Abstract
Cardiovascular disease is a leading cause of morbidity and mortality in the Western world. Studies of sortilin's influence on cardiovascular and metabolic diseases goes far beyond the genome wide association studies that have revealed an association between cardiovascular diseases and the 1p13 locus that encodes sortilin. Emerging evidence suggests a significant role of sortilin in the pathogenesis of vascular and metabolic diseases. This includes type II diabetes melitus via regulation of insulin resistance, and atherosclerosis through arterial wall inflammation and calcification, and dysregulated lipoprotein metabolism. Sortilin is also known for its functional role in neurological disorders. It serves as a key receptor for cytokines, lipids, and enzymes and participates in pathological cargo loading to and trafficking of extracellular vesicles. This article provides a comprehensive review of sortilin’s contributions to cardiovascular and metabolic diseases but focuses particularly on atherosclerosis. We summarize recent clinical findings that suggest sortilin may be a cardiovascular risk marker and also discuss sortilin as a potential drug target.
Keywords: sortilin, cardiovascular risk, atherosclerosis, calcification, clinical studies
Genetic aspects of sortilin biology: relationship between the sortilin locus and cardiovascular diseases
Atherosclerosis is a multifactorial disease. A combination of environmental and genetic factors contributes to the development of atherosclerosis. A large number of genome wide associations studies (GWAS) have been conducted in an attempt to identify novel candidate genes or loci involved in the generation of cardiac phenotypes ranging from high circulating levels of low density lipoprotein (LDL)-cholesterol (LDL-C),1–3 myocardial infarction4, 5 and various other aspects of atherosclerosis.6–9 Since 2007 new candidate genes affecting LDL-C have been identified through GWAS, including the 2 loci harboring CILP2-PBX4, and CELSR2-PSRC1-MYBPHL-SORT1.3
Several single nucleotide polymorphisms (SNPs) are in the region of the gene cluster CELSR2-PSRC1-MYBPHL-SORT1 at 1p13.3. All SNPs are located in the intergenic region between PSRC1 and CELSR2, and downstream of SORT1 and MYBPHL.10 SORT1 encodes sortilin, PSRC1 encodes proline/serine-rich coiled-coil protein 1, MYBPHL encodes Myosin Binding Protein H Like, and CELSR2 encodes cadherin EGF LAG seven-pass G-type receptor 2.
The major haplotype block, comprising rs599834, rs646776, rs629301, and rs12740374, associates with elevated LDL-C levels in many cohorts.1, 11, 12 The association of the genotype with the expression of the gene cluster SORT1-PSRC1-CELSR2 seems to be tissue-specific. Liver-based expression quantitative trait loci (eQLT) revealed that SORT1, CELSR2 and PSRC1 are co-regulated in the same direction by the above-mentioned SNPs.1, 13, 14 Schadt and colleagues used 427 human liver samples and demonstrated that the minor (protective) allele of rs599839 associates with increased hepatic SORT1 and CELSR2 and the expression of both genes correlated negatively with LDL-C. In this study decreased hepatic Psrc1 associated with the minor allele genotype and with decreased LDL-C.11 Later studies by Kathiresan et al. and Musunuru et al. showed that the presence of the minor (protective) allele of rs646776 associated with elevated hepatic expression of SORT1, CELSR2 and PSRC1 and these correlated negatively to LDL-C utilizing 60 and 960 human liver samples, respectively.1, 13 In other cohorts where the minor allele of rs599839 and rs646776 also associated with decreased LDL-C, the gene cluster SORT1-PSRC1-CELSR2 was analyzed in whole blood. Data from whole genome expression showed a significant increase in SORT1 expression associated with the minor allele of rs599839, while no association was detected between the genotype and PSRC1 and CELSR2.15 Whole blood qPCR data demonstrated an association of the minor allele with the expression of PSRC1, while there was no alteration in the expression of SORT1 and CELSR2.9 Of note, the expression quantitative trait loci-eQLT effect sizes are much smaller in whole blood than in liver and therefore might not be of functional consequence for sortilin biology. Further, tissue from abdominal aortic aneurysm (n=108) revealed no association of the rs599839 or rs12740374 genotype with sortilin protein expression.11 The GWAS studies were not able to pinpoint an exact molecular mechanism of any of the individual genes, but showed correlation with LDL-C. Furthermore, the SORT1 locus also associates with the following cardiac phenotypes: coronary artery disease,6, 9, 12, 16 early onset myocardial infarction,4, 5 abdominal aorta aneurism,8 coronary stenosis,6 coronary artery calcification,7 and aortic valve calcification.17 All these diseases are interconnected and could be linked through LDL-C. As these studies show association with LDL-C, it makes it difficult to determine whether sortilin has an additional direct effect on these cardiac phenotypes independent of LDL-C. This warrants more research to interrogate the direct mechanistic effects of SORT1 in atherosclerotic plaque development, calcification, and aneurism formation through direct genetic manipulation. Realtime information on the genetic variation of SORT1 and different human tissue gene expression can be found at the database http://gtexportal.org/.18
Sortilin in atherosclerosis
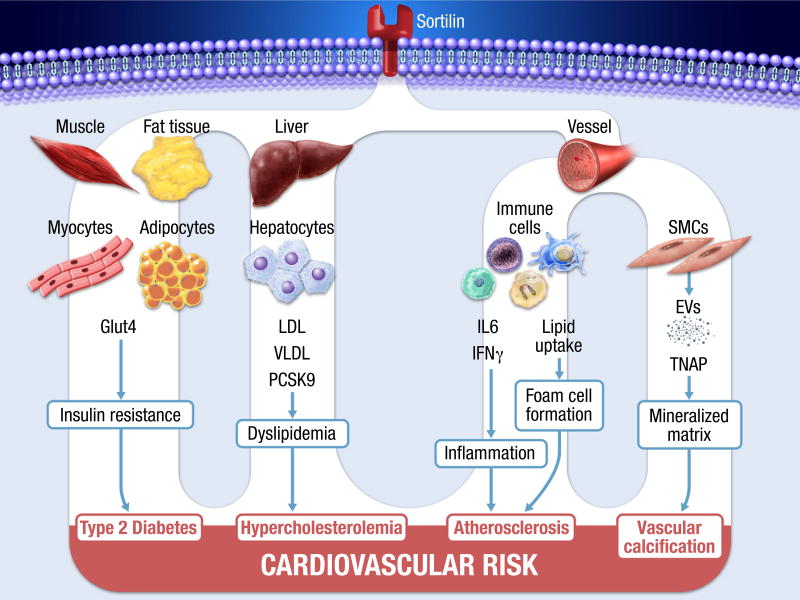
Sortilin is a member of the vacuolar protein sorting 10 protein family of sorting receptors. It is a 110 kDa single-pass type I transmembrane protein initially identified in brain tissue19 but now shown to be expressed in many cells types, including cardiovascular tissues. Sortilin is synthesized with a propeptide that is necessary for correct folding, and inhibition of premature ligand binding. The propeptide is cleaved off in the late part of the Trans-Golgi network.19 Recently sortilin was recognized as a major player in various stages of atherogenesis (Figure 1). Preclinical in vivo evidence suggests a significant role of sortilin in the pathogenesis of vascular and metabolic disorders, including atherosclerosis, through contributions to arterial wall inflammation20, 21 and calcification22, dysregulated lipoprotein metabolism,13, 23, 24 and type II diabetes melitus,25 – all cardiovascular risk factors.
Figure 1.
Multiple facets of sortilin contributing to cardiovascular risk. Sortilin locates 90% at intracellular membranes (ER/TGN/endosomes) and <10% at cell surface membranes. Sortilin participates in several pathophysiological mechanisms leading to increased cardiovascular risk.
Inflammation
The first experimental evidence for a link between sortilin and atherosclerosis was provided by Kjolby et al. who demonstrated reduced atherosclerotic plaque size caused by global deletion of sortilin in Ldlr-deficient mice.23 This finding was later supported by Patel et el. using sortilin-deficient mice in humanized Apobec1(−/−);hAPOB transgenic background.21
Both groups performed bone marrow transplantation from sortilin-deficient mice into irradiated Apoe- and Ldlr-deficient mice and showed a reduction in plaque size without changes in total cholesterol and LDL-C.20,21 Mechanistic studies revealed a role of sortilin in inflammatory response20,26,27 as well as foam cell formation,21 while macrophage recruitment was not affected.20,21 Mortenson et al. stimulated bone marrow cells isolated from sortilin-deficient and control mice towards proinflammatory macrophages using lipopolysaccharides and found reduced levels of interleukin 6 and interferon-γ secreted from sortilin-deficient macrophages and type 1 T helper cells, while other cytokines (e.g. tumor necrosis factor-α, interleukin 12) were similar between groups.20 Further, interleukin 6 levels were reduced in plasma of Apoe-deficient mice transplanted with sortlin-deficient cells and fed a Western diet for 9 weeks.20 Binding assays revealed an association of the extracellular domain of sortilin with both cytokines that was abolished by sortilin propeptide suggesting a binding to the tunnel structure.20 Patel et al. followed an in vivo approach and assessed the lipopolysaccharides-mediated inflammatory response in sortilin-deficient and control mice two and five hours post injection and did not find any difference in serum cytokine levels.21 Interestingly, sortilin/Ldlr-deficient bone marrow-derived macrophages differentiated by macrophage colony-stimulating factor demonstrated reduced foam cell formation after LDL stimulation.21 These finding were supported by in vivo foam cell formation assays on sortilin-deficient/Apobec1(−/−);hAPOB mice fed a western diet for 18 weeks. Peritoneal macrophages isolated from sortilin-deficient/Apobec1(−/−);hAPOB mice displayed reduced Oil Red O staining and cellular cholesterol.21 125I-LDL uptake studies using bone marrow-derived macrophages from sortilin-deficient mice revealed an LDL-receptor independent pathway in sortilin-mediated LDL uptake.21 Mortenson et al. also studied the role of sortilin in LDL uptake using fluorescently-labeled native, aggregated and oxidized LDL and flow cytometry.20 There was not difference in LDL uptake between sortilin-deficient bone marrow macrophages and control.20
These studies suggest that while sortilin-deficiency reduces plaque size, the impact on inflammatory response and foam cell formation varies depending on the mouse model and used methods. Whether these two different mechanistic findings are due to the different mouse genetic backgrounds is unclear and requires further investigation. Further studies on human macrophages are needed to conclude a consequence for translational aspects. For example, it would be imperative to assess the expression of sortilin in human pro- and anti-inflammatory macrophages and the expression levels in human atheroma to draw conclusion of the importance of sortilin in macrophage biology and provide mechanistic insights into the role of human sortilin in atherosclerosis development.
Dyslipidemia
It is well accepted that plasma lipid levels contribute to the pathogenesis of atherosclerosis. The controversy about sortilin’s role in hepatic lipid metabolism still exists and has been discussed in several reviews and editorials.10, 28–36
GWAS implicated sortilin in systemic cholesterol homeostasis. Human and mouse studies revealed hepatic sortilin as a protective protein attenuating circulating cholesterol levels.13 Possible underlying mechanisms include sortilin as a hepatic clearance receptor for LDL and as a sorting receptor for lipoprotein particles that reduced VLDL secretion. Two independent studies utilizing whole body sortilin-deficient mouse strains, however, showed reduced cholesterol levels.23, 24 Proposed sortilin functions associated with increased cholesterol levels include its receptor high-affinity for APOB100 to facilitate VLDL secretion from hepatocytes.25 In addition, sortilin was also identified as a high-affinity receptor for PCSK9;37 the latter binds LDLR during lysosomal degradation.38 This is of high clinical significance, since pharmacological inhibition of PCSK9 has become important in the treatment of patients with high LDL-C levels.39 In vivo loss-of and gain-of function mouse experiments showed a positive correlation of sortilin expression and circulating PCSK9 levels.37 The positive correlation of serum sortilin and PCSK9 was supported in human healthy subjects37 and non-coronary artery disease (CAD) patients.40 The impact of sortilin on cholesterol homeostasis warrants further investigation.
Vascular calcification
Vascular calcification contributes to and is an independent predictor of cardiovascular events. Computational modeling and clinical imaging studies suggest that subcellular microcalcifications in the fibrous cap of plaque enhances the risk of plaque rupture.41 Our group recently elucidated the mechanisms by which sortilin is involved in cardiovascular calcification. We showed that calcifying extracellular vesicles released by vascular smooth muscle cells (SMCs) are the smallest nidi to form microcalcification.42 In mediating the formation of calcifying extracellular vesicles on molecular level, sortilin facilitates the trafficking of the calcification protein tissue non-specific alkaline phosphatase into extracellular vesicles, thus resulting in formation of vesicles with high mineralization competence.22 Tissue non-specific alkaline phosphatase activity is sufficient to drive vascular calcification but is also necessary for bone mineralization.43 Therefore, inhibition of vascular sortilin may reduce vascular tissue non-specific alkaline phosphatase activity and thereby vascular calcification. Posttranslational modification of sortilin is one of the key mechanisms of vascular calcification.22 A C-terminal serine phosphorylation by Fam20C or casein kinase 2 is essential for sortilin trafficking in calcifying SMC. In vitro and in vivo loss-of function studies demonstrated a critical role of sortilin in the development of arterial calcification. Sortilin-deficient mice in an Ldlr-deficient background showed reduced vascular calcification without altering bone homeostasis. Bone marrow transplantation from sortilin-deficient mice to Ldlr-deficient mice demonstrated that vascular calcification was independent from infiltration of immune cells lacking sortilin. Importantly, our animal models did not show changes in cholesterol levels.22 We suggested that sortilin play a direct role in ectopic calcification, independent of potential remote effects as a consequence of its lipid metabolism function.22 Targeting C-terminal serine phosphorylation may serve as a therapeutic strategy to reduce vascular calcification.
Insulin resistance
The study of sortilin's influence on metabolic diseases goes far beyond the GWAS finding.
Different metabolic pathophysiological alterations such as hyperglycemia, hyperinsulinemia, and insulin resistance, contribute to the initiation and progression of atherosclerotic plaques.
Sortilin promotes the biogenesis of insulin-responsive GLUT4 storage vesicles in adipocytes and myocytes and impaired translocation of these vesicles is involved in the development of type II diabetes melitus.25, 44
Insulin resistance is a major cause of hepatic apolipoprotein apoB100/triglyceride overproduction in type II diabetes. Recent work suggests that sortilin plays a key role in this pathway by altering hepatic apoB100 metabolism in insulin-resistant conditions.45
Further, under diet-induced obesity, sortilin-deficient mice gained less body weight and had enhanced glucose uptake in insulin tolerance tests.46 This favorable metabolic phenotype in liver and adipose tissue was in part mediated by reduced acid sphingomyelinase activity,46 an enzyme that may regulate ceramide levels, a major modulator of insulin signaling.47
Circulating sortilin: Considering sortilin as a potential cardiovascular biomarker
Most studies have focused on modulating the expression of sortilin rather than exploring the effect of the soluble form. The main source of circulating sortilin that contributes to cardiovascular risk is not well understood. Experimental studies reported the ectodomain shedding of sortilin from neurons,48 tumor cells,49 and platelets.50 Sortilin can be shed by ADAM10.48 Others and we demonstrated that sortilin can be packed into and released from extracellular vesicles,22, 51 that could also contribute to circulating sortilin.
Only limited studies have assessed the circulating sortilin levels in patients with cardiovascular risk. The study by Japanese investigators was likely the first that revealed plasma sortilin levels in CAD patients demonstrating a 12%±27 reduction by statin treatment over 8 month in 90 CAD patients.52 There is growing evidence that statins increase plaque stabilization by promoting coronary macrocalcification; however the mechanism remains to be determined. We demonstrated that cellular smooth muscle cell sortilin promotes microcalcification; whether soluble sortilin has similar function and the effects of statins on microcalcification formation are unexplored. Future studies may focus on the understanding on how statins act on sortilin secretion and whether statin use, sortilin and vascular calcification are causally linked. Of note, changes in total cholesterol and LDL-C levels did not correlate with sortilin changes suggesting lipid independent mechanisms.52 Plasma sortilin levels were higher in patients with elevated cardiovascular risk but without CAD history as compared to CAD patients receiving aspirin therapy.50 Whether these results are an effect of the aspirin therapy or CAD history remains unclear, given that the authors demonstrated in vitro that aspirin suppresses sortilin release from activated platelets, and patients with increased cardiovascular risk but without CAD history had a higher platelet count. In addition, recent studies demonstrated an impact of aging on sortilin biology. Sortilin serum levels correlated negatively with age in men.22 In younger individuals, the presence of the minor (protective) allele of rs646776 associated with a greater genotype-specific difference in LDL-C levels than in older individuals.53 The authors however, did not report an age-cut. These data need further confirmation. Another two studies showed higher sortilin levels in statin-naïve CAD patients compared to non-CAD patients.40, 54
We recently demonstrated a significant association of serum sortilin levels with both abdominal aortic calcification and cardiovascular events in a cohort of men aged over 50 years old (n=830).55 In multivariate-adjusted analysis, the third and fourth quartiles of sortilin associated with 3.4-fold and 3.8-fold higher risk of cardiovascular events compared to the first quartile. This association was independent of traditional Framingham risk factors, including LDL-C and C-reactive protein as well as statin therapy.55 The above-mentioned studies have considerable limitations and well-designed larger studies are needed to confirm that serum sortilin levels may be a cardiovascular risk marker.
The role of sortilin-derived propeptide and its association with sortilin in cardiovascular disease is currently unknown. In a small cohort, Devander et al. showed decreased propeptide serum levels in patients with major depressive disorder when compared to healthy controls.56 A mouse study supported this finding and suggested that sortilin-derived propeptide possess anti-depressive effects.57 Whether the circulating propeptide has a specific biological function and how it traffics from the late Golgi compartment and enters the blood stream remain largely unknown. The propeptide exhibits high affinity to mature sortilin and hinders ligand binding (e.g., neurotensin).58 Determining whether the serum levels of soluble sortilin and sortilin-dervied propeptide are physiologically interrelated requires further investigations.
Sortilin also exist as a variant (17b) that is extracellular released in a truncated form and may act as a decoy receptor.59 Sortilin 17b splicing variant is formed by the inclusion of exon 17b into SORT mRNA.59 It’s regulation is reported in dementia.60 The contribution of soluble sortilin 17b to the circulating sortilin pool and the pathological consequences of abnormal splicing of sortilin in cardiovascular disease remain unknown.
There are several unanswered questions about sortilin biology that present an exciting research opportunity: What is the molecular mechanism contributing to release of circulating sortilin? Does soluble sortilin has a biological function (e.g., receptor-mediated signaling, endocytosis) or is it only a surrogate marker? What is the relative contribution of soluble versus vesicle-packed sortilin to the circulation?”
Sortilin pathway – Possible drug target?
The multiple contributions of sortilin to cardiovascular risk suggest sortilin as a potential therapeutic target for cardiovascular disease. Hampering ligand binding to the Vps10 domain pocket by small-molecules might be a feasible approach. Ligand binding has been shown to require furin-mediated propeptide cleavage.58 Neurotensin is a well-studied sortilin ligand that binds into the small binding pocket of the tunnel of the Vsp10 domain. Most ligand-sortilin binding cannot be blocked by neurotensin suggesting that other ligands prefer different binding sites.23 Nevertheless, the reaction of small-molecule ligand AF40431 and its optimized successor AF38469 with the neurotensin-binding site of sortilin was recently reported.61, 62 Whether the orally bioavailable AF38469 has a specific biological effect in vivo remains to be demonstrated.
A peptide library screen and mutation studies revealed specific amino acid residues that are critical for selective pro-neurotensin interaction without affecting other receptor functions.63 However, the authors in this study suggested that this peptide is unlikely to be suitable for therapeutic use due to its low affinity.
Sortilin intracellular domain exerts its function via posttranslational modification and ligand binding that impacts intracellular trafficking and sorting and thereby could serve as a drug target. We demonstrated by mutation studies that preventing sortilin phosphorylation at the C-terminal serine 825 reduces SMC calcification.22 The phosphorylation at serine 825 determines the intracellular sortilin location to either the trans-Golgi network (when phosphorylated) or the lysosomal system (when not phosphorylated). A phosphoproteome screen revealed additional potential phosphorylation at 819 and 821,64 but a functional consequence has not yet been reported. Li et al. identified a hepatic serine phosphorylation at 793 and 825 that is involved in dyslipidemia in type II diabetes.45, 65
An additional approach includes the modulation of sortilin expression via drug-based siRNAs, antibodies or small molecules. Recent findings from a proteomic screen identified the small signal peptide-binding drug cyclotriazadisulfonamide, an anti-HIV agent, as an inhibitor of sortilin expression.66 To our knowledge, no small molecules targeting other parts of Sortilin (tail, or stalk) have been reported.
Sortilin-directed therapy to reduce atherosclerotic/metabolic risk might have adverse effects on the nervous system. Sortilin is essential for proper neuronal functionality by controlling the trafficking and release of neurotrophins, and affect death signaling via p75NTR.67 However, recent evidence suggests sortilin as a risk factor for neurodegenerative diseases like Alzheimer’s disease and Frontotemporal dementia. Further understanding of the complex biology of sortilin in protein sorting and signaling may offer novel therapeutic strategies to combat cardiovascular and neuronal diseases.
Caution may be needed when using protein tags as an experimental tool in sortilin biology and in drug screening systems in order to avoid methodological errors. Wild type sortilin normally locates 90% inside the cell (ER/TGN/endosomes) and <10% at the cell surface.19 Adding tags to sortilin is likely to alter sortilin’s intracellular distribution and block GGA1/2/3 binding,68 consequently may alter pathways that are based on intracellular trafficking, endocytosis and ligand binding.
To our knowledge, there is no available drugs that could target sortilin and disturb ligand binding to e.g., IL6, ApoB, ApoE or PCSK9. However, the authors believe that sortilin could be a potential drug target. Since sortilin is a multi-ligand receptor, potential targeting strategies must be both tissue and pathway specific.
Concluding remarks
There are several biological mechanisms through which sortilin may contribute to regulation of cardiovascular risk. For the development of cardiovascular therapeutic strategies targeting sortilin and its pathways, the multiple functions of sortilin must be considered. Outside the brain, sortilin is best known for its activities in lipid metabolism. Recently demonstrated effects of sortilin on vascular inflammation and calcification have, however, been shown to be independent of lipids. As sortilin is acting simultaneously at multiple levels, its global inhibition could have substantial effect on cardiovascular diseases.22, 23 Future directions may include research on the pathways up-stream of cellular sortilin regulation that is currently less understood. Gaining a better understanding on the biological function of soluble sortilin (e.g., shedded, truncated or vesicular) that may serve as a signaling molecule; and the regulatory impact of posttranslational modification will broaden the options of sortilin’s druggablilty. In addition, while recent studies suggested a significant association of sortilin levels with both subclinical aortic atherosclerosis indices and cardiovascular events, larger clinical studies are needed to evaluate evidence for the clinical utility of sortilin as a diagnostic or therapeutic tool.
Supplementary Material
Acknowledgments
Source of funding: Dr. Elena Aikawa is supported by National Institutes of Health (NIH) grants R01HL 114805 and R01HL 136431. Dr. Claudia Goettsch is supported by the START-Program of the Faculty of Medicine, RWTH Aachen and German Research Foundation grant GO1801/5-1. Dr. Mads Kjolby is supported by the Danish Diabetes Academy, Novo Nordisk Foundation.
Footnotes
Disclosures: None
References
- 1.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samani NJ, Braund PS, Erdmann J, et al. The novel genetic variant predisposing to coronary artery disease in the region of the psrc1 and celsr2 genes on chromosome 1 associates with serum cholesterol. J Mol Med (Berl) 2008;86:1233–1241. doi: 10.1007/s00109-008-0387-2. [DOI] [PubMed] [Google Scholar]
- 3.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang AZ, Li L, Zhang B, Shen GQ, Wang QK. Association of snp rs17465637 on chromosome 1q41 and rs599839 on 1p13.3 with myocardial infarction in an american caucasian population. Ann Hum Genet. 2011;75:475–482. doi: 10.1111/j.1469-1809.2011.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myocardial Infarction Genetics C. Kathiresan S, Voight BF, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muendlein A, Geller-Rhomberg S, Saely CH, Winder T, Sonderegger G, Rein P, Beer S, Vonbank A, Drexel H. Significant impact of chromosomal locus 1p13.3 on serum ldl cholesterol and on angiographically characterized coronary atherosclerosis. Atherosclerosis. 2009;206:494–499. doi: 10.1016/j.atherosclerosis.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell CJ, Kavousi M, Smith AV, et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation. 2011;124:2855–2864. doi: 10.1161/CIRCULATIONAHA.110.974899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones GT, Bown MJ, Gretarsdottir S, et al. A sequence variant associated with sortilin-1 (sort1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet. 2013;22:2941–2947. doi: 10.1093/hmg/ddt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arvind P, Nair J, Jambunathan S, Kakkar VV, Shanker J. Celsr2-psrc1-sort1 gene expression and association with coronary artery disease and plasma lipid levels in an asian indian cohort. J Cardiol. 2014;64:339–346. doi: 10.1016/j.jjcc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Kjolby M, Nielsen MS, Petersen CM. Sortilin, encoded by the cardiovascular risk gene sort1, and its suggested functions in cardiovascular disease. Curr Atheroscler Rep. 2015;17:496. doi: 10.1007/s11883-015-0496-7. [DOI] [PubMed] [Google Scholar]
- 11.Schadt EE, Molony C, Chudin E, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace C, Newhouse SJ, Braund P, et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: Serum urate and dyslipidemia. Am J Hum Genet. 2008;82:139–149. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via sort1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innocenti F, Cooper GM, Stanaway IB, et al. Identification, replication, and functional fine-mapping of expression quantitative trait loci in primary human liver tissue. PLoS Genet. 2011;7:e1002078. doi: 10.1371/journal.pgen.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linsel-Nitschke P, Heeren J, Aherrahrou Z, et al. Genetic variation at chromosome 1p13.3 affects sortilin mrna expression, cellular ldl-uptake and serum ldl levels which translates to the risk of coronary artery disease. Atherosclerosis. 2010;208:183–189. doi: 10.1016/j.atherosclerosis.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Lee BS, Shin DJ, et al. A genome-wide association study of a coronary artery disease risk variant. J Hum Genet. 2013;58:120–126. doi: 10.1038/jhg.2012.124. [DOI] [PubMed] [Google Scholar]
- 17.Smith JG, Luk K, Schulz CA, et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312:1764–1771. doi: 10.1001/jama.2014.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consortium GT. The genotype-tissue expression (gtex) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen MB, Kjolby M, Gunnersen S, Larsen JV, Palmfeldt J, Falk E, Nykjaer A, Bentzon JF. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J Clin Invest. 2014;124:5317–5322. doi: 10.1172/JCI76002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, Millar J, Kruth H, Rader DJ. Macrophage sortilin promotes ldl uptake, foam cell formation, and atherosclerosis. Circulation research. 2015;116:789–796. doi: 10.1161/CIRCRESAHA.116.305811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016 doi: 10.1172/JCI80851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12:213–223. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Strong A, Ding Q, Edmondson AC, Millar JS, et al. Hepatic sortilin regulates both apolipoprotein b secretion and ldl catabolism. J Clin Invest. 2012;122:2807–2816. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of glut4 storage vesicles in 3t3-l1 adipocytes. Dev Cell. 2005;9:99–108. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Herda S, Raczkowski F, Mittrucker HW, Willimsky G, Gerlach K, Kuhl AA, Breiderhoff T, Willnow TE, Dorken B, Hopken UE, Rehm A. The sorting receptor sortilin exhibits a dual function in exocytic trafficking of interferon-gamma and granzyme a in t cells. Immunity. 2012;37:854–866. doi: 10.1016/j.immuni.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Yabe-Wada T, Matsuba S, Takeda K, Sato T, Suyama M, Ohkawa Y, Takai T, Shi H, Philpott CC, Nakamura A. Tlr signals posttranscriptionally regulate the cytokine trafficking mediator sortilin. Sci Rep. 2016;6:26566. doi: 10.1038/srep26566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linsel-Nitschke P, Samani NJ, Schunkert H. Sorting out cholesterol and coronary artery disease. N Engl J Med. 2010;363:2462–2463. doi: 10.1056/NEJMcibr1010765. [DOI] [PubMed] [Google Scholar]
- 29.Tall AR, Ai D. Sorting out sortilin. Circulation research. 2011;108:158–160. doi: 10.1161/RES.0b013e31820d7daa. [DOI] [PubMed] [Google Scholar]
- 30.Strong A, Rader DJ. Sortilin as a regulator of lipoprotein metabolism. Curr Atheroscler Rep. 2012;14:211–218. doi: 10.1007/s11883-012-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaker AM, Frishman WH. Sortilin: The mechanistic link between genes, cholesterol, and coronary artery disease. Cardiol Rev. 2014;22:91–96. doi: 10.1097/CRD.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 32.Strong A, Patel K, Rader DJ. Sortilin and lipoprotein metabolism: Making sense out of complexity. Curr Opin Lipidol. 2014;25:350–357. doi: 10.1097/MOL.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westerterp M, Tall AR. Sortilin: Many headed hydra. Circulation research. 2015;116:764–766. doi: 10.1161/CIRCRESAHA.115.306036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortensen MB, Kjolby M, Bentzon JF. Sortilin and atherosclerosis. Oncotarget. 2015;6:19352–19353. doi: 10.18632/oncotarget.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparks CE, Sparks RP, Sparks JD. The enigmatic role of sortilin in lipoprotein metabolism. Curr Opin Lipidol. 2015;26:598–600. doi: 10.1097/MOL.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt V, Willnow TE. Protein sorting gone wrong--vps10p domain receptors in cardiovascular and metabolic diseases. Atherosclerosis. 2016;245:194–199. doi: 10.1016/j.atherosclerosis.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Gustafsen C, Kjolby M, Nyegaard M, Mattheisen M, Lundhede J, Buttenschon H, Mors O, Bentzon JF, Madsen P, Nykjaer A, Glerup S. The hypercholesterolemia-risk gene sort1 facilitates pcsk9 secretion. Cell Metab. 2014;19:310–318. doi: 10.1016/j.cmet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD. Secreted pcsk9 decreases the number of ldl receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116:2995–3005. doi: 10.1172/JCI29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban D, Poss J, Bohm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62:1401–1408. doi: 10.1016/j.jacc.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 40.Hu D, Yang Y, Peng DQ. Increased sortilin and its independent effect on circulating proprotein convertase subtilisin/kexin type 9 (pcsk9) in statin-naive patients with coronary artery disease. Int J Cardiol. 2017;227:61–65. doi: 10.1016/j.ijcard.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz JL, Weinbaum S, Aikawa E, Hutcheson JD. Zooming in on the genesis of atherosclerotic plaque microcalcifications. J Physiol. 2016;594:2915–2927. doi: 10.1113/JP271339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutcheson JD, Goettsch C, Bertazzo S, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–343. doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchet R, Millan JL, Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol Biol. 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- 44.Ariga M, Nedachi T, Katagiri H, Kanzaki M. Functional role of sortilin in myogenesis and development of insulin-responsive glucose transport system in c2c12 myocytes. J Biol Chem. 2008;283:10208–10220. doi: 10.1074/jbc.M710604200. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Matye DJ, Li T. Insulin resistance induces posttranslational hepatic sortilin 1 degradation in mice. J Biol Chem. 2015;290:11526–11536. doi: 10.1074/jbc.M115.641225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabinowich L, Fishman S, Hubel E, Thurm T, Park WJ, Pewzner-Jung Y, Saroha A, Erez N, Halpern Z, Futerman AH, Zvibel I. Sortilin deficiency improves the metabolic phenotype and reduces hepatic steatosis of mice subjected to diet-induced obesity. J Hepatol. 2015;62:175–181. doi: 10.1016/j.jhep.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab. 2015;26:538–550. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Evans SF, Irmady K, Ostrow K, Kim T, Nykjaer A, Saftig P, Blobel C, Hempstead BL. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J Biol Chem. 2011;286:29556–29567. doi: 10.1074/jbc.M111.219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navarro V, Vincent JP, Mazella J. Shedding of the luminal domain of the neurotensin receptor-3/sortilin in the ht29 cell line. Biochem Biophys Res Commun. 2002;298:760–764. doi: 10.1016/s0006-291x(02)02564-0. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa K, Ueno T, Iwasaki T, Kujiraoka T, Ishihara M, Kunimoto S, Takayama T, Kanai T, Hirayama A, Hattori H. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis. 2016;249:110–115. doi: 10.1016/j.atherosclerosis.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 51.Wilson CM, Naves T, Vincent F, Melloni B, Bonnaud F, Lalloue F, Jauberteau MO. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J Cell Sci. 2014;127:3983–3997. doi: 10.1242/jcs.149336. [DOI] [PubMed] [Google Scholar]
- 52.Nozue T, Hattori H, Ogawa K, Kujiraoka T, Iwasaki T, Michishita I. Effects of statin therapy on plasma proprotein convertase subtilisin/kexin type 9 and sortilin levels in statin-naive patients with coronary artery disease. J Atheroscler Thromb. 2016;23:848–856. doi: 10.5551/jat.33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shirts BH, Hasstedt SJ, Hopkins PN, Hunt SC. Evaluation of the gene-age interactions in hdl cholesterol, ldl cholesterol, and triglyceride levels: The impact of the sort1 polymorphism on ldl cholesterol levels is age dependent. Atherosclerosis. 2011;217:139–141. doi: 10.1016/j.atherosclerosis.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh TJ, Ahn CH, Kim BR, Kim KM, Moon JH, Lim S, Park KS, Lim C, Jang H, Choi SH. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovasc Diabetol. 2017;16:92. doi: 10.1186/s12933-017-0568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goettsch C, Iwata H, Hutcheson JD, O'Donnell CJ, Chapurlat R, Cook NR, Aikawa M, Szulc P, Aikawa E. Serum sortilin associates with aortic calcification and cardiovascular risk in men. Arterioscler Thromb Vasc Biol. 2017 doi: 10.1161/ATVBAHA.116.308932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devader C, Roulot M, Moreno S, Minelli A, Bortolomasi M, Congiu C, Gennarelli M, Borsotto M, Heurteaux C, Mazella J. Serum sortilin-derived propeptides concentrations are decreased in major depressive disorder patients. J Affect Disord. 2017;208:443–447. doi: 10.1016/j.jad.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 57.Mazella J, Petrault O, Lucas G, Deval E, Beraud-Dufour S, Gandin C, El-Yacoubi M, Widmann C, Guyon A, Chevet E, Taouji S, Conductier G, Corinus A, Coppola T, Gobbi G, Nahon JL, Heurteaux C, Borsotto M. Spadin, a sortilin-derived peptide, targeting rodent trek-1 channels: A new concept in the antidepressant drug design. PLoS Biol. 2010;8:e1000355. doi: 10.1371/journal.pbio.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munck Petersen C, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J. 1999;18:595–604. doi: 10.1093/emboj/18.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prudencio M, Jansen-West KR, Lee WC, Gendron TF, Zhang YJ, Xu YF, Gass J, Stuani C, Stetler C, Rademakers R, Dickson DW, Buratti E, Petrucelli L. Misregulation of human sortilin splicing leads to the generation of a nonfunctional progranulin receptor. Proc Natl Acad Sci U S A. 2012;109:21510–21515. doi: 10.1073/pnas.1211577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohagheghi F, Prudencio M, Stuani C, Cook C, Jansen-West K, Dickson DW, Petrucelli L, Buratti E. Tdp-43 functions within a network of hnrnp proteins to inhibit the production of a truncated human sort1 receptor. Hum Mol Genet. 2016;25:534–545. doi: 10.1093/hmg/ddv491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen JL, Schroder TJ, Christensen S, Strandbygard D, Pallesen LT, Garcia-Alai MM, Lindberg S, Langgard M, Eskildsen JC, David L, Tagmose L, Simonsen KB, Maltas PJ, Ronn LC, de Jong IE, Malik IJ, Egebjerg J, Karlsson JJ, Uppalanchi S, Sakumudi DR, Eradi P, Watson SP, Thirup S. Identification of the first small-molecule ligand of the neuronal receptor sortilin and structure determination of the receptor-ligand complex. Acta Crystallogr D Biol Crystallogr. 2014;70:451–460. doi: 10.1107/S1399004713030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schroder TJ, Christensen S, Lindberg S, Langgard M, David L, Maltas PJ, Eskildsen J, Jacobsen J, Tagmose L, Simonsen KB, Biilmann Ronn LC, de Jong IE, Malik IJ, Karlsson JJ, Bundgaard C, Egebjerg J, Stavenhagen JB, Strandbygard D, Thirup S, Andersen JL, Uppalanchi S, Pervaram S, Kasturi SP, Eradi P, Sakumudi DR, Watson SP. The identification of af38469: An orally bioavailable inhibitor of the vps10p family sorting receptor sortilin. Bioorg Med Chem Lett. 2014;24:177–180. doi: 10.1016/j.bmcl.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 63.Serup Andersen O, Boisguerin P, Glerup S, Skeldal S, Volkmer R, Willnow TE, Nykjaer A, Andersen OM. Identification of a linear epitope in sortilin that partakes in pro-neurotrophin binding. J Biol Chem. 2010;285:12210–12222. doi: 10.1074/jbc.M109.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rigbolt KT, Prokhorova TA, Akimov V, Henningsen J, Johansen PT, Kratchmarova I, Kassem M, Mann M, Olsen JV, Blagoev B. System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Sci Signal. 2011;4:rs3. doi: 10.1126/scisignal.2001570. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Bi L, Hulke M, Li T. Fish oil and fenofibrate prevented phosphorylation-dependent hepatic sortilin 1 degradation in western diet-fed mice. J Biol Chem. 2014;289:22437–22449. doi: 10.1074/jbc.M114.548933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Puyenbroeck V, Claeys E, Schols D, Bell TW, Vermeire K. A proteomic survey indicates sortilin as a secondary substrate of the er translocation inhibitor cyclotriazadisulfonamide (cada) Mol Cell Proteomics. 2017;16:157–167. doi: 10.1074/mcp.M116.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for prongf-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 68.Cramer JF, Gustafsen C, Behrens MA, Oliveira CL, Pedersen JS, Madsen P, Petersen CM, Thirup SS. Gga autoinhibition revisited. Traffic. 2010;11:259–273. doi: 10.1111/j.1600-0854.2009.01017.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.