Abstract
Background
Given concern for hernia mesh infection, surgeons often use biologic mesh which may provide reduced risk of infection but at the cost of decreased repair durability. We evaluated mesh coating to provide sustained release of antibiotics to prevent prosthetic mesh infection and also allow a durable repair.
Materials and methods
Cyclodextrin-based polymer was crosslinked onto multifilament polyester mesh and loaded with vancomycin (1.75 mg/cm2). Pigs received modified meshes (n =6) or normal, untreated meshes (n =4), which were implanted into acute 10 × 5 cm ventral hernia, then directly inoculated with 106 colony-forming unit (CFU) of methicillin-resistant Staphylococcus aureus (MRSA). These were compared to animals receiving normal, uninfected mesh. All mesh was secured in an underlay bridge manner, and after 30 d, the abdominal wall was removed for quantitative bacterial culture and biomechanical analysis.
Results
All animals survived 30 d. All six animals with coated mesh cleared MRSA infection. The four control animals did not clear MRSA (P =0.005). Quantitative bacterial load was higher in standard mesh versus drug-delivery mesh group (2.34×104 versus 80.9 CFU/gm). These data were log10-transformed and analyzed by Welch’s t-test (P = 0.001). Minimum number of CFUs detectable by assay (300) was used instead of zero. Biomechanical analysis of controls (1.82 N/mm infected; 1.71 N/mm uninfected) showed no difference to the modified meshes (1.31 N/mm) in tissue integration (P = 0.15).
Conclusions
We successfully prevented synthetic mesh infection in a pig model using a cyclodextrin-based polymer to locally deliver vancomycin to the hernia repair site and clearing antibiotic-resistant bacteria. Polymer coating did not impact the strength of the hernia repair.
Keywords: Hernia, Infection, Mesh, Pig, Synthetic, Polyester, Treatment, Prevention, Cyclodextrin, Coating, Vancomycin
Introduction
The advent of prosthetic materials has revolutionized hernia surgery by significantly reducing hernia recurrence rates. The ideal properties of prosthetic mesh have changed little since it was first introduced providing a durable repair while reducing the risk of potential complications including infection, chronic inflammation, and intra-abdominal adhesions. Despite advances in some of these areas, we lack adequate options for the prevention of prosthetic mesh infection.1,2
Mesh infections can present up to a year following implantation, yet are often thought to be the result of contamination at the time of implantation.2 Current options available for treating such an infection rely on high doses of systemic antibiotics, which provide varied penetration into the local tissue.3 In addition, such therapies put patients at risk for associated complications including Clostridium difficile and the development of resistant organisms.4 Despite the most aggressive treatment, prosthetic device infections frequently require the removal of the device.
Therefore, instead of treating infections, many surgeons have turned to prevention of infections. Current mainline therapy includes perioperative antibiotics with the goal of obtaining sufficient local tissue levels to prevent bacterial growth. As an adjunct, some investigators are evaluating the potential for drug-delivery polymers which can be used to coat prosthetic devices in an attempt to provide sustained local drug levels. Initial studies have shown varied success5,6 due to the fact that these polymers often rely on diffusion alone for drug release, resulting in a highly nonlinear profile with a rapid burst of the majority of drug on the order of hours to days, leaving very little behind to be delivered on later dates. This diffusion-based, biphasic release has the potential consequence of too much drug at the initial time points and too little drug at later time points, a perfect storm for generating drug-resistant bacteria. Given the nearly year-long window for hernia mesh infection, we hypothesize that a longer, more sustained delivery profile is necessary to kill bacteria and prevent infections.
Our group has shown success in developing an affinity-based polymer that provides controllable and sustained release of antibiotics from weeks to months. This work utilizing both in vitro and in vivo models demonstrated our ability to coat a sample (~0.7 × 0.7 cm) of prosthetic mesh and prevent a subcutaneous Staphylococcus aureus infection in rodents.7–9 The aim of the current study was to expand upon our previous work and evaluate the ability of a polymer-coated mesh loaded with vancomycin (VM) to prevent methicillin-resistant S aureus (MRSA) infection while providing a durable ventral hernia repair.
Methods
Creation of modified meshes
A 30% (wt/wt) cyclodextrin prepolymer (CD) solution in 0.2 M potassium hydroxide was mixed with ethylene glycol diglycidyl ether (EGDGE) at a molar ratio of 1:0.7 CD: EGDGE. A 15 cm × 10 cm piece of polyester mesh (Parietex TET, Covidien, Mansfield, MA) was uniformly coated with 20 mL of the CD-EGDGE solution; the polymer was allowed to crosslink via base-catalyzed epoxide ring opening on the polyester mesh for 5 d at room temperature in a sealed stainless steel tray. The polymerized CD (pCD)–coated meshes were removed and washed extensively in distilled water for 3 d replacing the water periodically to wash out excess reagents. After washing, each mesh was partially dried and VM was loaded by incubating polymer-coated mesh in a 5% (wt/vol) aqueous VM solution for 2 d. After 2 d, the mesh was briefly rinsed to remove excess, unbound drug; exposed to UV light 20 min on each side to sterilize; and then kept in a sealed plastic container until used. Extensive chemical and physical characterization of these and similar meshes were previously reported.7,8
Bacteria
A clinical strain of MRSA (Xen30, Caliper LifeSciences, Hopkinton, MA) was cultured overnight, diluted 1:50 and then placed in a 37°C shaker allowing the bacterial to reach a concentration of 108 colony-forming unit (CFU)/mL based on optical density. This solution was diluted utilizing serial dilutions in sterile 0.9% normal saline (NS) to obtain a concentration of 106 CFU/mL. One cubic centimeters of fluid was then used to inoculate the mesh after it has been secured in place and prior to closing the wound as described below.
Animals–surgical repair
Female Yorkshire pigs (30–35 kg; Pineview Farms, Valley City, OH) were acclimated to our facility for 7 d prior to surgery. Due to large differences between abdominal tissue composition between males and females, in this preliminary study, only female animals were used. All animal care and operative procedures were performed in accordance with the US Public Health Service Guide for the Care of Laboratory Animals (NIH Publication 85-23, 1985) and were performed with the prior approval of the Case Western Reserve University Institutional Animal Care and Use Committee. Induction of surgical anesthesia consisted of intramuscular injection of Telazol (6-8 mg/kg), followed by endotracheal intubation, and maintenance of anesthesia with inhaled isoflurane (2%-5%). Postoperative pain control was obtained using local injection of Marcaine (5% diluted 1:10) followed by a fentanyl patch (25 μg) for the first 72 h. Prior to any surgery and at the time of necropsy, the abdominal wall was clipped and prepped with 70% chlorhexidine solution. Animals received a single, preoperative dose of systemic antibiotics (Baytril 7.5 mg/kg).
Surgical repair began by creating a 10-cm midline laparotomy centered over the umbilicus. The platysma muscle was then freed from the rectus and external oblique muscle. Rectus muscle (~2.5 cm) was removed from both the left and right sides utilizing electrocautery to create a 10 cm × 5 cm final defect. Animals were then randomly assigned to repair using either pCD-coated mesh loaded with VM or control polyester mesh (Parietex TET, Covidien, Mansfield, MA). The defect was repaired in an underlay bridge fashion utilizing a 15 cm × 10 cm piece of mesh, which provided 2.5 cm of mesh tissue overlap in each direction. Of the animals which were inoculated with MRSA, six animals received the cyclodextrin polymer-coated, drug-loaded meshes, which four animals received standard, unmodified meshes. These were compared to animals which also underwent ventral hernia repair using standard, unmodified meshes but were not infected.
Mesh was secured with interrupted 0-polypropylene transfascial sutures placed every 2-3 cm. Prior to closure of the wound, the animals in the infection groups were inoculated with 106 CFUs of MRSA, directly into the mesh, as prepared above. The subcutaneous tissue was closed with a running 2-0 polyglactin suture followed by the skin with a subcutaneous 4-0 polyglactin suture.
Necropsy
Animals were euthanized 30 d after hernia repair. The abdominal wall was clipped and prepped with 70% chlorhexidine solution to remove skin flora. A full thickness abdominal wall explant was performed using sterile technique. Two samples were obtained from each animal for biomechanical testing. Two additional samples were obtained for quantitative cultures.
Bacterial culture
All samples were weighed, placed in 10 mL sterile NS, ho-mogenized, and then serially diluted 10-fold in NS. The original suspension and all dilutions were plated in 100 μL volume on TSA plates with 5% sheep blood and incubated for 24 h at 37°C. Bacterial growth was quantified from plates showing 30-300 CFU per plate and expressed as CFU/g of specimen based on the weight of each specimen. Bacterial clearance, as a percentage, was defined as the number of animals with no MRSA growth on cultures divided by the total number of animals for a particular group.
Biomechanics
Samples (n = 2 per animal) were cut into a dumbbell shape using a standardized tissue press centering the press over the mesh-tissue interface (7 mm wide by 40 mm long). Within 4 h of euthanasia, uniaxial mechanical testing was performed on an Instron 5543 Frame (Instron, Norwood, MA) with FlexTest SE controller (MTS, Eden Prairie, MN) in a saline bath at 36°C. All specimens were preconditioned for 10 cycles at 50 g followed by a test of constant velocity ramp to failure at 4 mm/s. Tensile strength of the mesh-tissue interface was defined as the normalized maximum stress (maximum force divided by specimen width; Newtons/mm width). Mesh-tissue interface samples from pigs that were not infected was previously analyzed using this technique and used for baseline comparison.
Data analysis
Data were analyzed using Stata version 10. Percent bacterial clearance and average bacterial counts (CFU/g) were calculated following culture studies. For statistical purposes, when no MRSA was detected from bacterial culture, normal microbiology procedure is to set the value not at “0” but at the minimum number of colonies reproducibly detectable (300 CFU). The data were log10-transformed prior to statistical analysis.10 Mean tensile strength (N/mm) was compared across the two study groups. Welch’s unequal variances t-test and Fisher’s exact tests were used when appropriate. A P-value < 0.05 was considered significant for all tests.
Results
Ten pigs were included in the study; four control animals underwent repair with noncoated polyester mesh, and six animals underwent repair with pCD-coated mesh loaded with VM. There were no complications during the postoperative period, and all animals survived for the entire study duration.
Bacterial analysis
After 30 d, all control animals repaired with standard polyester mesh showed subjective signs of bacterial contamination (Fig. 1A and B), whereas all pCD-coated mesh was free of any visible signs of contamination (Fig. 1C and D). When evaluating bacterial clearance, all pCD-coated samples (6/6) successfully cleared the inoculated MRSA and had no detectable growth on bacterial culture. In contrast, none (0/4) of the noncoated polyester animals were able to clear the bacterial load (P = 0.005) and had an average MRSA growth of 2.34 × 104 CFU/gm versus no detectable growth in the pCD-coated mesh (average 80.9 CFU/gm). The bacterial counts from the control group ranged from 8400 to 56,700 CFU/g (Table 1) as a result of this variance all quantitative culture data were log10-transformed prior to statistical analysis via Welch’s t-test accounting for the heteroscedasticity.10 After log10-transformation, the pCD-coated mesh was significantly different from the polyester mesh control (P = 0.001; Table 1).
Fig. 1.
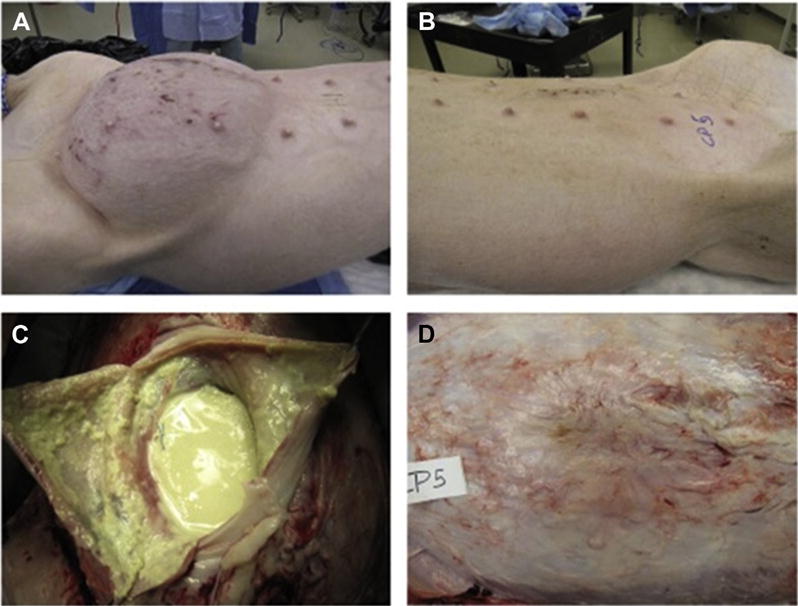
Images taken at the study end point (30 d). These images are representative of animals in the infected/untreated mesh condition (A and B) and in the antibiotic delivery mesh condition (C and D). Control animals with no infection appeared similar to (C and D) and are not shown. Infected/untreated animals showed extensive signs of infection as well as inflammatory/immunological response (B) in addition to significant abdominal distension (A). Animals receiving antibiotic delivery meshes showed excellent hernia repair (C) and minimal inflammation and scarring on the abdominal flap (D). (Color version of figure is available online.)
Table 1.
Bacterial counts from all infected animals in this study.
| Animal number | Unmodified meshes (CFU) | Drug-delivery meshes (CFU) |
|---|---|---|
| Control 1 | 200,334 | |
| Control 2 | 84,875 | |
| Control 3 | 84,004 | |
| Control 4 | 567,362 | |
| Experimental 1 | 300* | |
| Experimental 2 | 300* | |
| Experimental 3 | 300* | |
| Experimental 4 | 300* | |
| Experimental 5 | 300* | |
| Experimental 6 | 300* | |
| Average | 2.34×104 | 300* |
The four control animals receiving a standard, unmodified mesh showed extensive infection at the study end point (30 d), with an average of 2.34 × 104 CFU. The six experimental animals receiving antibiotic delivery meshes all showed no surviving bacteria. Even though no bacteria were detected, as per microbiological standards, all conditions are set at 300 CFU, which is the minimum detectable units for these experimental conditions.
Samples with 0 colonies were set to the minimum detectable unit of 300 CFU.
Biomechanical analysis
Through biomechanical analysis, we determined no significant difference in the tensile strength of the mesh-tissue interface in all tested conditions (Fig. 2). The samples included (1) noncoated, infected meshes (1.82 N/mm), (2) noncoated, uninfected meshes (1.71 N/mm), and (3) the pCD-coated mesh (1.31 N/mm respectively; P = 0.15). All tested samples had failure points at the mesh-tissue interface. Future studies can investigate longer term mechanical strength differences as tissue remodeling continues to progress after 30 d.
Fig. 2.
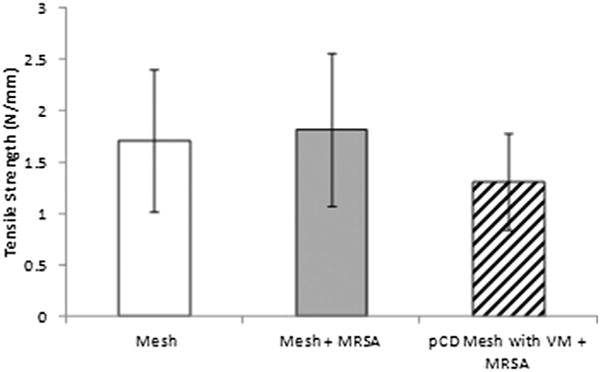
Mechanical testing (tissue integration strength) of samples at the study end point (30 d). Mechanical pull-strength testing was performed on all samples for the three conditions: (1) Uninfected control animals receiving normal mesh implants with no introduced infection; (2) infected control animals receiving normal mesh implants but receiving 106 CFU of MRSA; and (3) experimental conditions where animals receive antibiotic delivery mesh implants, as well as 106 CFU of MRSA. There was no significant difference between the three conditions (P = 0.15), and all tested samples had failure points at the mesh-tissue interface.
Discussion
Prosthetic mesh infections remain a significant challenge in hernia surgery. This study represents our ongoing work evaluating the use of pCD coating for the prevention of prosthetic mesh infections. We previously demonstrated that coating a piece of polyester mesh with pCD loaded with VM will successfully prevent a MRSA mesh infection at 30 d when directly inoculated. This work serves as an initial investigation into using this antibiotic delivery technology in the setting of a ventral hernia repair. Furthermore, the coating of polyester mesh with pCD polymer does not significantly alter the ability of the mesh to provide a durable ventral hernia repair.
Since Luijendijk et al.11 first reported the role of using prosthetic mesh at the time of hernia repair, prosthetic mesh has become a mainstay for surgeons. Despite advances in mesh design and manufacturing,12 few advances have been made in the prevention of prosthetic mesh infections with recent studies putting the risk of mesh infection anywhere from 10% to 25%.13,14 Additionally, some studies have reported mesh infections to occur out to 33 mo after implantation.15 Of increasing concern is the widespread prevalence of MRSA infections both in the community as well as in the hospital.1,16 With the potential for MRSA to form a biofilm, which can be extremely difficult to eradicate, many people have turned to using biologic grafts in these patients.1 However, the ability to prevent mesh infections from occurring in the first place may be more worthwhile.
Currently, DualMesh Plus (Gore, Flagstaff, AZ) is the only commercially available synthetic mesh with the primary goal of reducing prosthetic mesh infections. DualMesh Plus is manufactured from polytetrofluorethylene loaded with chlorhexidine diacetate and silver carbonate relying on the principle of highly nonlinear, diffusion-based, “burst-type” release where the drug diffuses rapidly from high drug concentration in the mesh into the local tissue. However, DualMesh Plus’s duration of activity is limited to only 14 d, and the release pattern of the drug is rapid and nonlinear resulting in a high percentage of the release taking place within the first hours to days.9,17 This rapid release has been associated with systemic inflammatory effects that can often have a deleterious clinical response,18 and clinical data are not clear whether this mesh provides decreased infection rates and prevention of biofilm formation.1,12,19,20 Recent approval of XenMatrix AB (Bard, Murray Hill, NJ) made another antimicrobial hernia mesh solution available to clinicians. XenMatrix is a biologic (porcine dermal graft) coated with minocycline and rifampin. Due to the difference in mesh mechanics, resorption, and immune/inflammatory response, it is difficult to make a direct comparison between this biologic mesh, and the synthetic meshes studied here. However, the approval of this mesh demonstrates the strong need for a successful solution in this product space.
The pCD polymer used in this study takes advantage of a technology referred to as affinity-based drug release. The rate limiting step in release is based primarily on the chemical affinity between the drug of choice (in this study VM) and the CD comprising the polymer. The rate of antibiotic release can be tailored by adjusting the composition of the pCD polymer (e.g., switching types of CD–α, β, or γ CD whose rings comprise different numbers of glucose residues; or altering crosslinking) or choice of different antibiotics. In addition to prosthetic hernia meshes, this pCD-based delivery polymer can be used to coat other substrates such as orthopedic devices such as total knee replacement implants.
The initial challenge in designing a pCD-coated mesh was obtaining a uniform, thin film followed by optimizing the release profile to provide longer bactericidal activity, which is important clinically. Initial in vitro studies using pCD-coated polyester mesh demonstrated continued antibacterial activity upward of 40 d when bound with a hydrophobic drug such as rifampin. Given the nature of CD’s hydrophobic pocket, when the pCD polymer is bound with a more hydrophilic drug such as VM, the length of antimicrobial activity was somewhat shorter but still provided lethal doses of antibiotic for more than 14 d.8 The clinical role of MRSA in prosthetic mesh infections warranted further investigation into the use of VM with the pCD polymer.19,21
The initial in vivo study investigated the ability of pCD-coated mesh loaded with VM to resist S aureus infection in a mouse subcutaneous model.7 Samples of pCD-coated mesh loaded with VM were compared against standard (i.e., noncoated) samples of mesh treated with local VM flush. At both 2 and 4 wk, S aureus infection persisted in the VM flush group but was undetectable in the pCD-coated mesh group. In addition to demonstrating the ability of CD polymer to resist S aureus infection in vivo, this work also provided initial insight into safety and the nontoxic nature of a CD polymer-coated mesh as no gross toxicity or excessive foreign body response was observed. However, this study did not evaluate the potential of the pCD-coated mesh to repair an actual hernia defect, and what effect the drug-loaded, coated mesh may have on the desired mesh-tissue integration.
The goal of the current pilot study was to evaluate the use of a pCD-coated mesh in the setting of an infected ventral hernia repair. The animals repaired with pCD-coated mesh loaded with VM were able to clear the MRSA infection 100% of the time in contrast to the control polyester mesh, which none of the animals (0/4) were able to clear the 106 CFU bacterial load (P = 0.001), and without impacting the durability of the repair. This study only investigated out to 30 d. It is possible that infection was only repressed, and not fully eradicated, so future work will explore longer term end points. Nevertheless, in all animals (6/6), there was no detectable growth at 30 d. Presumably if any bacteria were present, but suppressed they would have been identified during outgrowth.
An additional benefit of the pCD polymer coating over systemic antibiotic administration is the significant dose reduction of antibiotics required. In this study, each hernia defect was repaired with a 15 cm × 10 cm (area of 150 cm2) piece of pCD-coated mesh loaded with 1.75 mg/cm2 VM. Therefore, the total VM dose was approximately 262.5 mg of VM, which is approximately one-fourth of a single intravenous dose of VM given to a patient with a mesh infection. This need for less drug will significantly reduce systemic levels, limit the potential for costly adverse reactions, and provide the lowest possibility for driving antibiotic resistance by delivering the drug in the most effective manner possible–directly to the defect site. The intent of this kind of local delivery is to provide sufficient quantity to eradicate local bacteria but not reach high systemic levels.
Any mesh used in ventral hernia repair must provide durable repair with good tissue ingrowth into the mesh. Although this study represents a relatively small group of animals (n = 10), the pCD polymer coating did not significantly interfere with the tensile strength of the mesh-tissue interface when measured biomechanically (P = 0.15). The underlay bridge form of hernia repair used in this work is not necessarily the clinically preferred method of repair but does serve as a worst-case scenario when evaluating the ability of the mesh to provide a durable repair and clear bacterial contamination. Similarly, we used an unmodified or standard mesh as the infected control to provide a worst-case scenario, and a situation similar to what would be seen clinically.
This study serves as an initial evaluation of the feasibility for using pCD-coated mesh in the setting of a ventral hernia repair. Further investigation into the local and systemic levels of drug released from the mesh is warranted. In addition, this study only evaluated a single time point of 30 d. Although we have previously shown long-term (>40 d) inhibitory action in vitro,8 later in vivo time points should be undertaken to investigate long-term antimicrobial activity. In addition, while long-term biocompatibility was not specifically investigated, the short-term observations showed no concerns in regard to toxicity or foreign body response, comparable to what was seen in the rodent studies. Nevertheless, evaluation of foreign body response to the pCD polymer coating will be critical to successful long-term hernia repair.
Prosthetic mesh infection following ventral hernia repair remains a significant problem despite significant advances in mesh design. This study utilizes an affinity-based drug-delivery system for the prevention of mesh infection in a ventral hernia model. Additional work evaluating the long-term effects and the biocompatibility at cellular and molecular levels is ongoing.
Acknowledgments
Research reported in this publication was supported by the Coulter-Case Translation and Innovation Partnership and in part by National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM121477.
Disclosures
Michael J. Rosen is a Bard speaker, Ariste Medical board member, and has received research support from W.L. Gore & Associates, Inc. and from Miromatrix Medical, Inc.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of CCTIP or the National Institutes of Health.
Author contributions: T.R.T., S.T.Z., and H.A.v.R. developed the polymer, synthesized it as mesh coating, and experimentally determined its characteristics. They helped with analysis and interpretation of data and they edited the manuscript. J.A.B., D.M.K., and M.J.R. helped conceive, design, and conduct the study and analyzed and interpreted the data. They drafted and edited the manuscript. All authors gave approval for submission of this manuscript.
References
- 1.Brown RH, Subramanian A, Hwang CS, Chang S, Awad SS. Comparison of infectious complications with synthetic mesh in ventral hernia repair. Am J Surg. 2013;205:182–187. doi: 10.1016/j.amjsurg.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Tolino MJ, Tripoloni DE, Ratto R, Garcia MI. Infections associated with prosthetic repairs of abdominal wall hernias: pathology, management and results. Hernia. 2009;13:631–637. doi: 10.1007/s10029-009-0541-y. [DOI] [PubMed] [Google Scholar]
- 3.Barza M, Cuchural G. General principles of antibiotic tissue penetration. J Antimicrob Chemother. 1985;15:59–75. doi: 10.1093/jac/15.suppl_a.59. [DOI] [PubMed] [Google Scholar]
- 4.Johanesen PA, Mackin KE, Hutton ML, et al. Disruption of the gut microbiome: clostridium difficile infection and the threat of antibiotic resistance. Genes (Basel) 2015;6:1347–1360. doi: 10.3390/genes6041347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chilukuri DM, Shah JC. Local delivery of vancomycin for the prophylaxis of prosthetic device-related infections. Pharm Res. 2005;22:563–572. doi: 10.1007/s11095-005-2497-7. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Alonso A, Garcia-Criado FJ, Parreno-Manchado FC, et al. Study of the efficacy of coated VICRYL plus antibacterial suture (coated Polyglactin 910 suture with Triclosan) in two animal models of general surgery. J Infect. 2007;54:82–88. doi: 10.1016/j.jinf.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Harth KC, Rosen MJ, Thatiparti TR, et al. Antibiotic-releasing mesh coating to reduce prosthetic sepsis: an in vivo study. J Surg Res. 2010;163:337–343. doi: 10.1016/j.jss.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 8.Thatiparti TR, Shoffstall AJ, von Recum HA. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials. 2010;31:2335–2347. doi: 10.1016/j.biomaterials.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 9.Thatiparti TR, von Recum HA. Cyclodextrin complexation for affinity-based antibiotic delivery. Macromol Biosci. 2010;10:82–90. doi: 10.1002/mabi.200900204. [DOI] [PubMed] [Google Scholar]
- 10.Jarvis B, Hedges AJ, Corry JE. Assessment of measurement uncertainty for quantitative methods of analysis: comparative assessment of the precision (uncertainty) of bacterial colony counts. Int J Food Microbiol. 2007;116:44–51. doi: 10.1016/j.ijfoodmicro.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 12.Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ. In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg. 2012;16:2139–2144. doi: 10.1007/s11605-012-1992-5. [DOI] [PubMed] [Google Scholar]
- 13.Balique JG, Benchetrit S, Bouillot JL, et al. Intraperitoneal treatment of incisional and umbilical hernias using an innovative composite mesh: four-year results of a prospective multicenter clinical trial. Hernia. 2005;9:68–74. doi: 10.1007/s10029-004-0300-z. [DOI] [PubMed] [Google Scholar]
- 14.Harrell AG, Novitsky YW, Kercher KW, et al. In vitro infectability of prosthetic mesh by methicillin-resistant Staphylococcus aureus. Hernia. 2006;10:120–124. doi: 10.1007/s10029-005-0056-0. [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer C, Andersen PV, Juul P, Qvist N. Late mesh rejection as a complication to transabdominal preperitoneal laparoscopic hernia repair. Surg Endosc. 1998;12:1164–1165. doi: 10.1007/s004649900807. [DOI] [PubMed] [Google Scholar]
- 16.Hicks CW, Blatnik JA, Krpata DM, Novitsky YW, Rosen MJ. History of methicillin-resistant Staphylococcus aureus (MRSA) surgical site infection may not be a contraindication to ventral hernia repair with synthetic mesh: a preliminary report. Hernia. 2014;18:65–70. doi: 10.1007/s10029-012-1035-x. [DOI] [PubMed] [Google Scholar]
- 17.Langer R. Implantable controlled release systems. Pharmacol Ther. 1983;21:35–51. doi: 10.1016/0163-7258(83)90066-9. [DOI] [PubMed] [Google Scholar]
- 18.Cobb WS, Paton BL, Novitsky YW, et al. Intra-abdominal placement of antimicrobial-impregnated mesh is associated with noninfectious fever. Am surg. 2006;72:1205–1208. discussion 1208–1209. [PubMed] [Google Scholar]
- 19.Bellows C, Smith A. In vitro study of biofilm growth on biologic prosthetics. Pol J Microbiol. 2014;63:409–414. [PubMed] [Google Scholar]
- 20.Cevasco M, Itani KM. Ventral hernia repair with synthetic, composite, and biologic mesh: characteristics, indications, and infection profile. Surg Infect (Larchmt) 2012;13:209–215. doi: 10.1089/sur.2012.123. [DOI] [PubMed] [Google Scholar]
- 21.Ousley J, Baucom RB, Stewart MK, et al. Previous methicillin-resistant Staphylococcus aureus infection independent of body site increases odds of surgical site infection after ventral hernia repair. J Am Coll Surg. 2015;221:470–477. doi: 10.1016/j.jamcollsurg.2015.04.023. [DOI] [PubMed] [Google Scholar]


