Abstract
Macrophages play a central role in the normal healing process after tissue injury and the host response to foreign objects such as biomaterials. The process leading to macrophage adhesion and activation on protein-adsorbed substrates is complex and unresolved. While the use of primary cells offers clinical relevancy, macrophage cell lines offer unique advantages such as availability and relatively homogeneous phenotype as models to probe the molecular mechanism of cell–surface interaction. Our goal was to better characterize the effect of the culture condition and surface-associated ligands on the extent of U937 adhesion. Tyrosine phosphorylation of intracellular proteins was surveyed as a basis to seek a greater understanding of the molecular mechanism involved in mediating U937 adhesion on various ligand-adsorbed surfaces. U937 viability and adhesion on tissue culture polystyrene (TCPS) increased with (i) increasing serum level, (ii) decreasing tyrosine phosphorylation inhibitor AG18 concentration, or (iii) increasing culture time. The adsorption of various adhesion proteins such as fibronectin and peptide ligands (i.e., RGD, PHSRN) on TCPS did not significantly increase the adherent density of U937 when compared with albumin and PBS ligand controls. However, ligand identity and the presence of phorbol myristate acetate dramatically affected the extent (i.e., increase or decrease) and the identity (i.e., molecular weight) of phosphotyrosine proteins in adherent U937 in a time-dependent manner. The extent and identity of phosphotyrosine proteins did not exhibit a clear AG18 dose dependency, rather the level of tyrosine phosphorylation for a distinct group of proteins was either increased or decreased for a given AG18 concentration. r 2004 Elsevier Ltd. All rights reserved.
Keywords: AG18, RGD, PHSRN, PMA
1. Introduction
Macrophages play a central role in the normal healing process after tissue injury and the host response to foreign objects such as biomaterials and pathogens [1,2]. The process leading to macrophage adhesion and activation on protein-adsorbed substrates is complex. For example, the concentration, composition, and conformation of adsorbed proteins on biomaterials depend mainly on the surface chemistry and topography, the chemicophysical property and concentration of each protein, time, and mechanical forces such as fluid shear in a dynamic fashion [3]. In addition to the role of protein conformation in modulating receptor recognition and cellular function, inconsistencies in the literature exist between the type and amount of adsorbed proteins and the subsequent cellular behavior [4,5]. For example, monocyte adhesion on a poorly adhesive surface is enhanced by adsorbed IgG [6], while others have shown that fibrinogen is more critical for phagocyte adhesion on biomaterials [7]. Others and we have demonstrated that macrophage adhesion is mainly modulated by the substrate and the extracellular matrix protein such as fibronectin [8,9].
To better understand the association between surface-bound ligands with extracellular membrane receptors and the subsequent selective intracellular signaling events in macrophages, others and we have relied on the use of primary human blood derived monocytes [10,11]. The clinical relevance of using primary cells to the host foreign body response of biomaterials is obvious. However, primary cells are limited in supply and availability, laborious to isolate, costly, and have tremendous donor-to-donor variation in terms of adhesion, phenotypic expression, and tyrosine phosphorylation. The molecular and cellular effect of the isolation process is also not well understood and characterized. Macrophage-like cell lines such as IC-21 (murine) [12] and U937 (human) [13] are routinely employed for various biomedical research applications and offer an alternative in biomaterial-related investigation. The advantages of cell lines are ready availability, high proliferative potential, less expensive, extended culture lifetime, and relatively homogeneous phenotype. The goal of the current research is to better characterize the effect of culture condition (i.e., amount of serum, culture time, presence of phorbol myristate acetate (PMA), dose dependency of tyrosine phosphorylation inhibitor AG18) and surface-associated ligands on the extent of U937 adhesion. Furthermore, others have demonstrated that fibronectin–integrin interaction mainly results in the phosphorylation of two intracellular kinase families: protein tyrosine kinase and protein serine/threonine kinase in various types of cells. Thus, tyrosine phosphorylation of intracellular proteins is surveyed as a basis to seek a greater understanding of the molecular mechanism involved in mediating U937 adhesion on surfaces adsorbed with fibronectin-derived ligands.
2. Materials and methods
2.1. Cell line and reagents
Human monocytic cell line, CRL-1593.2/U-937 (American Type Culture Collection, ATCC), was maintained in RPMI 1640 supplemented with 5% fetal bovine serum (FBS). Reagents were obtained from the following commercial sources: RPMI 1640 medium and FBS (ATCC); human serum fibronectin, human serum albumin and hexaglycine (G6), PMA, tyrosine protein phosphorylation inhibitor tyrphostin A23 (AG18) (Sigma); G3RGDG and G3PHSRNG oligopeptides were synthesized by the Biotechnology Center, University of Wisconsin-Madison at >95% purity as analyzed by HPLC and mass spectrometry; trypsin/neutralization solution/HBSS kit (CAMBREX); M-PER® Mammalian Protein Extraction Reagent, Halt™ Protease Inhibitor Cocktail, BCA Protein Assay Reagent kit® (PIERCE); immobilized phosphotyrosine monoclonal antibody (9419) (Cell Signaling Technology); ultra-pure stacking gel buffer, resolving gel buffer concentrate, 40% (w/v) acrylamide:bisacrylamide (37.5:1) (GIBCO-BRL); and 10× Tris/glycine/SDS buffer Bio-Safe™ Coomassie, 10-Well Ready Gel®, Tris-HCl Gel®, 10% Resolving Gel®, 4% Stacking® Gel (BIO-RAD).
2.2. Determination of the culture condition and adhesion assay
To minimize the possible unknown effect of components in FBS on mediating cell adhesion and tyrosine phosphorylation, we first determined the lowest concentration of FBS necessary to maintain U937 viability. Cells were incubated in suspension for 24 h in medium supplemented with 0%, 2.5%, 5%, 7.5%, or 10% FBS with or without 50 ng/ml PMA. Cell viability was quantified by trypan blue exclusion test [14]. Cell viability significantly decreased in the absence of FBS, but the cell viability in the medium with 5% FBS reached 90% of that with 10% FBS in the absence of PMA (Fig. 1). Hence, 5% FBS was chosen for all subsequent U937 cell culture to minimize the unknown effects of FBS upon adhesion and tyrosine phosphorylation. Following similar methodology, 50 ng/ml was the lowest concentration of PMA that significantly induced U937 adhesion on tissue culture polystyrene (TCPS) in the presence of 5% FBS and was chosen for all subsequent experiments. Cells cultured in the presence of PMA and 5% FBS showed a viability of 100% compared to cells grown in PMA and 10% FBS (Fig. 1). Morphologically, multi-pseudopodia formation and homotypic aggregation were two most apparent characteristics of adherent U937 cells on TCPS in the presence of 50 ng/ml PMA and 5% FBS.
Fig. 1.
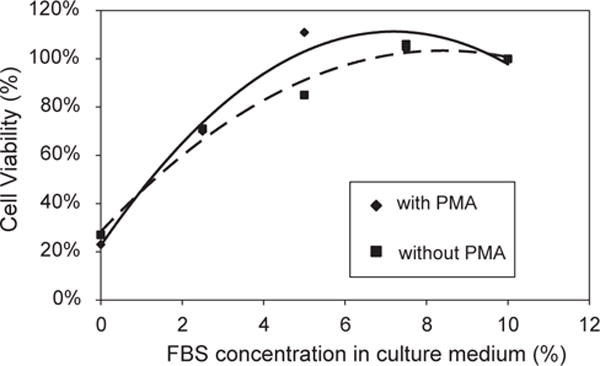
Trend line showing the effect of FBS concentration and 50 ng/ml PMA on U937 viability at 24 h of the culture. The addition of PMA increased cell viability and cell viability with 5% FBS(PMA+) reached 100% of that with 10% FBS (n=2–4, error bars omitted for clarity) (broken line: without PMA; solid line: with PMA).
To determine the effect of ligand-adsorbed surfaces in mediating cell adhesion, commercially available tissue culture polystyrene (TCPS, flask of a surface area of 75 cm2, Falcon BD®) was prepared 24 h prior to cell C for 24 h. PBS only was used as the ligand control. Flasks were rinsed twice with cold PBS prior to use. Source cells (passage between 3 and 10) were collected by centrifugation at 500 × g for 10 min at 37°C, rinsed with PBS, resuspended in RPMI 1640 supplemented with 5% FBS, AG18 (at 0, 20, 40, 60, or 80 μM), and PMA (50 ng/ml). Cells were seeded at a seeding density of 1.7 × 105/cm2 of surface area onto treated TCPS flask and incubated for 2, 4, 12, 20, or 24 h. At each culture time, samples were rinsed with cold PBS to remove nonadherent cells and the adherent cell density was quantified using a computer-assisted inverse contrast microscope (Nikon Eclipse TE300).
2.3. Preparation of cell lysates and immunoprecipitation
Tyrosine phosphorylation of intracellular proteins in adherent U937 on various ligand preadsorbed TCPS was surveyed. Briefly, 12 or 24 h after seeding following the above adhesion assay protocol (seeding density: 1.7 × 105cells/cm2 of sample area with a total area of 150 cm2/sample, with AG18 at various concentration as above with 50 ng/ml PMA and 5% FBS) non-adherent cells were removed via gently washing with the culture medium. Adherent cells were rinsed once with cold PBS, incubated with 0.025% trypsin/EDTA, trypsin neutralizing solution, Hepes-buffered saline solution at 37°C for 5 min for each reagent then centrifuged at 1000 × g for 10 min at 37°C. Cell pellet was rinsed with ice-cold PBS and 0.5 ml/sample of ice-cold cell lysis buffer with protease inhibitor cocktail was added. Lysates were placed on ice for 1 h, microcentrifuged for 10 min at 4°C, and sonicated for 5 s. The amount of total protein in the supernatant was quantified with an established BCA assay following the supplier’s instruction. For each sample, 550 μg of total protein was mixed with immobilized phosphotyrosine antibody (v:v=10:1 as recommended by the supplier) in a 1.5 ml Eppendorf and gently rocked overnight at 4°C. Samples were gently washed three times prior to resuspension in 20 μl 3 × SDS sample buffer, centrifuged at 14,000 × g for 3 min, heated to 80–85°C in a water bath for 2–5 min, and ~20 μl of the resulting solution sample was loaded and resolved on a 10% SDS-PAGE gel (70~80 V in stacking gel and 110 V in resolving gel). Gels were stained overnight with Coomassie Blue and each band was quantified by densitometry (Molecular Dynamics, ImageQuant Software). The intensity of each band was standardized as follows: relative band intensity = [(raw intensity of a band) − (background of that lane)]/[(intensity of the marker on the same gel) − (background of the marker lane)].
2.4. Statistical analysis
Cell adhesion study was repeated independently two to four times (n=2–4) and the data were analyzed by one- or two-way ANOVA at p<0.05 (SigmaStat). Tyrosine phosphorylation assays for each test condition (i.e., ligand identity, culture time, AG18 concentration) was independently repeated two to four times (n = 2–4) and the data were analyzed by ANOVA at p<0.05.
3. Results and discussion
3.1. Cell adhesion assays
A time course of U937 adhesion at 0, 4, 12, 20, and 24 h was first determined to quantify the kinetics of cell adhesion on TCPS in the presence of 5% FBS and 50 ng/ml of PMA. Adherent cell density increased with increasing time and reached a plateau by 24 h (Fig. 2). In order to obtain a sufficient number of adherent cells and amount of proteins for the subsequent phosphotyrosine protein immunoprecipitation and to minimize processes such as proliferation which might askew the protein analysis, culture times of 12 and 24 h were chosen to determine the modulatory effect of surface-associated ligands on cell adhesion and intracellular tyrosine phosphorylation. The doubling time for U937 cells is between 48 and 72 h in the media with 5% FBS, so the adherent cell measurements at 12 and 24 h are primarily dependent on cell adhesion rather than proliferation.
Fig. 2.

A time course of adherent U937 density on TCPS (seeding density: 1.7 × 105 cells/cm2 with 5% FBS and 50 ng/ml PMA, no AG18 was added, n=2–4).
The effect of various ligand-adsorbed surfaces on adherent cell density was analyzed in the presence of 0, 20, 40, 60, and 80 μM AG18. With no AG18 added to the culture medium, adherent cell density on all surfaces at 24 h was significantly higher than that at 12 h on respective ligand-adsorbed surfaces. No statistical significance was found among various ligand-adsorbed surfaces when compared with PBS control at 12 and 24 h and at each respective AG18 concentration level (Table 1). However, adherent cells on fibronectin-adsorbed surfaces showed a flatter morphology with more pseudopodial extension when compared with those on PBS- or other ligand-adsorbed surfaces at both time points. With the addition of 20, 40, 60, or 80 μM AG18, adherent cell density at 12 h on all surfaces was, in general, comparable to each other and to that of respective surface with 0 μM AG18. At 24 h, adherent cell density decreased significantly on all ligand-adsorbed surfaces in the presence of 20, 40, 60, or 80 μM AG18 when compared to that of respective surfaces with 0 μM AG18, but the effect was not ligand-or dosage-dependent (Table 1 and Fig. 3).
Table 1.
Adherent U937 density (×100 cells/cm2) treated with various AG18 concentrations on ligand-adsorbed TCPS
| AG18 concentration (μM) | Culture time (h) | Ligand identity
|
|||||
|---|---|---|---|---|---|---|---|
| PBS | Albumin | Fibronectin | G6 | G3RGDG | G3PHSRNG | ||
| 0 | 12 | 110±2 | 112±17 | 100±1 | 102±1 | 107±13 | 105±3 |
| 24 | 142±51↑ | 150±26↑ | 127±11↑ | 146±17↑ | 134±8↑ | 141±1↑ | |
| 20 | 12 | 109±9 | 62±12 | 95±4 | 99±2 | 96±8 | 100±6 |
| 24 | 70±11 ↓ | 75±0↓ | 68±2↓ | 65±5↓ | 70±2↓ | 73±1↓ | |
| 40 | 12 | 113±10 | 55±1↓ | 97±4 | 102±9 | 96±6 | 105±5 |
| 24 | 70±14↓ | 68±7↓ | 70±20↓ | 71±23↓ | 65±4↓ | 67±14↓ | |
| 60 | 12 | 112±2 | 109±3 | 108±13 | 107±11 | 99±9 | 100±10 |
| 24 | 76±10↓ | 78±10↓ | 84±8↓ | 84±8↓ | 79±3↓ | 77±4↓ | |
| 80 | 12 | 133±16 | 123±8 | 125±9↑ | 107±9 | 104±11 | 108±5 |
| 24 | 72±2↓ | 75±2↓ | 80±8↓ | 72±4↓ | 69±10↓ | 68±5↓ | |
All values expressed in mean±s.d. and analyzed at p<0.05 (ANOVA of average values taken from two to four independent experiments with a culture condition of 50 ng/ml PMA, 5% FBS, seeding density of 1.7 × 105 cells/cm2 surface area) vs. 0 μM AG18 of respective surfaces of 12 h culture as indicated by ↑, increase or ↓, decrease.
No significant difference (p<0.05 ANOVA) was found between various ligand-adsorbed surfaces when compared with PBS controls at respective AG18 concentration level and each culture time.
Fig. 3.

Dose–response curve of AG18 of adherent U937 on various ligand adsorbed surfaces cultured with 50 ng/ml PMA at 24 h. Adherent cell number decreased significantly (p<0.05 ANOVA) with increase of AG18 concentration from 0 to 20 μM and was comparable thereafter up to 80 μM. No significant difference was observed among various surfaces adsorbed with: ●, PBS; ▲, albumin; ■, fibronectin; × G6; *, G3RGDG; ◊, G3PHSRNG (n=2–4, error bars omitted for clarity).
3.2. Survey of phosphotyrosine proteins regulated by PMA
The effect of PMA on protein tyrosine phosphorylation in adherent U937 on TCPS was determined. This effect established the extent of regulation and the identity of phosphotyrosine proteins due to the culture condition only and not due to ligand-modified surface. The presence of 50 ng/ml PMA, necessary to induce U937 adhesion, increased the tyrosine phosphorylation of ~200, ~140, ~100, ~85, ~65, ~42, and ~29 kDa proteins, while decreased that of ~160, ~63, and ~23 kDa proteins in adherent cells when compared with cells without PMA treatment (i.e., cells in suspension) (Fig. 4).
Fig. 4.
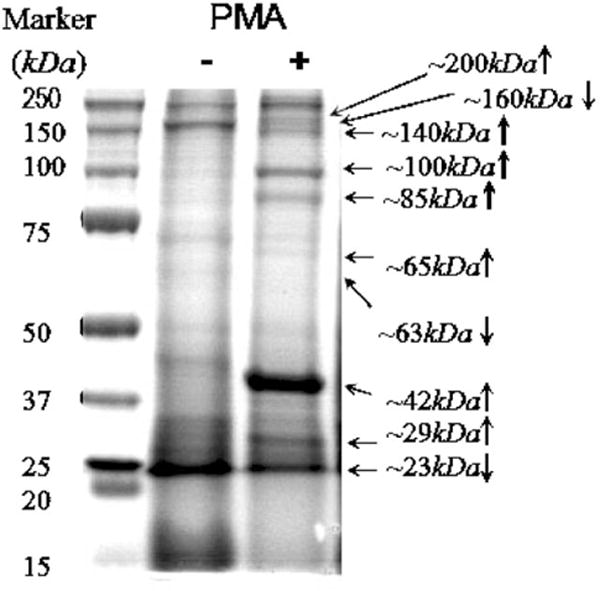
Effect of 50 ng/ml PMA on phosphotyrosine proteins in adherent U937 on TCPS at 24 h of culture. Significant differences (p < 0.05) were observed between samples with or without 50 ng/ml PMA based on the result of IP-SDS-PAGE electrophoresis staining by Coomassie Blue (shown by arrows) and analyzed with densitometry.
PMA mediates many biological functions in different cell types, such as tissue specific cell proliferation or growth arrest, apoptosis, differentiation, activation, secretion, adhesion, and migration by stimulating different signaling pathways, among which the phospholipases Cgamma2-protein kinase C-MAPK cascade is frequently reported [15,16]. In addition, nuclear factor kappaB and activating factor 1 have also been identified as PMA targets. Several other transcription factors, including six-CREB, E2F, CETP/CRE, c-Rel, Msp1, Pax6, GATA, NF-E1, ISRE, Elk, and c-Jun induced by PMA, have been profiled in HeLa cells [17]. Our data showed that U937 adhered to all treated surfaces in the presence of PMA, without which no cell adhesion occurred. We hypothesized that some of the adhesion-related cellular components would have been induced or activated during this adhesion process. Cross-referencing with the Swiss-Prot® database and TrEMBL® (http://us.expasy.org), several potential adhesion-related phosphotyrosine proteins matching the molecular weight range are likely candidate targets of PMA in U937. Proteins that could be upregulated include: non-receptor tyrosine protein kinase TYK2 (134 kDa) [18], adapter-related protein complex 2 alpha 2 subunit (104 kDa), epidermal growth factor receptor substrate 15 (99 kDa) [19], CD97 antigen (82 kDa) [20], mitogen-activated protein kinase 6 (83 kDa) [21], paxillin (65 kDa) [22], transcription factor NF-E2 45 kDa subunit (41 kDa) [23], mitogen-activated protein kinase 1 (41 kDa) [24], mitogen-activated protein kinase 3 (43 kDa), and myristoylated alanine-rich C-kinase substrate (31 kDa) [25]. Plausible downregulated proteins include: mitogen-activated protein kinase 4 (63 kDa) [24] and nuclear transcription factor Y subunit beta (23 kDa) [26], hinting that these phosphotyrosine proteins might serve as constraints in cell adhesion and/or cell spreading.
3.3. Survey of phosphotyrosine proteins regulated by surface ligands
Expression profile of phosphotyrosine proteins in adherent cells on various ligand-adsorbed TCPS surfaces was analyzed. At 12 h without AG18, albumin-adsorbed surfaces increased the tyrosine phosphorylation of ~70 kDa (a) proteins, whereas tyrosine phosphorylation of ~52 kDa (b) and ~100 kDa (c) proteins was decreased on G6- and G3PHSRNG-adsorbed surfaces, respectively, when compared with PBS-treated surface control (Table 2, band designation within parenthesis in Fig. 5A). In the presence of 20 μM AG18, fibronectin-adsorbed surfaces increased the tyrosine phosphorylation of ~160 kDa (d) proteins when compared with the PBS control. Tyrosine phosphorylation of ~52 kDa (e) proteins on G3PHSRNG-adsorbed surfaces was increased by 2-folds when compared with that in the absence of AG18. At 40 μM AG18, surface-ligand identity did not significantly affect tyrosine phosphorylation, whereas at 60 μM AG18, fibronectin- and G3RGDG-adsorbed surfaces, respectively, increased the tyrosine phosphorylation of ~90, ~52 and ~42, and increased ~52 kDa proteins when compared to PBS-treated controls. In the presence of 80 μM AG18, albumin- and fibronectin-adsorbed surfaces, respectively, decreased the tyrosine phosphorylation of ~85 kDa proteins and increased that of ~42 kDa proteins when compared to PBS-treated controls.
Table 2.
Densitometric analysis of differentially (increase (↑) or decrease (↓)) expressed phosphotyrosine proteins (kDa) in adherent U937 on various ligand adsorbed TCPS (vs. PBS-treated control surface) treated with AG18 at 12 h
| AG18 concentraion (μM) | Ligand identity
|
||||
|---|---|---|---|---|---|
| Albumin | Fibronectin | G6 | G3RGDG | G3PHSRNG | |
| 0 | ↑~70 (a) | NS | ↓~52 (b) | NS | ↓~100 (c) |
| 20 | NS | ↑~160 (d) | NS | NS | ↑~52 (e) |
| 40 | NS | NS | NS | NS | NS |
| 60 | NS | ↑~90,~52,~42 | NS | ↑~52 | NS |
| 80 | ↓~85 | ↑~42 | NS | NS | NS |
NS: no significant difference (p<0.05) when compared with PBS controls at respective AG18 concentration level. Letter in () indicates band designation in Fig. 5A. Bold print indicates proteins that are differentially regulated when compared to cells treated with PMA-only (Fig. 4).
All values expressed in mean±s.d. and analyzed at p<0.05 (ANOVA of average values taken from two to four independent experiments (n = 2–4) with a culture condition of 50 ng/ml PMA, 5% FBS, seeding density of 1.7 × 105 cells/cm2 surface area) vs. respective PBS controls of respective AG 18 concentration level.
Fig. 5.
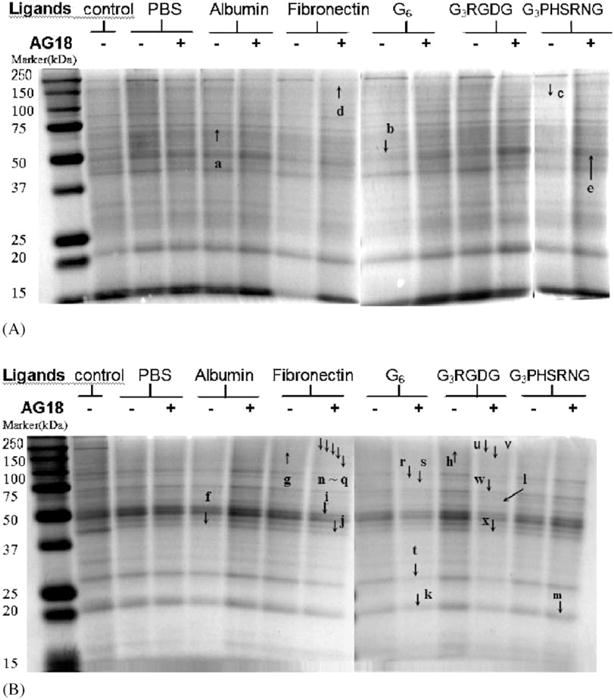
Protein tyrosine phosphorylation in adherent U937 on various ligand adsorbed surfaces with or without 20 μM AG18 at 12 h (A) or 24 h (B) of culture. Arrows e and n–x show the differences between samples with and without 20 μM AG18 from the same surface (p<0.05). Arrows a–d and f–m indicate the differences between ligand-adsorbed surfaces and PBS-treated controls at given concentration of AG18 (p<0.05) as determined from densitometric data.
At 24 h, the extent and the identity of phosphotyrosine proteins in adherent U937 as mediated by surface ligand were significantly different from that of 12 h. In the absence of AG18, albumin-adsorbed surfaces decreased the tyrosine phosphorylation of ~42 kDa (f) proteins, whereas fibronectin- and G3RGDG-adsorbed surfaces increased the tyrosine phosphorylation of ~200 kDa (g, h) proteins when compared with PBS-treated controls (Table 3, band designation within parenthesis in Fig. 5B). In the presence of 20 μM AG18, fibronectin-adsorbed surfaces decreased the tyrosine phosphorylation of ~52 kDa (i) and ~42 kDa (j) proteins, whereas G6-, G3RGDG-, and G3PHSRNG-adsorbed surfaces, respectively, decreased the tyrosine phosphorylation of ~23 (k) and ~65 kDa (l) proteins and increased that of ~23 kDa (m) proteins when compared with PBS-treated controls. In the presence of 40 μM AG18, fibronectin-adsorbed surfaces increased the tyrosine phosphorylation of ~23 kDa proteins, whereas G6-adsorbed surfaces decreased that of ~42 kDa proteins when compared with PBS-treated controls. Ligand identity did not affect the extent and type of protein tyrosine phosphorylation in the presence of 60 or 80 μM AG18 when compared with PBS–TCPS control (Table 3).
Table 3.
Densitometric analysis of differentially (increase (↑) or decrease (↓)) expressed phosphotyrosine proteins (kDa) in adherent U937 on various ligand adsorbed TCPS (vs. PBS-treated control surface) treated with AG18 at 24 h
| AG18 concentraion (μm) | Ligand identity
|
||||
|---|---|---|---|---|---|
| Albumin | Fibronectin | G6 | G3RGDG | G3PHSRNG | |
| 0 | ↓~42 (f) | ↑~200 (g) | NS | ↑~200 (h) | NS |
| 20 | NS | ↓~52 (i), ~42 (j) | ↓~23 (k) | ↓~65 (I) | ↑~23 (m) |
| 40 | NS | ↑~23 | ↓~42 | NS | NS |
| 60 | NS | NS | NS | NS | NS |
| 80 | NS | NS | NS | NS | NS |
NS: no significant difference (p<0.05) when compared with PBS controls at respective AG18 concentration level. Letter in () indicates band designation in Fig. 5B. Bold print indicates proteins that are differentially regulated when compared to cells treated with PMA only (Fig. 4).
All values expressed in mean±s.d. and analyzed at p<0.05 (ANOVA of average values taken from two to four independent experiments (n=2–4) with a culture condition of 50 ng/ml PMA, 5% FBS, seeding density of 1.7 × 105 cells/cm2 surface area) vs. respective PBS controls of respective AG 18 concentration level.
The RGD loop in the 10th type III domain of fibronectin is near the N-terminus of this domain [27] and the interaction with PHSRN synergy regions of domain 9 may stabilize a particular conformation of the RGD loop or provide a specific context that allows recognition by some integrins and not others [28]. In our study, although adherent cell density on all ligand-adsorbed surfaces were comparable, the extent (i.e., increase or decrease) and the identity (i.e., molecular weight) of intracellular phosphotyrosine proteins in adherent U937 were surface-ligand dependent. In order to determine which proteins were tyrosine phosphorylated in the presence of various adsorbed ligands, those regulated by PMA as a critical culture component had to be considered first. We observed in adherent U937 on TCPS that the extent of tyrosine phosphorylation increased for ~200, ~140, ~100, ~85, ~65, ~42, and ~29 kDa proteins, whereas decreased for ~160, ~63, and ~23 kDa proteins in the presence of PMA. Hence, modulated proteins that were other than those listed above and/or showing opposing trend (i.e., increased in the presence of PMA but decreased in the presence of PMA and ligand-adsorbed substrate) were likely contributed by the surface-ligand identity (shown in bold type in Tables 2 and 3). For example, proteins such as those of ~160, ~90, ~85, ~70, ~52–50, ~42, and ~23 kDa were differentially modulated by various ligand-adsorbed surface either at 12 or 24 h of culture for a given AG18 concentration level. Of a particular note, the modulation of ~42 and ~23 kDa proteins by albumin-, fibronectin-, and G3PHSRNG-adsorbed surfaces at 24 h showed an opposite trend when compared to PMA treatment only (shown in bold type in Table 3). The upregulation of tyrosine phosphorylation of ~52 kDa proteins by G3PHSRN-, G3RGDG-, and fibronectin-adsorbed surfaces at 12 h even in the presence of AG18 suggested the critical nature of these proteins in mediating cell adhesion on these ligand-adsorbed substrates. Others had shown that a 50 kDa integrin-associated protein (CD47) in several cell types is physically associated with alpha(v)beta3 and beta3 integrin subunits [29,30] and is activated via tyrosine phosphorylation upon the complexation of these integrins with extracellular matrix proteins such as fibronectin [31]. CD47 activation is required for the integrin-regulated calcium influx critical in the subsequent integrin-regulated cellular function, such as cytoskeletal rearrangement, cell spreading, and movement [32,33]. Although the evidence and the function of tyrosine phosphorylated ~160, ~85, ~70, ~42, and ~23 kDa proteins in adherent U937 are unclear, our current data indicate the existence of unique and novel tyrosine phosphorylation events triggered by a specific cell– surface interaction.
3.4. Survey of phosphotyrosine proteins regulated by AG18 concentration
For a given ligand-adsorbed surface, various AG18 concentrations differentially modulated tyrosine phosphorylation in adherent U937. In general, the extent and the identity of phosphotyrosine proteins did not exhibit a clear AG18 dose dependency, rather the level of tyrosine phosphorylation for a distinct group of proteins was either increased or decreased for a given AG18 concentration (Table 4). At 12 h of culture on PBS-treated controls, tyrosine phosphorylation of proteins such as ~85, ~42, and ~23 kDa was decreased in the presence of 60–80 μM of AG18 when compared with cells treated with 0 μM of AG18. On albumin-adsorbed surfaces, tyrosine phosphorylation of proteins such as ~23 kDa was decreased in the presence of 60–80 μM of AG18, whereas that of five other proteins was decreased in the presence of 80 μM of AG18, when compared with cells treated with 0 μM of AG18. On fibronectin-adsorbed surfaces, tyrosine phosphorylation of ~23 kDa proteins was decreased in the presence of 60–80 μM of AG18, whereas that of four other proteins was increased in the presence of 60 μM of AG18. On G6-adsorbed surfaces, tyrosine phosphorylation of proteins such as ~200, ~42, and ~23 kDa proteins was decreased in the presence of 60–80 μM of AG18, whereas that of two other proteins was increased in the presence of 40 μM of AG18. On G3RGDG adsorbed surfaces, tyrosine phosphorylation of proteins such as ~42, and ~23 kDa proteins was decreased in the presence of 60– 80 μM of AG18. On G3PHSRNG-adsorbed surfaces, tyrosine phosphorylation of proteins such as ~42, and ~23 kDa proteins was decreased in the presence of 60–80 μM of AG18, whereas that of ~52 (e) and ~100 kDa was increased in the presence of 20 and 40 μM of AG18, respectively, when compared to cells treated with 0 μM of AG18 (Table 4, band designation within parenthesis in Fig. 5A).
Table 4.
Densitometric analysis of differentially (increase (↑) or decrease (↓)) expressed phosphotyrosine proteins (kDa) in U937 treated with various concentrations of AG18 (vs. 0 μM AG18) adherent on ligand-adsorbed TCPS at 12 h
| Ligands | AG18 concentration (μM)
|
|||
|---|---|---|---|---|
| 20 | 40 | 60 | 80 | |
| None (PBS) | NS | NS | ↓~90,~85,~42,~23 | ↓~170,~85,~65,~52,~42,~23 |
| ↑~140 | ||||
| Albumin | NS | NS | ↓~42,~23 | ↓~170,~85,~70,~65,~52,~23 |
| Fibronectin | NS | NS | ↓~23 | ↓~23 |
| ↑~140,~100,~90,~85 | ||||
| G6 | NS | ↑~100,~85 | ↓~200,~85,~70,~65, | ↓~200,~42,~23 |
| ↓ ~52,~42,~23 | ||||
| G3RGDG | NS | NS | ↓~70,~65,~52,~42, ↓ ~23 | ↓~200,~42,~23 |
| G3PHSRNG | ↑~52 (e) | ↑~100 | ↓~42,~23 | ↓~200,~42,~23 |
NS: no significant difference (p<0.05) when compared with 0 μM AG18 of respective surfaces. Letter in () indicates band designation in Fig. 5. Bold print indicates proteins that are differentially regulated when compared to cells treated with PMA only (Fig. 4). All values expressed in mean±s.d. and analyzed at p<0.05 (ANOVA of average values taken from two to four independent experiments (n = 2–4) with a culture condition of 50 ng/ml PMA, 5% FBS, seeding density of 1.7 × 105 cells/cm2 surface area) vs. 0 μM AG18 of respective surfaces.
At 24 h, the extent and the identity of phosphotyrosine proteins in adherent U937 as mediated by various AG18 concentrations were significantly different from that of 12 h for a given ligand-adsorbed surface. Again, the extent and identity of phosphotyrosine proteins did not exhibit a clear AG18 dose dependency (Table 5, band designation within parenthesis in Fig. 5). On PBS-treated controls, tyrosine phosphorylation of proteins such as ~42 kDa was decreased in the presence of 40–60 μM of AG18, whereas that of ~200 and ~23 kDa was increased from 40 to 60 and from 60–80 μM of AG18, respectively, when compared to cells treated with 0 μM of AG18. On albumin-adsorbed surfaces, tyrosine phosphorylation of proteins such as ~23 kDa was increased in the presence of 40–80 μM of AG18. On fibronectin-adsorbed surfaces, tyrosine phosphorylation of ~200 (n), ~160 (o), ~130 (p), and ~100 kDa (q) proteins was decreased in the presence of 20 μM of AG18, whereas that of two other proteins was increased in the presence of 80 μM of AG18 (Table 5, band designation within parenthesis in Fig. 5). On G6- adsorbed surfaces, tyrosine phosphorylation of proteins such as ~85 (r), ~70 (s), ~23 kDa (t) proteins was decreased in the presence of 20 μM of AG18, whereas that of ~23 kDa was increased in the presence of 60 μM of AG18. On G3RGDG-adsorbed surfaces, tyrosine phosphorylation of proteins such as ~200 (u), ~130 (v), ~70 (w), ~42 kDa (x) proteins was decreased in the presence of 20 μM of AG18, whereas that of four other proteins was increased in the presence of 80 μM of AG18. On G3PHSRNG-adsorbed surfaces, tyrosine phosphorylation of proteins such as ~52 kDa proteins was decreased in the 40–60 μM of AG18, whereas that of ~42 kDa was increased in the presence of 60–80 μM of AG18.
Table 5.
Densitometric analysis of differentially (increase (↑) or decrease (↓)) expressed phosphotyrosine proteins (kDa) in U937 treated with various concentrations of AG18 (vs. 0 μM AG18) adherent on ligand-adsorbed TCPS at 24 h
| Ligands | AG18 concentration (μM)
|
|||
|---|---|---|---|---|
| 20 | 40 | 60 | 80 | |
| None (PBS) | NS | ↓~65, ~42, ~23 | ↓~42 | |
| ↑~200 | ↑~200,~23 | ↑~23 | ||
| Albumin | NS | ↓ ~52 | NS | |
| ↑~200,~160,~23 | ↑~23 | |||
| Fibronectin | ↓~200 (n),~160 (o) | ↓~23 | NS | |
| ↓~130 (p),~100 (q) | ↑~160 | ↑~42,~23 | ||
| G6 | ↓~85 (r),~70 (s), ~23 (t) | ↓~23 | ↑~23 | ↓ ~70,~23 |
| G3RGDG | ↓ ~200 (u),~130 (v), | ↓~23 | ↓~70 | |
| ↓ ~70 (w), ~42 (x) | ↑~200 | ↑~110,~70, ~42,~23 | ||
| G3PHSRNG | NS | ↓~52 | ↑~52 | |
| ↑~200,~160 | ↑~42,~23 | ↑~42 | ||
NS: no significant difference (p<0.05) when compared with 0 μM AG18 of respective surfaces. Letter in () indicates band designation in Fig. 5. Bold print indicates proteins that are differentially regulated when compared to cells treated with PMA only (Fig. 4). All values expressed in mean±s.d. and analyzed at p<0.05 (ANOVA of average values taken from two to four independent experiments (n = 2–4) with a culture condition of 50 ng/ml PMA, 5% FBS, seeding density of 1.7 × 105 cells/cm2 surface area) vs. 0 μMAG18 of respective surfaces.
Others have observed that AG18 attenuates TNF-alpha-induced U937 adhesion and growth [34]. In our study, we observed that AG18 attenuates the adhesion and spreading of U937 at 24 h of culture on albumin-, fibronectin-, hexaglycine-, G3RGDG- and G3PHSRNG-adsorbed surfaces (Table 1, Fig. 3). AG18 is generally employed as a broad-spectrum tyrosine protein phosphorylation inhibitor [35,36], and this was confirmed in our study where a decrease in tyrosine phosphorylation was observed for several proteins (i.e., ~200, ~85, ~65, and ~42 kDa) in the presence of AG18 and in a surface-ligand-dependent manner (shown in bold type in Tables 4 and 5) with PMA added to the culture medium. However, these proteins were increased by PMA only in U937 adherent on TCPS (Fig. 4). Our results suggest that these phosphotyrosine proteins are positively involved in cell adhesion and/or cell spreading. Conversely, the tyrosine phosphorylation of ~160 and 23 kDa proteins was decreased in the presence of PMA (Fig. 4) but increased in the presence of PMA and AG18 (shown in bold type in Tables 4 and 5). This phenomenon suggests that an inhibitory role in meditating cell adhesion and/or cell spreading. We observed that the presence of 20 μM AG18 statistically attenuated 11 phosphotyrosine proteins, while the presence of 80 μM AG18 enhanced nine phosphotyrosine proteins. In addition, the extent of tyrosine phosphorylation of certain proteins (i.e., ~200, ~160, ~70, and ~23 kDa) statistically increased at certain AG18 concentration while decreased at another concentration. This unexpected duality of AG18 suggests phosphotyrosine proteins other than AG18 targets might be phosphorylated or dephosphorylated to compensate the overreaction to AG18 up to 40 μM concentration.
4. Conclusion
The effect of several culture parameters (i.e., FBS and AG18 concentrations, time) on the viability and adhesion of U937 on TCPS was determined. The adsorption of various adhesion protein and peptide ligands did not significantly increase the adherent density of U937 when compared with ligand controls. However, ligand identity and the presence of PMA dramatically affected the extent (i.e., increase or decrease) and the identity (i.e., molecular weight) of phosphotyrosine proteins in adherent U937 in a culture-time-dependent manner. Furthermore, the extent and identity of phosphotyrosine proteins did not exhibit a clear AG18 dose dependency, rather the level of tyrosine phosphorylation for a distinct group of proteins was either increased or decreased for a given AG18 concentration.
Acknowledgments
This work was supported in part by NIH Grant EB-00290.
References
- 1.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–40. [PubMed] [Google Scholar]
- 2.Desevaux C, Girard C, Lenaerts V, Dubreuil P. Characterization of subcutaneous Contramid implantation: host response and delivery of a potent analog of the growth hormone-releasing factor. Int J Pharm. 2002;232(1–2):119–29. doi: 10.1016/s0378-5173(01)00912-7. [DOI] [PubMed] [Google Scholar]
- 3.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res. 2003;66A(2):247–59. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 4.Pettit DK, Hoffman AS, Horbett TA. Correlation between corneal epithelial cell outgrowth and monoclonal antibody binding to the cell binding domain of adsorbed fibronectin. J Biomed Mater Res. 1994;28(6):685–91. doi: 10.1002/jbm.820280605. [DOI] [PubMed] [Google Scholar]
- 5.Jenney CR, Anderson JM. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J Biomed Mater Res. 2000;49(4):435–47. doi: 10.1002/(sici)1097-4636(20000315)49:4<435::aid-jbm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Tang L, Lucas AH, Eaton JW. Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G. Lab Clin Med. 1993;122(3):292–300. [PubMed] [Google Scholar]
- 7.Tang L, Eaton JW. Inflammatory responses to biomaterials. Am J Clin Pathol. 1995;103(4):466–71. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- 8.Kao WJ, Lee D, Schense JC, Hubbell JA. Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J Biomed Mater Res. 2001;55(1):79–88. doi: 10.1002/1097-4636(200104)55:1<79::aid-jbm110>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Shen M, Horbett TA. The effects of surface chemistry and adsorbed proteins on monocyte/macrophage adhesion to chemically modified polystyrene surfaces. J Biomed Mater Res. 2001;57(3):336–45. doi: 10.1002/1097-4636(20011205)57:3<336::aid-jbm1176>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Kao WJ, Liu Y. Intracellular signaling involved in macrophage adhesion and FBGC formation as mediated by ligand-substrate interaction. J Biomed Mater Res. 2002;62(4):478–87. doi: 10.1002/jbm.10317. [DOI] [PubMed] [Google Scholar]
- 11.Babina M, Henz BM. All-trans retinoic acid down-regulates expression and function of beta2 integrins by human monocytes: opposite effects on monocytic cell lines. Eur J Immunol. 2003;33(3):616–25. doi: 10.1002/eji.200323367. [DOI] [PubMed] [Google Scholar]
- 12.Gambelli F, Di P, Niu X, Friedman M, Hammond T, Riches DW, Ortiz LA. Phosphorylation of tumor necrosis factor receptor 1 (p55) protects macrophages from silica-induced apoptosis. J Biol Chem. 2004;179(3):2020–9. doi: 10.1074/jbc.M309763200. (Epub Oct 21, 2003 ahead of print). [DOI] [PubMed] [Google Scholar]
- 13.Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003;2(12):1273–84. [PubMed] [Google Scholar]
- 14.Skowronek M, Roterman, Konieczny L, Stopa B, Rybarska J, Piekarska B, Gorecki A, Krol M. The conformational characteristics of Congo red, Evans blue and Trypan blue. Comput Chem. 2000;24(3–4):429–50. doi: 10.1016/s0097-8485(99)00089-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Lin WK, Cha JG, An NH, Yoo SJ, Park JH, Kim HM, Lee YM. The activation of PI3-K and PKC zeta in PMA-induced differentiation of HL-60 cells. Cancer Lett. 2001;171(1):79–85. doi: 10.1016/s0304-3835(01)00505-5. [DOI] [PubMed] [Google Scholar]
- 16.Lopez S, Peiretti F, Morange P, Laouar A, Fossat C, Bonardo B, Huberman E, Juhan-Vague I, Nalbone G. Activation of plasminogen activator inhibitor—1 synthesis by phorbol esters in human promyelocyte HL-60—roles of PKCbeta and MAPK p42. Thromb Haemost. 1999;81(3):415–22. [PubMed] [Google Scholar]
- 17.Jiang X, Norman M, Li X. Use of an array technology for profiling and comparing transcription factors activated by TNFalpha and PMA in HeLa cells. Biochem Biophys Acta. 2003;1642(1–2):1–8. doi: 10.1016/s0167-4889(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 18.Petricoin E, III, David M, Igarashi K, Benjamin C, Ling L, Goelz S, Finbloom DS, Larner AC. Inhibition of alpha interferon but not gamma interferon signal transduction by phorbol esters is mediated by a tyrosine phosphatase. Mol Cell Biol. 1996;16(4):1419–24. doi: 10.1128/mcb.16.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos-Gonzalez R, Glenney JR., Jr Temperature-dependent tyrosine phosphorylation of microtubule-associated protein kinase in epidermal growth factor-stimulated human fibroblasts. Cell Regul. 1991;2(8):663–73. doi: 10.1091/mbc.2.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichler W, Hamann J, Aust G. Expression characteristics of the human CD97 antigen. Tissue Antigens. 1997;50(5):429–38. doi: 10.1111/j.1399-0039.1997.tb02897.x. [DOI] [PubMed] [Google Scholar]
- 21.Kreideweiss S, Ahlers C, Nordheim A, Ruhlmann A. Ca2+-induced p38/SAPK signalling inhibited by the immunosuppressant cyclosporin A in human peripheral blood mononuclear cells. Eur J Biochem. 1999;265(3):1075–84. doi: 10.1046/j.1432-1327.1999.00830.x. [DOI] [PubMed] [Google Scholar]
- 22.Ku H, Meier KE. Phosphorylation of paxillin via the ERK mitogen-activated protein kinase cascade in EL4 thymoma cells. J Biol Chem. 2000;275(15):11333–40. doi: 10.1074/jbc.275.15.11333. [DOI] [PubMed] [Google Scholar]
- 23.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF–E2-related factor 2. Proc Natl Acad Sci USA. 2001;98(1):379. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zhang J, Yi XJ, Yu FS. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp Eye Res. 2004;78(1):125–36. doi: 10.1016/j.exer.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Smith L, Porzig H, Lee HW, Smith JB. Phorbol esters down-regulate expression of the sodium/calcium exchanger in renal epithelial cells. Am J Physiol. 1995;269(2 Part 1):C457. doi: 10.1152/ajpcell.1995.269.2.C457. [DOI] [PubMed] [Google Scholar]
- 26.Sriraman V, Sharma SC, Richards JS. Transactivation of the progesterone receptor gene in granulosa cells: evidence that Sp1/ Sp3 binding sites in the proximal promoter play a key role in luteinizing hormone inducibility. Mol Endocrinol. 2003;17(3):436–49. doi: 10.1210/me.2002-0252. [DOI] [PubMed] [Google Scholar]
- 27.Leahy DJ, Aukhil I, Erickson HP. A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84(1):155–64. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 28.Grant RP, Spitzfaden C, Altroff H, Campbell ID, Mardon HJ. Structural requirements for biological activity of the ninth and tenth FIII domains of human fibronectin. J Biol Chem. 1997;272(10):6159–66. doi: 10.1074/jbc.272.10.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271(1):21–4. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Frazier WA, Gao AG, Dimitry J, Chung J, Brown EJ, Lindberg FP, Linder ME. The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi. J Biol Chem. 1999;274(13):8554–60. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem. 2001;276(43):40156–66. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 32.Shahan TA, Fawzi A, Bellon G, Monboisse JC, Kefalides NA. Regulation of tumor cell chemotaxis by type IV collagen is mediated by a Ca(2+)-dependent mechanism requiring CD47 and the integrin alpha(V)beta(3) J Biol Chem. 2000;275(7):4796–802. doi: 10.1074/jbc.275.7.4796. [DOI] [PubMed] [Google Scholar]
- 33.Rebres RA, Green JM, Reinhold MI, Ticchioni M, Brown EJ. Membrane raft association of CD47 is necessary for actin polymerization and protein kinase C theta translocation in its synergistic activation of T cells. J Biol Chem. 2001;276(10):7672–8. doi: 10.1074/jbc.M008858200. (Epub 2000 December 12). [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Chou C, Sun Y, Huang W. Tumor necrosis factor alpha-induced activation of downstream NF-kappaB site of the promoter mediates epithelial ICAM-1 expression and monocyte adhesion. Involvement of PKCalpha, tyrosine kinase, and IKK2, but not MAPKs, pathway. Cell Signal. 2001;13(8):543–53. doi: 10.1016/s0898-6568(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 35.Soltoff SP. Evidence that tyrphostins AG10 and AG18 act as mitochondrial uncouplers and alter phosphorylation-dependent cell signaling. J Biol Chem. 2004;279(12):10910–8. doi: 10.1074/jbc.M305396200. [DOI] [PubMed] [Google Scholar]
- 36.Eimerl S, Orly J. Regulation of steroidogenic genes by insulin-like growth factor-1 and follicle-stimulating hormone: differential responses of cytochrome P450 sidechain cleavage, steroidogenic acute regulatory protein, and 3beta-hydroxysteroid dehydrogenase/isomerase in rat granulosa cells. Biol Reprod. 2002;67(3):900–10. doi: 10.1095/biolreprod.101.002170. [DOI] [PubMed] [Google Scholar]


