Abstract
Objective
We present a patient with unilateral sudden sensorineural hearing loss (SSNHL) who was found to have a vascular loop in the ipsilateral internal auditory canal (IAC), and we review the literature regarding this association. Underlying pathophysiologic factors surrounding microvascular compression of the vestibulocochlear nerve are poorly understood and make treatment recommendations, especially the option of microvascular decompression, difficult if not controversial. The current report represents an attempt to understand this clinical entity as discussed in the current literature.
Case summary: A 77-year-old female with a long history of progressive right-sided hearing loss and episodic vertigo developed unilateral right SSNHL, tinnitus, vertigo, and disequilibrium. She was originally diagnosed with a vestibular schwannoma on magnetic resonance imaging (MRI) and was referred to our institution for Gamma Knife radiosurgery. Repeat MRI demonstrated a loop of the anterior inferior cerebellar artery (AICA) compressing the vestibulocochlear nerve within the right IAC. There was no evidence of a schwannoma on the repeat MRI. She was not offered radiosurgery, and she elected conservative management.
Conclusion
Vascular compression of cranial nerves can lead to neuronal dysfunction, and this has been rarely described in patients involving the vestibulocochlear nerve complex. There is evidence that microvascular decompression (MVD) of the vestibulocochlear nerve can be effective in selected patients who exhibit pulsatile tinnitus or disabling positional vertigo in the setting of a vascular loop within the ipsilateral IAC, but available evidence at this time does not support MVD for SSNHL.
Keywords: Hearing loss, Internal auditory canal, Cerebellopontine angle, Vessel loop, Anterior inferior cerebellar artery, Vascular malformation, Vestibular schwannoma, Microvascular decompression, Magnetic resonance imaging
1 INTRODUCTION
The cerebellopontine angle (CPA) is a cerebrospinal fluid-filled, triangular space located at the junction of the lateral pons and anterior cerebellum. The CPA contains cranial nerves V-VIII, the superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), and draining veins. Anterolateral to the CPA, the internal auditory canal (IAC), a bony channel situated along the posterior face of the petrous bone, transmits the facial nerve from the CPA to the temporal bone and ultimately the face, and the vestibulocochlear nerve (VCN) from the cochlea and vestibular apparatus to the brainstem.[1]
The anterior inferior cerebellar artery (AICA) arises from the basilar artery and courses variably posterolaterally in the CPA or along the underside of the cerebellum supplying the anterior cerebellum including the flocculus, middle cerebellar peduncle, and inferolateral pons. It gives rise to the labyrinthine (internal auditory) artery, which supplies the cochlea and vestibular system. A terminal artery, the labyrinthine artery is the sole blood supply of the labyrinth and an area of the brainstem and cerebellum. There is high variability in the course of the AICA within the CPA.[2] The prevalence of AICA loops within the IAC has been reported to be between 13-40% in post-mortem dissections[3, 4] and 14-34% by magnetic resonance imaging (MRI).[5, 6]
The most common pathology of the VCN in the CPA and IAC is a vestibular schwannoma (VS), a neoplasm of Schwann cells that is responsible for 5-10% of all intracranial tumors.[7] Patients with VS commonly present with progressive unilateral sensorineural hearing loss and tinnitus, but can also present with SSNHL, symptoms of vestibular dysfunction, facial numbness/pain, and facial paresis.[8] However, there are other causes for cranial nerve dysfunction in this area – both neoplastic and non-neoplastic such as compression from vascular malformations[9-13] and arterial loops.[14] These more unusual lesions can lead to confusion in diagnosis due to the similar symptomatology and MRI findings of vascular compression of the VCN.
In 1975, Janetta proposed that compression of the VCN by the AICA within the IAC could be responsible for symptoms of hearing loss, tinnitus, vertigo, and hemifacial spasm;[15] however, this has been a point of much controversy. Hemifacial spasm is an accepted clinical sequela of vascular compression of the facial nerve. While some studies demonstrate a correlation between symptoms related to microvascular compression of the VCN complex and VCN dysfunction,[16, 17] others do not recognize this correlation and classify AICA loops within the IAC as normal variants.[3, 5, 18] There is also uncertainty as to why these symptoms manifest in some patients with AICA loops in the IAC but not others. Theories to explain this phenomenon include elongation of arteries with age, arteriosclerosis, development of arachnoid adhesions between the artery and nerve, and hypoxic damage to the cochlea and vestibular system due to arterial compression.[19, 20]
We present a woman with unilateral sudden sensorineural hearing loss, tinnitus, disequilibrium, and vertigo who was mistakenly diagnosed with a vestibular schwannoma and referred to our institution for radiosurgery. On repeat imaging, she was found to have an AICA loop in close proximity to the VCN within the IAC on the ipsilateral side. The purpose of this report is to caution clinicians about the possibility of an intracanalicular vascular loop masquerading as a VS and to discuss AICA loop compression of the VCN as a potential cause for hearing loss.
2 CASE REPORT
A 77-year-old woman with a history of breast cancer in remission, transient ischemic attack, atrial fibrillation, progressive right-sided hearing loss over several years, and intermittent vertigo for 10-15 years was diagnosed in March 2013 with a right IAC “mass” by MRI after suffering sudden complete right-sided hearing loss, tinnitus, and aural fullness, as well as an episode of vertigo, while taking a shower. Originally, she was treated for otitis media, and when her symptoms persisted, she was referred to an otolaryngologist who documented profound sensorineural hearing loss of the right ear (Figure 1). She also had mild high frequency sensorineural hearing loss of the left ear. She did not have facial weakness, numbness, hemifacial spasm, visual symptoms, or speech difficulty. Her first MRI demonstrated an ovoid mass lesion measuring 9.1 x 2.7 mm in the mid-portion of the right IAC. The mass had minimal contrast enhancement but was thought to be a VS despite the fact that the degree of enhancement was atypical for a VS. At this point, she was referred to a neurosurgeon, who then referred her to our institution for evaluation for Gamma Knife radiosurgery (GKRS) for treatment of a presumed VS.
Figure 1.
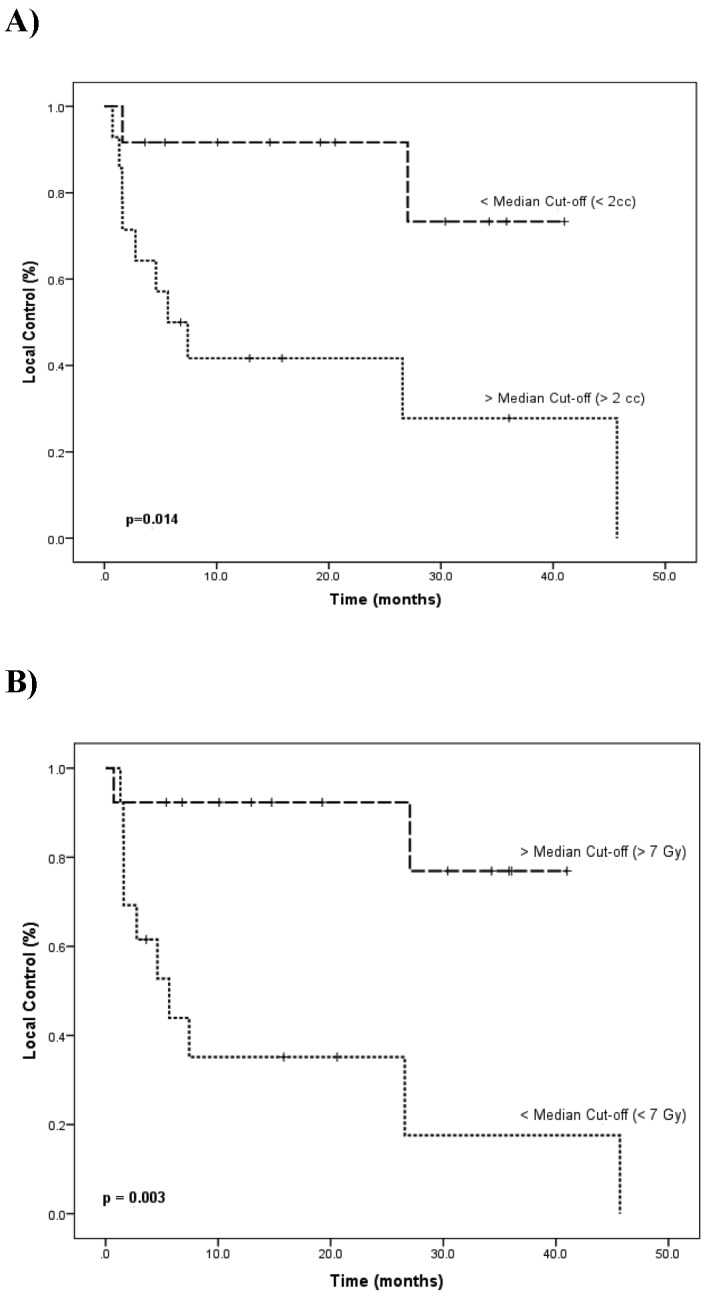
Results of pure tone audiometry. X corresponds to left ear and O corresponds to right ear.
The patient was evaluated at our institution in April 2013. At that time, she continued to complain of right-sided hearing loss, tinnitus, and disequilibrium. On examination, she demonstrated minimal hearing on her right side, a mildly positive Romberg’s sign, and she was unable to tandem walk. Due to the large slice thickness of her previous MRI resulting in poor imaging of the IAC, as well as the minimal enhancement of the mass, a repeat MRI with IAC protocol (thin section, T1 with and without gadolinium enhancement, T2, and constructive interference steady state sequences [CISS]) was ordered.
Her repeat MRI in May 2013 demonstrated asymmetry of the right IAC with no enhancement and a loop of the right AICA within the IAC (Figure 2). These findings were not identified on the left side. Based on the new MRI findings, she was offered conservative management. A follow-up MRI was scheduled for 6 months later due to ambiguity as to whether or not there was an underlying tumor. This was performed in December 2013 and demonstrated the same finding of an ectatic blood vessel coursing along the vestibulocochlear complex in the right IAC, without enhancement (Figure 3). At this point, she had not undergone any sort of non-surgical treatment and continued to complain of right-sided hearing loss, intermittent right-sided otalgia, tinnitus, and mild imbalance (without falls or pronounced dizziness). A follow-up MRI was scheduled for 12 months later.
Figure 2.
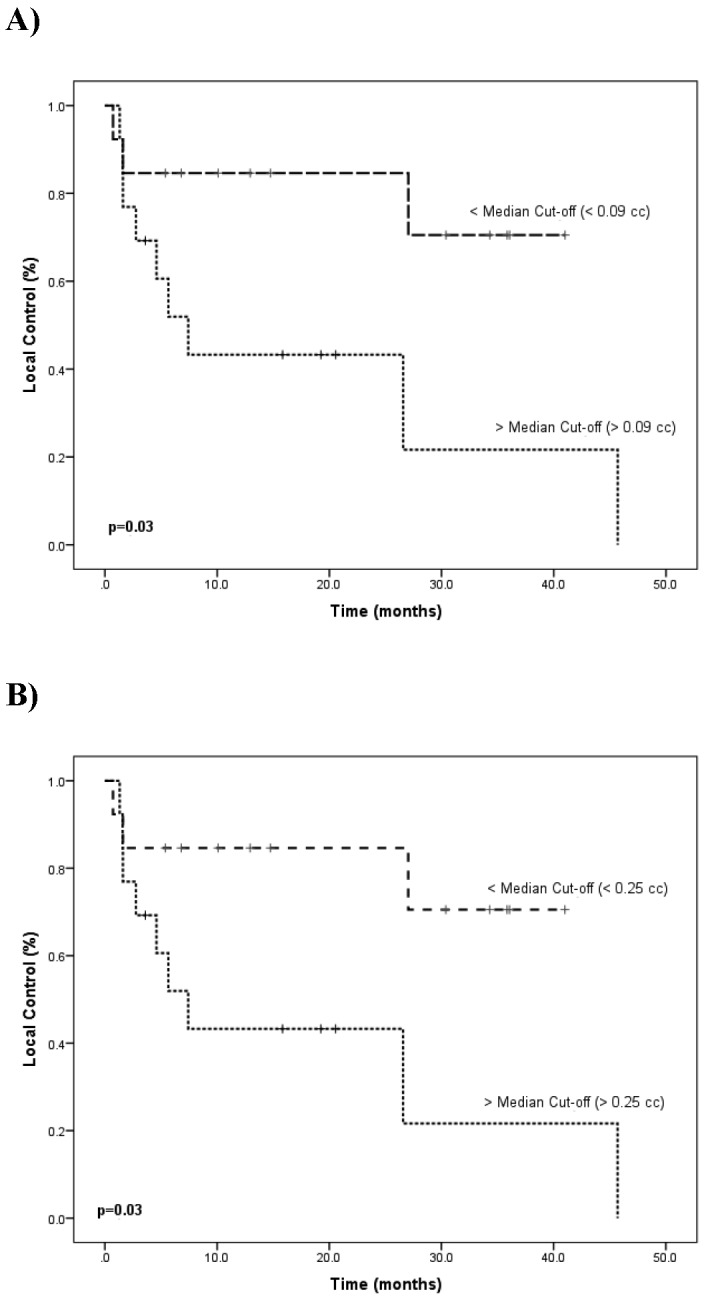
Axial T2 SPACE sequence MRI from May 2013 demonstrating a classic loop of the AICA (arrow) within the IAC resulting in distortion of the VCN on the right side.
Figure 3.
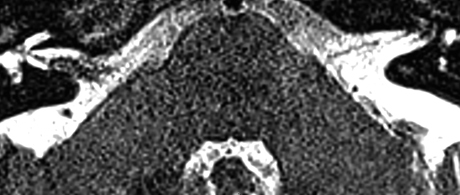
Axial T2 SPACE sequence MRI from December 2013 demonstrating an unchanged finding of a classic loop of the AICA within the IAC resulting in distortion of the VCN on the right side.
3 DISCUSSION
This patient demonstrates the potential difficulty distinguishing a VS from an intracanalicular vascular loop of AICA on MRI. Several older case reports describe patients undergoing surgical intervention for VS that were found to have a vascular loop with vascular compression of the VCN instead.[14, 21, 22] This confusion is due to similar symptomatology and MRI findings between VS and AICA compression of CN VIII in the IAC, as well as the rarity of vascular loop compression as a cause for unilateral hearing loss. Nevertheless, modern MRI techniques including CISS sequences and IAC protocols with and without contrast should be able to differentiate between the two.
A study of 1821 patients with sensorineural or mixed hearing loss with MRI of the IAC showed that 7% had findings on MRI to explain their hearing loss, of which 41% had VS, 30% had inflammation of the middle ear and mastoid, 13% had ischemic foci in the brainstem, 8% had other CPA masses or tumors, 5% had vascular loop compression, 3% had inner ear dysplasia, and 1% had chronic cryptococcal meningitis.[23] These results demonstrate that a “mass” found within the CPA should not be assumed to be a VS; MRI can be an effective tool in screening for sources of sensorineural hearing loss due to CN VIII compression and other causes.
In many cases of SSNHL, however, there are no clear findings on MRI to explain the patient’s symptoms. A study of 54 SSNHL patients reported MRI abnormalities in 31 (57%) patients; only 6 of these patients had MRI findings that correlated with the clinical picture (two labyrinthine hemorrhages, one cochlear inflammation, one acoustic neuroma, one arachnoid cyst of the CPA, and one case of white matter lesions in the pons suggestive of multiple sclerosis).[24]
Auditory brainstem response testing (ABR) may serve as an adjunct in the diagnosis of vascular compression of the eighth cranial nerve. Studying ABRs in patients with compression of the vestibulocochlear nerve, Möller et al. found an interwave latency delay between waves I and III on the affected side of most patients.[25] Using Möller’s criteria, Schwaber et al found that 42 of 56 (75%) patients with suspected compression of the vestibulocochlear nerve demonstrated abnormal ABR’s, and 11 of 13 patients that underwent surgical intervention in their series were confirmed to have compression.[26] Markowski et al also used Möller’s criteria to show that 86% of patients with evidence of AICA compression of the VCN on MRI had abnormal ABRs.[27] De Ridder et al followed 78 patients with unilateral tinnitus and suspected vascular compression of the VCN. They found that a decrease in peak II and a lengthening of the interpeak latency of waves I-III were associated with tinnitus and hearing loss at the tinnitus frequency, respectively. In patients who underwent MVD, postoperative improvements in tinnitus and hearing loss at the tinnitus frequency were associated with peak II and interpeak latency of waves I-III normalization, respectively.[28]
While vascular compression of the VCN was believed to be the cause of the patient’s symptoms, some would argue that this is not the primary cause of her complaints. In their post-mortem study of 1327 human temporal bones, Reisser et al. did not find a correlation between AICA loops in the IAC and unexplained hearing loss, tinnitus, vertigo, or Meniere’s disease.[3] This finding is also supported by Gorrie et al. and Sirikci et al. in their studies of 58 and 140 patients with unexplained hearing loss.[5, 18]
However, in a recent meta-analysis of three case-control studies comparing symptomatic and asymptomatic ears in patients with sensorineural hearing loss and/or non-pulsatile tinnitus, patients with unilateral hearing loss were twice as likely to have vascular loops in the symptomatic ear. In addition, this report reviewed two case-control studies comparing non-pulsatile and pulsatile tinnitus and found that patients with pulsatile tinnitus were 80 times more likely to have a contacting vascular loop than those with non-pulsatile tinnitus.[16] Similarly, Kazawa et al. demonstrated a correlation between patients with direct extension or looping of the AICA into the IAC on MRI and hearing loss on that particular side.[17] Taken together, these studies imply a strong correlation between tinnitus/hearing loss and vascular loops, but they do not show causation.
Most case reports that we found demonstrated improvement of vestibulocochlear symptoms after microvascular decompression (MVD). The results from these case reports are summarized in Table 1. As a caveat, these studies are case reports, and no study is more recent than 2007. We did not find a report of MVD of the VCN in the literature more recent than 2008.
Table 1.
Reported cases of unilateral hearing loss due to vascular loop compression in the IAC treated with MVD
| Reference | Patient | Presentation | Exam/Imaging | Course | Surgical Findings | Intervention | Outcomes |
| Shalit and Reichenthal. 1978[21] | 44 M | 1 year progressive left-sided hearing loss and tinnitus | CT: some enlargement of left internal meatal canal | Left suboccipital craniectomy due to concern for intracanalicular VIII tumor | Constriction of VIII by AICA loop | AICA loop moved medially away from VIII | Complete resolution of hearing loss and tinnitus |
| Applebaum and Valvassori. 1984[19] | 37 F | 5 years left-sided progressive hearing loss, tinnitus, and vertigo | Pneumo-CT: left AICA entering IAC and looping around VIII | Left suboccipital craniectomy | AICA entering IAC and compressing VIII | Separation of AICA and VIII with Teflon felt | NR |
| Roland et al. 1995[39] | 50 M | Left-sided mild hearing loss and severe tinnitus | ABR: increased I-III interpeak interval on left compared to right | Left suboccipital craniotomy | VIII compressed by PICA | Autologous muscle interposed between PICA and VIII | Complete relief of tinnitus and improved hearing |
| Roland et al. 1995[39] | 55 M | Right-sided mild hearing loss and severe tinnitus | Absent stapedius reflexes in right ear, audiometric evaluation showed high-frequency hearing loss on right side | Right suboccipital craniotomy | VIII compressed by AICA | Autologous muscle interposed between AICA and VIII | Marked improvement of tinnitus and stapedial reflexes, returned to normal 1 year postoperatively |
| Herzog et al. 1997[22] | 63 M | 1.5 years left-sided hearing loss and vertigo | MRI: enhancement in left IAC | Worsening hearing loss and disequilibrium with new hemifacial spasm over 6 weeks à translabyrinthe approach to IAC | AICA between VII and VIII | Displacement of AICA from VII using Teflon pledget | Complete resolution of disequilibrium and hemifacial spasm |
| Herzog et al. 1997[22] | 63 M | 1 year right-sided hearing loss with sudden complete hearing loss and vertigo | MRI: bulbous enhancing lesion in IAC | Translabyrinthe approach to IAC | AICA coursing laterally though IAC, posterior to VII and VIII | Elevated AICA away from VII and VIII | Dizziness subsided over 4 weeks, facial nerve function gradually improved over 6 months |
| Okamura et al. 2000[29] | 39 F | 10 years intermittient vertigo with right-sided tinnitus and hearing loss, 40 months phonophobia | Abnormal ABR’s | Surgical intervention | AICA compressing cochlear nerve, PICA attached to inferior vestibular third | AICA loop displaced medially, PICA displaced with Teflon-cushion | Complete resolution of high-pitched tinnitus (low-pitched tinnitus continued), phonophobia, and vertigo, improvement in hearing |
| Scoleri et al. 2001[40] | 33 M | Several years vertigo, fluctuating sensorineural hearing loss, tinnitus, and aural fullness, diagnosed with Meriere’s disease | Audiometric testing showed sensorineural hearing loss in left ear, MRI: normal | Elected for vestibular nerve section procedure via retromastoid approach | Main trunk of AICA bisected vestibulocochlear nerve | Vestibular nerve divided and 6 mm segment removed, MVD of cochlear division with Teflon sponge between it and AICA | Resolution of vertigo but continued progressive hearing loss 4 years postoperatively |
| Sakas et al. 2007[41] | 52 F | 6 years right-sided otalgia, hearing loss, tinnitus, and vertigo | MRI: AICA curved into IAC and compressed VII and VIII | Retromastoid craniotomy | AICA compressing VII and VIII | AICA loop mobilized and Teflon narrow band placed between it and VII/VIII | Complete resolution of otalgia after 6 months, improvement in hearing, tinnitus, and vertigo |
Abbreviations: CT = computed tomography, MRI = magnetic resonance imaging, MRA = magnetic resonance angiography, MVD = microvascular decompression, ABR = auditory brainstem response, AICA = anterior inferior cerebellar artery, PICA = posterior inferior cerebellar artery, CPA = cerebellopontine angle, IAC = internal auditory canal, VII = facial nerve, VIII = vestibuolocochlear nerve, NR = not reported
Okamura et al. reported their findings in a case series of MVD performed on 19 patients with slowly progressive hearing loss and tinnitus thought to be due to vascular compression of the VCN (12 of whom had compression due to the AICA) showing that 64% of patients with hearing loss of 20dB or more had improvement of at least 5dB, and 94% of patients with high-pitch, pulsatile tinnitus had improvement postoperatively.[29] Ryu et al. similarly reported hearing improvement in 8/11 (73%) patients with low-frequency, progressive hearing loss of 20dB or more.[30] Brackmann et al., in a study of 20 patients with disabling positional vertigo who underwent retrosigmoid craniotomy for MVD, showed that 80% of patients had improvement in their symptoms.[31] Similarly, Möller et al. reported a cure rate of approximately 80% after performing MVD for disabling positional vertigo in 207 patients.[32] These studies seem to indicate that MVD is effective in curing disabling positional vertigo as a result of vascular compression of the eighth cranial nerve, but hearing loss should not be expected to reverse significantly after treatment. Defining the clinical picture of disabling positional vertigo and establishing criteria for diagnosis, and ultimately surgical decompression for this clinical entity, remain enigmatic and challenging.
Thus, when is MVD indicated in cases of vascular compression of the VCN? The neurosurgeon and neurotologist must take into account ABR results and MRI findings within the CPA and IAC, as well as the patient’s history, in order to determine the chance that vascular compression is the primary cause of the patient’s symptoms. Gorrie et al. demonstrated a significant correlation between symptoms of VCN dysfunction and presence of an AICA loop within the IAC when the AICA traversed between the seventh and eighth nerves, but interestingly not above or below these nerves.[18] Okamura and Chadha both found strong correlations between pulsatile tinnitus and AICA compression of the VCN.[16, 29] Finally, based on the findings of their studies, Applebaum and Valvasorri suggested consideration of vascular loop compression over tumor mass effect in patients with unilateral hearing loss, spontaneous nystagmus, and normal caloric testing.[19, 33] Thus, if a patient’s MRI demonstrates that the AICA is extending directly or looping into the IAC and contacting the VCN (especially if it courses between the seventh and eighth cranial nerves), and the patient is complaining of unilateral hearing loss and pulsatile tinnitus on the ipsilateral side, in addition to disabling positional vertigo, MVD may be indicated.
However, there are potential complications from MVD of the vestibulocochlear nerve that must be taken into account, which include hearing loss,[34-36] loss of vestibular function,[31] ischemia from damage to the AICA,[29] avulsion rupture of the internal auditory artery,[37] and cerebellar hemorrhage,[38] potentially devastating complications. More extensive studies, perhaps even a correlated temporal bone histopathology study, are needed to more accurately weigh the risks and benefits of surgery in this scenario.
4 CONCLUSION
We describe a woman with unilateral SSNHL, tinnitus, otalgia, vertigo, and disequilibrium possibly caused by compression of the VCN by a loop of AICA within the IAC. The patient was originally diagnosed with a VS based on MRI findings, but directed and repeat imaging did not confirm VS and instead demonstrated an AICA loop. While there is controversy regarding the relationship between AICA loops within the IAC and VCN compression syndromes, several studies have proven the benefit of MVD in select cases. Further studies are required to determine the efficacy of this surgical treatment.
Footnotes
Disclosures/Conflicts of Interest/Funding: None
REFERENCES
- 1. Rhoton AL., Jr The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery 2000; 47: S93-129. [DOI] [PubMed] [Google Scholar]
- 2. Kim HN, Kim YH, Park IY, Kim GR, Chung IH. Variability of the surgical anatomy of the neurovascular complex of the cerebellopontine angle. The Annals of otology, rhinology, and laryngology 1990; 99: 288-96. [DOI] [PubMed] [Google Scholar]
- 3. Reisser C, Schuknecht HF. The anterior inferior cerebellar artery in the internal auditory canal. The Laryngoscope 1991; 101: 761-6. [DOI] [PubMed] [Google Scholar]
- 4. Mazzoni A, Hansen CC. Surgical anatomy of the arteries of the internal auditory canal. Archives of otolaryngology 1970; 91: 128-35. [DOI] [PubMed] [Google Scholar]
- 5. Sirikci A, Bayazit Y, Ozer E, Ozkur A, Adaletli I, Cuce MA, Bayram M. Magnetic resonance imaging based classification of anatomic relationship between the cochleovestibular nerve and anterior inferior cerebellar artery in patients with non-specific neuro-otologic symptoms. Surgical and radiologic anatomy : SRA 2005; 27: 531-5. [DOI] [PubMed] [Google Scholar]
- 6. De Carpentier J, Lynch N, Fisher A, Hughes D, Willatt D. MR imaged neurovascular relationships at the cerebellopontine angle. Clinical otolaryngology and allied sciences 1996; 21: 312-6. [DOI] [PubMed] [Google Scholar]
- 7. De-Monte F. Neoplasms and the cranial nerves of the posterior fossa. Surgery of the Cranial Nerves of the Posterior Fossa. Park Ridge, IL: American Association of Neurological Surgeons; 1993:253-4. [Google Scholar]
- 8. Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery 1997; 40: 1-9; discussion 10. [DOI] [PubMed] [Google Scholar]
- 9. Shim HJ, Song DK, Lee SW, Lee DY, Park JH, Shin JH, Kim S. A case of unilateral sensorineural hearing loss caused by a venous malformation of the internal auditory canal. International journal of pediatric otorhinolaryngology 2007; 71: 1479-83. [DOI] [PubMed] [Google Scholar]
- 10. Patel PN, Connor S, Brew S, Gleeson MJ. An arteriovenous malformation within the internal acoustic meatus and cerebellopontine angle cistern. The Journal of laryngology and otology 2011; 125: 1275-8. [DOI] [PubMed] [Google Scholar]
- 11. Di Rocco F, Paterno V, Safavi-Abbasi S, El-Shawarby A, Samii A, Samii M. Cavernous malformation of the internal auditory canal. Acta neurochirurgica 2006; 148: 695-7. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira D, Mendes V, Vide A, Costa JD. Developmental venous anomaly of the internal auditory canal in a child with unilateral sensorineural hearing loss--a rare association. Pediatric radiology 2012; 42: 1021-3. [DOI] [PubMed] [Google Scholar]
- 13. Wuertenberger CJ, Rosahl SK. Vertigo and tinnitus caused by vascular compression of the vestibulocochlear nerve, not intracanalicular vestibular schwannoma: review and case presentation. Skull base : official journal of North American Skull Base Society [et al] 2009; 19: 417-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brookler KH, Hoffman RA. Acoustic neuroma or vascular loop? The American journal of otology 1979; 1: 32-6. [PubMed] [Google Scholar]
- 15. Jannetta PJ. Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surgical forum 1975; 26: 467-9. [PubMed] [Google Scholar]
- 16. Chadha NK, Weiner GM. Vascular loops causing otological symptoms: a systematic review and meta-analysis. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 2008; 33: 5-11. [DOI] [PubMed] [Google Scholar]
- 17. Kazawa N, Togashi K, Ito J. The anatomical classification of AICA/PICA branching and configurations in the cerebellopontine angle area on 3D-drive thin slice T2WI MRI. Clinical imaging 2013; 37: 865-70. [DOI] [PubMed] [Google Scholar]
- 18. Gorrie A, Warren FM, 3rd, de la Garza AN, Shelton C, Wiggins RH., 3rd Is there a correlation between vascular loops in the cerebellopontine angle and unexplained unilateral hearing loss? Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2010; 31: 48-52. [DOI] [PubMed] [Google Scholar]
- 19. Applebaum EL, Valvassori GE. Auditory and vestibular system findings in patients with vascular loops in the internal auditory canal. The Annals of otology, rhinology & laryngology Supplement 1984; 112: 63-70. [DOI] [PubMed] [Google Scholar]
- 20. Jannetta PJ. Neurovascular compression in cranial nerve and systemic disease. Annals of surgery 1980; 192: 518-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shalit MN, Reichenthal E. Anomalous anterior inferior cerebellar artery simulating intracanalicular acoustic nerve tumor. Surgical neurology 1978; 10: 337-9. [PubMed] [Google Scholar]
- 22. Herzog JA, Bailey S, Meyer J. Vascular loops of the internal auditory canal: a diagnostic dilemma. The American journal of otology 1997; 18: 26-31. [PubMed] [Google Scholar]
- 23. Kwan TL, Tang KW, Pak KK, Cheung JY. Screening for vestibular schwannoma by magnetic resonance imaging: analysis of 1821 patients. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine 2004; 10: 38-43. [PubMed] [Google Scholar]
- 24. Cadoni G, Cianfoni A, Agostino S, Scipione S, Tartaglione T, Galli J, Colosimo C. Magnetic resonance imaging findings in sudden sensorineural hearing loss. The Journal of otolaryngology 2006; 35: 310-6. [DOI] [PubMed] [Google Scholar]
- 25. Moller MB. Results of microvascular decompression of the eighth nerve as treatment for disabling positional vertigo. The Annals of otology, rhinology, and laryngology 1990; 99: 724-9. [DOI] [PubMed] [Google Scholar]
- 26. Schwaber MK, Hall JW. Cochleovestibular nerve compression syndrome. I. Clinical features and audiovestibular findings. The Laryngoscope 1992; 102: 1020-9. [DOI] [PubMed] [Google Scholar]
- 27. Markowski J, Gierek T, Kluczewska E, Witkowska M. Assessment of vestibulocochlear organ function in patients meeting radiologic criteria of vascular compression syndrome of vestibulocochlear nerve--diagnosis of disabling positional vertigo. Medical science monitor : international medical journal of experimental and clinical research 2011; 17: CR169-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Ridder D, Heijneman K, Haarman B, van der Loo E. Tinnitus in vascular conflict of the eighth cranial nerve: a surgical pathophysiological approach ABR to changes. Progress in brain research 2007; 166: 401-11. [DOI] [PubMed] [Google Scholar]
- 29. Okamura T, Kurokawa Y, Ikeda N, Abiko S, Ideguchi M, Watanabe K, Kido T. Microvascular decompression for cochlear symptoms. Journal of neurosurgery 2000; 93: 421-6. [DOI] [PubMed] [Google Scholar]
- 30. Ryu H, Uemura K, Yokoyama T, Nozue M. Indications and results of neurovascular decompression of the eighth cranial nerve for vertigo, tinnitus and hearing disturbances. Advances in oto-rhino-laryngology 1988; 42: 280-3. [DOI] [PubMed] [Google Scholar]
- 31. Brackmann DE, Kesser BW, Day JD. Microvascular decompression of the vestibulocochlear nerve for disabling positional vertigo: the House Ear Clinic experience. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2001; 22: 882-7. [DOI] [PubMed] [Google Scholar]
- 32. Moller MB, Moller AR, Jannetta PJ, Jho HD, Sekhar LN. Microvascular decompression of the eighth nerve in patients with disabling positional vertigo: selection criteria and operative results in 207 patients. Acta neurochirurgica 1993; 125: 75-82. [DOI] [PubMed] [Google Scholar]
- 33. Applebaum EL, Valvasorri G. Internal auditory canal vascular loops: audiometric and vestibular system findings. The American journal of otology 1985; Suppl: 110-3. [PubMed] [Google Scholar]
- 34. Fuse T, Moller MB. Delayed and progressive hearing loss after microvascular decompression of cranial nerves. The Annals of otology, rhinology, and laryngology 1996; 105: 158-61. [DOI] [PubMed] [Google Scholar]
- 35. Moller AR, Moller MB. Does intraoperative monitoring of auditory evoked potentials reduce incidence of hearing loss as a complication of microvascular decompression of cranial nerves? Neurosurgery 1989; 24: 257-63. [DOI] [PubMed] [Google Scholar]
- 36. Yap L, Pothula VB, Lesser T. Microvascular decompression of cochleovestibular nerve. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies 2008; 265: 861-9. [DOI] [PubMed] [Google Scholar]
- 37. Sekiya T, Moller AR. Cochlear nerve injuries caused by cerebellopontine angle manipulations. An electrophysiological and morphological study in dogs. Journal of neurosurgery 1987; 67: 244-9. [DOI] [PubMed] [Google Scholar]
- 38. Sakaki T, Morimoto T, Miyamoto S, Kyoi K, Utsumi S, Hyo Y. Microsurgical treatment of patients with vestibular and cochlear symptoms. Surgical neurology 1987; 27: 141-6. [DOI] [PubMed] [Google Scholar]
- 39. Roland PS, Fell W, Meyerhoff W. Surgical Decompression of the Eighth Nerve for Tinnitus. The international tinnitus journal 1995; 1: 139-46. [PubMed] [Google Scholar]
- 40. Scoleri P, Widner SA, Cass SP. An anatomic variant of the anterior inferior cerebellar artery in a patient with Meniere’s disease. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2001; 22: 519-25. [DOI] [PubMed] [Google Scholar]
- 41. Sakas DE, Panourias IG, Stranjalis G, Stefanatou MP, Maratheftis N, Bontozoglou N. Paroxysmal otalgia due to compression of the intermediate nerve: a distinct syndrome of neurovascular conflict confirmed by neuroimaging. Case report. Journal of neurosurgery 2007; 107: 1228-30. [DOI] [PubMed] [Google Scholar]


