%) | 24 (49.0) |
| Female | |
| Race, n (%) | |
| African American | 9 (18.4) |
| Caucasian | 39 (79.6) |
| BMI, kg/m2 | 26.7 (6.1) |
| Smoking status, n (%) | |
| Current smoker | 15 (30.6) |
| Former smoker | 33 (67.3) |
| Pack-years | 50.0 (28.7) |
| CCI, n (%) | |
| 0-2 | 26 (60.5) |
| 3-6 | 17 (39.5) |
| Tumor size, cm | 2.8 (1.4) |
| SUV | 10.4 (7.3) |
| FEV1, % predicted | 59.8 (20.0) |
| DLCO, % predicted | 44.5 (18.3) |
| Tumor location, n (%) | |
| RUL | 12 (26.1) |
| RML | 3 (6.5) |
| RLL | 6 (13.0) |
| LUL | 21 (45.7) |
| LLL | 4 (8.7) |
| Central location, n (%) | 14 (28.6) |
| Cardiac involvement, n (%) | 2 (4.1) |
| Histology, n (%) | |
| Adenocarcinoma | 17 (34.7) |
| Squamous cell carcinoma | 13 (26.5) |
| Small cell carcinoma | 2 (4.1) |
| Non-diagnostic/never-biopsied | 17 (34.7) |
| Clinical stage, n (%) | |
| IA | 37 (75.5) |
| IB | 8 (16.3) |
| IIA | 4 (8.2) |
| KPS | 81.2 (9.3) |
| Radiation dose, n (%) | |
| 30 Gy | 6 (12.2) |
| 34 Gy | 3 (6.1) |
| 48 Gy | 4 (8.2) |
| 50 Gy | 24 (49.0) |
| 60 Gy | 12 (24.5) |
| Fractions, n (%) | |
| 1 | 9 (18.8) |
| 3 | 7 (14.6) |
| 4 | 5 (10.4) |
| 5 | 27 (56.2) |
| Local failure, n (%) | 2 (4.1) |
6MWD = 6-minute walk distance; 6MWT = 6-minute walk test; BDS = Borg dyspnea score; BMI = body-mass index; CCI = Charlson comorbidity index; DBP = diastolic blood pressure; DLCO = diffusion capacity of carbon monoxide; FEV1 = forced expiratory volume within 1 second; HR = heart rate; HRR = heart rate recovery; KPS = Karnofsky performance status; LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe; SBP = systolic blood pressure; SUV = standardized uptake value
A total of 32 (65%) patients had impaired HRR following the 6MWT (Table 2). Patients had a mean systolic blood pressure (SBP) of 134 mmHg and diastolic blood pressure (DBP) of 75 mmHg prior to 6MWT; their respective values after 6MWTs were 155 mmHg and 81 mmHg. Oxygen saturation decreased by 2.4% on average during the test. The mean 6MWD was 63% predicted and BDS was 3.1. Fifteen (31%) patients were deceased at the end of data collection. Of those who died, 14 (93%) had impaired HRR.
Table 2.
6MWT measurements
| Measurements | Value |
|---|---|
| Blood pressure, mmHg | |
| Pre-6MWT SBP | 134.5 (19.2) |
| Pre-6MWT DBP | 74.9 (9.6) |
| Post-6MWT SBP | 155.4 (23.4) |
| Post-6MWT DBP | 81.2 (11.8) |
| Change in SBP | +21.0 (21.9) |
| Change in DBP | +6.3 (11.2) |
| Oxygen saturation, % | |
| Pre-6MWT | 95.3 (2.7) |
| During 6MWT | 92.9 (3.2) |
| Post 6MWT | 95.3 (3.6) |
| Distance | |
| 6MWD, ft | 963.6 (256.6) |
| 6MWD, % predicted | 63.3 (18.7) |
| BDS | 3.1 (1.9) |
| Heart rate, beats/min | |
| Resting HR | 81.2 (14.4) |
| 6MWT HR | 105.4 (16.0) |
| HR-1min | 94.1 (15.6) |
| HRR | -11.3 (8.7) |
| Impaired HRR, n (%) | 32 (65.3) |
Values are expressed as means and standards of deviations, unless specified
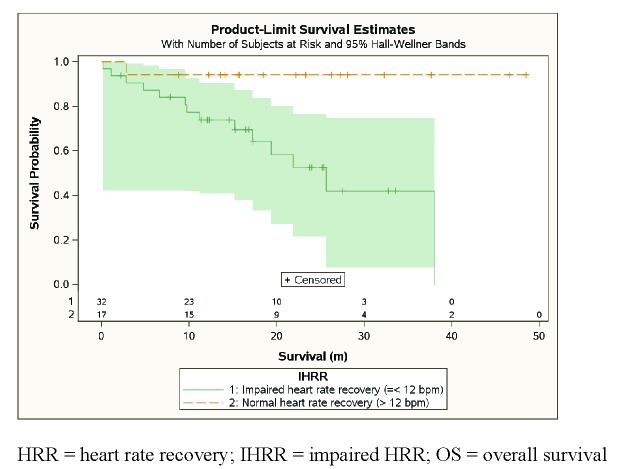
In univariable Cox regression analysis, impaired HRR was significantly associated with an increased risk of death (HR 11.0, CI 1.42 – 84.4, p = 0.004) (Table 3, Figure 1). Among those with impaired HRR, the 2-year OS rate and median survival time (MST) were 52.6% and 21.8 months, respectively. Among those with normal HRR, the 2-year OS rate was 94.1%; only one patient died (the survival time was 2.8 months). For the entire cohort, the 2-year OS rate was 67.3%, median follow-up 15.6 months, and MST 25.7 months. Other variables which had statistically significant associations with survival included smoking status (p = 0.005), post-6MWT DBP (p = 0.014), and BDS (p = 0.018).
Table 3.
Univariable survival analyses
| Variable | HR (95% CI) | p value |
|---|---|---|
| Age | 1.02 (0.96, 1.08) | 0.51 |
| Gender Female: male |
1.24 (0.44, 3.51) | 0.69 |
| Race Not Caucasian: Caucasian |
0.88 (0.25, 3.14) | 0.85 |
| BMI | 0.91 (0.82, 1.02) | 0.11 |
| Smoking status | 0.005 | |
| Former: current | 1.53 (0.42, 5.58) | |
| Never: current | 21.0 (1.76, 251) | |
| Pack years | 0.99 (0.97, 1.01) | 0.38 |
| CCI 3-6: CCI 0-2 | 1.28 (0.45, 3.60) | 0.65 |
| Tumor size | 1.33 (0.99, 1.78) | 0.050 |
| SUV | 0.98 (0.91, 1.07) | 0.66 |
| FEV1, % predicted | 1.01 (0.99, 1.03) | 0.37 |
| DLCO, % predicted | 0.98 (0.95, 1.02) | 0.38 |
| Histology | 0.95 | |
| Squamous: adenocarcinoma | 1.08 (0.29, 4.02) | |
| Small cell: adenocarcinoma | 1.80 (0.21, 15.6) | |
| Clinical stage | 0.093 | |
| IB: IA | 1.19 (0.32, 4.45) | |
| IIA: IA | 3.93 (1.04, 14.8) | |
| KPS | 0.95 (0.91, 1.00) | 0.055 |
| Local failure | 1.04 (0.12, 8.77) | 0.97 |
| Total dose | 0.98 (0.93, 1.03) | 0.47 |
| Fractions | 1.12 (0.83, 1.52) | 0.45 |
| Pre-6MWT SBP | 1.00 (0.97, 1.02) | 0.77 |
| Pre-6MWT DBP | 0.96 (0.91, 1.01) | 0.15 |
| Post-6MWT SBP | 0.98 (0.95, 1.00) | 0.10 |
| Post-6MWT DBP | 0.94 (0.90, 0.99) | 0.014 |
| Resting HR | 0.98 (0.94, 1.02) | 0.35 |
| 6MWD | 0.999 (0.997, 1.001) | 0.50 |
| 6MWD, % pred | 1.00 (0.97, 1.03) | 0.88 |
| BDS | 1.33 (1.04, 1.69) | 0.018 |
| HR during 6MWT | 0.98 (0.94, 1.01) | 0.19 |
| HR-1 min after 6MWT | 1.00 (0.96, 1.03) | 0.85 |
| HRR | 1.08 (0.998, 1.16) | 0.054 |
| Impaired HRR | 11.0 (1.42, 84.4) | 0.004 |
CI = confidence interval; HR = hazard ratio; 6MWD = 6-minute walk distance; 6MWT = 6-minute walk test; BDS = Borg dyspnea score; BMI = body-mass index; CCI = Charlson comorbidity index; DBP = diastolic blood pressure; DLCO = diffusion capacity of carbon monoxide; FEV1 = forced expiratory volume within 1 second; HR = heart rate; HRR = heart rate recovery; KPS = Karnofsky performance status; LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe; SBP = systolic blood pressure; SUV = standardized uptake value
Figure 1.
Kaplan-Meier estimates of HRR and OS
In multivariable analyses (MVA), which included all variables whose p-values were < 0.10 from univariable analyses (additionally included tumor size, KPS, and clinical stage), impaired HRR was significantly associated with an increased risk of death (HR 15.8, CI 1.96 – 128.0, p = 0.010). Post-6MWT DBPs and BDSs were also significantly associated with mortality (Table 4). No other variables were statistically significant in MVA.
Table 4.
Statistically significant variables from multivariable survival analyses
| Variable | HR (95% CI) | p value |
|---|---|---|
| Post 6MWT DBP | 0.93 (0.88, 0.97) | 0.002 |
| BDS | 1.36 (1.07, 1.73) | 0.013 |
| Impaired HRR | 15.8 (1.96, 128.0) | 0.010 |
CI = confidence interval; HR = hazard ratio; 6MWD = 6-minute walk distance; 6MWT = 6-minute walk test; BDS = Borg dyspnea score; BMI = body-mass index; CCI = Charlson comorbidity index; DBP = diastolic blood pressure; DLCO = diffusion capacity of carbon monoxide; FEV1 = forced expiratory volume within 1 second; HR = heart rate; HRR = heart rate recovery; KPS = Karnofsky performance status; LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe; SBP = systolic blood pressure; SUV = standardized uptake value
DISCUSSIONS
In this study, we investigated the association between HRR and survival in patients receiving SBRT for treatment of early-stage lung cancer. We found that impaired HRR was significantly associated with worse survival in this group. Our cohort of patients had similar age, tumor histology, and clinical stage compared to other series on patients with medically inoperable, early-stage lung cancer treated with SBRT [3, 18-21]. The MST of 26 months and 2-year OS rate of 67% were also comparable with outcomes from published series on SBRT patients.
Heart rate recovery is traditionally thought to reflect an individual’s physical fitness. Impaired HRR has previously been shown to be predictive of mortality independent of coronary artery disease [16, 22], in those with chronic heart failure [23], and in recent years, in those with idiopathic pulmonary fibrosis (IPF) [24]. To the best of our knowledge, our study is the first to describe an association between impaired HRR and mortality in patients with early-stage lung cancer receiving SBRT.
The decision to treat medically inoperable, early-stage lung cancer patients can be challenging. Patients who are too ill to benefit from aggressive treatment should be identified to avoid unnecessary treatment and treatment-related harms. It has been difficult to identify on an individual basis from the group of medically inoperable patients as a whole. There exist only a few prognostic variables in this select group of patients. HRR is a new variable with prognostic value that can be used for risk-stratification in this group of patients.
Impaired HRR, however, does not appear to portend a prognosis poor enough to withhold treatment. In our series, patients with impaired HRR has a MST of 22 months and 2-year OS rate of 53%, better than outcomes for untreated patients (the same rates for those with untreated stage I NSCLC are ~ 9-14 months and 20-30% [25], and among those ≥ 75 years of age, 15-20% [5]). This comparison should be made with caution as our patient cohort only included those who had thorough clinical evaluation, including staging, and deemed healthy enough to undergo curative treatment. While impaired HRR alone might not drive patient selection, it is possible that a more strict definition than ≤ 12 beats per minute might improve the predictive performance, or that HRR in combination with other predictive factors could lead to a more robust selection model.
The BDSs and post-6MWT DBPs were also associated with survival in our cohort of patients. Dyspnea, including as defined by the BDS, has been associated with mortality in patients with pulmonary disease in previous studies [26-28]. Results from our multivariable survival analysis suggested that for every 1 point increase in the BDS, there is a 36% increased relative risk of death. This variable, therefore, can also be useful in predicting mortality in this patient group. No previous studies, however, have reported post exercise BP as a predictor of mortality. Post exercise BP is partly modulated by parasympathetic activity, the same mechanism by which HRR is modulated. A higher post-6WMT DBP could therefore, theoretically increase the risk of death, through the same mechanism by which impaired HRR predicts mortality. Our results suggested that higher post-6MWT DBP is associated with a decreased risk of death (and not an increased risk as suggested by the proposed mechanism). Other BP measurements, including post-6MWT SBP and SBP, and changes in SBP and DPB following the 6MWT, did not predict mortality.
Other 6MWT variables which have been implicated with survival in patients with underlying pulmonary diseases include oxygen saturation/desaturation [29-31] and 6MWD [32-35]. These variables were not found to be associated with survival in our study, possibly due to the small sample size. Clinical variables previously known to be associated with survival in lung cancer patients include age, gender, KPS, CCI, and clinical stage. These variables did not have a statistically significant association with survival in our series, again, possibly due to our small sample size.
The 6MWT is a simple and relatively low-cost test in which HRR can be readily measured. Performance of a 6MWT is more common in many pulmonary function labs than those suggested by current guidelines (i.e. the shuttle walk test or formalized stair climbing test) [14]. We propose that the 6MWT, coupled with HRR evaluation, is another variable which can be incorporated into the physiologic evaluation of patients with lung cancer being considered for SBRT. We plan to develop a risk prediction model which incorporates HRR, other 6MWT variables, and previously identified clinical prognostic variables in a clinical algorithm to guide treatment decision-making. Such model can include patient-related factors (e.g. CCI), lung lesion characteristics (e.g. PET-SUV), and patient fitness (e.g. 6MWT variables).
Our study is limited by its retrospective nature. The small sample size makes it difficult to gauge the actual hazard ratio for impaired HRR and death. While helpful in predicting survival, we did not include quality of life, another important evaluation in the pretreatment assessment of patients with lung cancer. It is unclear if impaired HRR predicts mortality in other lung cancer patients, including those who are candidates for surgical treatment, and those who are unable to undergo 6MWT due to poor fitness. Our study has several strengths. We included many of the variables previously known to be associated with survival in the lung cancer patient population, including in the population receiving SBRT. We tested for potential differences in prognostic variables between our patient cohort and other patients treated with SBRT in the same time period and found no difference in these variables, which allowed us to generalize our findings to other SBRT patients. Survival data were collected and maintained by our own department, and therefore, are highly accurate compared to data obtained from public databases such as the social security death index.
We conclude that impaired HRR is associated with worse survival in patients with early-stage lung cancer who are treated with SBRT.
Abbreviations
6MWT = 6-minute walk test; 6MWD = 6-minute walk distance; AIC = Akaike Information Criterion; BDS = Borg dyspnea score; BP = blood pressure; CCI = Charlson comorbidity index; CI = confidence interval; DBP = diastolic blood pressure; DLCO = diffusion capacity of carbon monoxide; FEV1 = forced expiratory volume in 1 second; HR = hazard ratio; HRR = heart rate recovery; IPF = idiopathic pulmonary fibrosis; KPS = Karnofsky performance status; MST = median survival time; MVA = multivariable analysis; NSCLC = non-small cell lung cancer; OS = overall survival; PET-SUV = positron emission tomography standardized uptake value; SBP = systolic blood pressure; SBRT = stereotactic body radiotherapy
References
- 1. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e278S-313S. [DOI] [PubMed] [Google Scholar]
- 2. Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012; 142: 1620-35. [DOI] [PubMed] [Google Scholar]
- 3. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol 2010; 95: 32-40. [DOI] [PubMed] [Google Scholar]
- 5. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010; 28: 5153-9. [DOI] [PubMed] [Google Scholar]
- 6. Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J Thorac Oncol 2012; 7: 542-51. [DOI] [PubMed] [Google Scholar]
- 7. Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 72: 404-9. [DOI] [PubMed] [Google Scholar]
- 8. Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009; 4: 838-44. [DOI] [PubMed] [Google Scholar]
- 9. SH Ou, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients : a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007; 110: 1532-41. [DOI] [PubMed] [Google Scholar]
- 10. Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009; 88: 362,70; discussion 370-1. [DOI] [PubMed] [Google Scholar]
- 11. Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2002; 52: 1047-57. [DOI] [PubMed] [Google Scholar]
- 12. Kopek N, Paludan M, Petersen J, Hansen AT, Grau C, Hoyer M. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol 2009; 93: 402-7. [DOI] [PubMed] [Google Scholar]
- 13. Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011; 6: 1496-504. [DOI] [PubMed] [Google Scholar]
- 14. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e166S-90S. [DOI] [PubMed] [Google Scholar]
- 15.ATS Committee Proficiency on Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. J Am Respir Crit Care Med 2002; 166: 111-7. [DOI] [PubMed] [Google Scholar]
- 16. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341: 1351-7. [DOI] [PubMed] [Google Scholar]
- 17. Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010; 76: 326-32. [DOI] [PubMed] [Google Scholar]
- 18. Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009; 27: 3290-6. [DOI] [PubMed] [Google Scholar]
- 19. Bradley JD, El Naqa I, Drzymala RE, Trovo M, Jones G, Denning MD. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 2010; 77: 1146-50. [DOI] [PubMed] [Google Scholar]
- 20. Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009; 75: 677-82. [DOI] [PubMed] [Google Scholar]
- 21. Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012; 82: 967-73. [DOI] [PubMed] [Google Scholar]
- 22. Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42: 831-8. [DOI] [PubMed] [Google Scholar]
- 23. Tang YD, Dewland TA, Wencker D, Katz SD. Post-exercise heart rate recovery independently predicts mortality risk in patients with chronic heart failure. J Card Fail 2009; 15: 850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swigris JJ, Swick J, Wamboldt FS, et al. Heart rate recovery after 6-min walk test predicts survival in patients with idiopathic pulmonary fibrosis. Chest 2009; 136: 841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raz DJ, Zell JA, SH Ou, Gandara DR, Anton-Culver H, Jablons DM. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007; 132: 193-9. [DOI] [PubMed] [Google Scholar]
- 26. Golpe R, Perez-de-Llano LA, Mendez-Marote L, Veres-Racamonde A. Prognostic value of walk distance, work, oxygen saturation, and dyspnea during 6-minute walk test in COPD patients. Respir Care 2013; 58: 1329-34. [DOI] [PubMed] [Google Scholar]
- 27. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121: 1434-40. [DOI] [PubMed] [Google Scholar]
- 28. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005-12. [DOI] [PubMed] [Google Scholar]
- 29. Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008; 134: 746-52. [DOI] [PubMed] [Google Scholar]
- 30. Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. J Am Respir Crit Care Med 2006; 174: 803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paciocco G, Martinez FJ, Bossone E, Pielsticker E, Gillespie B, Rubenfire M. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J 2001; 17: 647-52. [DOI] [PubMed] [Google Scholar]
- 32. Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant 2011; 30: 982-9. [DOI] [PubMed] [Google Scholar]
- 33. Budweiser S, Heidtkamp F, Jorres RA, et al. Predictive significance of the six-minute walk distance for long-term survival in chronic hypercapnic respiratory failure. Respiration 2008; 75: 418-26. [DOI] [PubMed] [Google Scholar]
- 34. Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med 2006; 100: 1734-41. [DOI] [PubMed] [Google Scholar]
- 35. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. J Am Respir Crit Care Med 2000; 161: 487-92. [DOI] [PubMed] [Google Scholar]