Abstract
Group B coxsackieviruses (CVBs) are a group of common human pathogens producing various clinical symptoms. Although the virology of CVB is well known, there is limited information on viral pathogenesis and the relationship between clinical symptoms and viral phenotype, particularly for CVB type 2 (CVB2). In 2004 in Korea, two CVB2 strains were isolated: CB2/04/279 from stool of an acute myocarditis patient with heart failure and CB2/04/243 from an aseptic meningitis patient. In this study, a high degree of homology was observed between the CB2/04/279 and CB2/04/243 full genome sequences. The two Korean CVB2 isolates had 93.1% homology compared to 82.1%–82.5% nucleotide sequence identity with the cardiovirulence-associated reference CVB strain Ohio-1 (CVB/O). CVB2-induced pathogenesis was analyzed, focusing on virus-induced pathology of various tissues in 4-week-old BALB/c inbred male mice. Myocarditis developed and extensive pancreatic inflammation was observed in all mice infected with CB2/04/279 or CVB/O, but not in animals infected with CB2/04/243. This is the first report of the full-genomic sequence and pathogenesis of the CVB2 strain isolated from an acute myocarditis patient in Korea.
Keywords: cardiovirulence, coxsackievirus B2, myocarditis, pathogenesis
Introduction
Group B coxsackieviruses (CVB1 to 6) belong to the Human enterovirus B (HEV-B) species of the Picornaviridae family, which is a large and complex family [21]. CVBs were recognized as causes of human heart disease shortly after their description in the early 1950s and, on the grounds of isolation and serology, they remain the most common enteroviruses associated with human cardiomyopathies [2,3,4,6,27].
Although many CVB infections are mild and generally produce only cold-like symptoms, these viruses can also cause serious diseases such as meningitis, myocarditis, local myositis, paralysis, cardiomyopathy, hepatitis, pancreatitis, orchitis, and encephalitis [5,9,10,11,22]. In serious cases, CVB2 can cause cardiomyopathy or meningitis. However, the mechanisms of viral pathogenesis in conditions such as myocarditis and meningitis by CVB2 remain unclear [1,14,23].
Two clinical isolates of CVB2 obtained in 2004 in Korea were used in this study. The two CVB2 strains were isolated from clinical specimens obtained from patients with aseptic meningitis (CB2/04/243) and acute myocarditis (CB2/04/279). In this study, we examined CVB2-induced myocarditis and pancreatitis in mice and compared the complete genomic sequences of the two isolated strains of CVB2 exhibiting different clinical features. The pathologic and virologic changes were evaluated in various tissues in 4-week-old BALB/c inbred male mice.
Materials and Methods
Viruses and cells
The CVB2 strains were isolated from clinical specimens obtained from a Korean patient with aseptic meningitis (CB2/04/24 from a throat swab) and another with acute myocarditis (CB2/04/279 from a stool) in 2004. Isolation was accomplished by using susceptible cell lines such as Vero (African green monkey kidney) and BGM (buffalo green monkey) cells. Viruses were propagated in Vero cells grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, USA) supplemented with L-glutamine and 5% fetal bovine serum (FBS) (Invitrogen). Inoculated cells were incubated until complete cytopathic effect was observed and then stored at −80℃. To release viruses, cell culture supernatants were frozen and thawed three times and then stored at −80℃ until use. Three CVB2 strains were used in this study: CB2/04/243 (GenBank accession No. EF174468), CB2/04/279 (GenBank accession No. EF174469), and a reference strain (Ohio-1 strain; American Type Cell Collection No. VR-29; GenBank accession No. AF085363) of CVB2 (CVB/O) [16,20].
Viral RNA extraction
Viral RNA was extracted from infected cell culture supernatant or from homogenized tissue by using a viral RNA purification kit (MagExtractor-Viral RNA, Code No. NPK-401; TOYOBO, Japan). Supernatant (300 µL) was mixed with 700 µL lysis buffer and then vortexed for 10 sec after adding 50 µL of magnetic beads, vortexing for an additional 10 sec and then placing the tube on a magnetic stand (TOYOBO). The supernatant was then removed from the beads, which were then washed with washing solution I (700 µL), followed by washing solution II (900 µL) to remove unwanted material. The beads were added to diethylpyrocarbonate (DEPC)-sterilized water and then incubated for 2 min at 65℃. Finally, viral RNA was eluted from the beads with DEPC water and stored at −80℃ until use.
Reverse transcriptase-polymerase chain reaction (RT-PCR) and long-distance polymerase chain reaction (LD PCR)
Complementary DNA (cDNA) was transcribed with Superscript III reverse transcriptase (Invitrogen) in 50 µL reaction mixtures containing RNA, a reverse primer (Oligo dT18mer; Invitrogen), 2.5 mM deoxynucleoside triphosphates (dNTPs), and 200 U/µL Superscript III RT in a buffer containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, and 2 mM dithiothreitol. The RT reaction mixtures were incubated at 50℃ for 60 min, inactivated by incubation at 75℃ for 10 min, and chilled at 4℃. For LD PCR, the HL PCR PreMix Kit (Bioneer, Korea) was used in 20 µL reaction volumes containing 2 µL of cDNA. A full-length genome (7,411 base pairs) was amplified with the primer pair S (5′-TTAAAACAFCCTGTGGGTTG-3′)/3END (5′-CGCACCGAATGCGGAGAATTTAC-3′) [13]. The LD PCR was carried out using the following cycling conditions: 1 min at 94℃; 35 cycles of 30 sec at 94℃, 5 min 30 sec at 68℃; and then 10 min at 68℃.
Sequence analysis of the viral genome
The CVB2 genome was sequenced by using a primer walking strategy, which primarily uses full-length products as templates in the sequencing reaction. The extreme 5′-end sequence of the CVB2 genome was determined by using the 5′ RACE system for Rapid Amplification of cDNA Ends ver. 2.0 (Invitrogen). Sequencing templates of the 5′-end were generated by RT-PCR using gene-specific primer 1 (GSP1) (5′-TCAAT TGTCACCATAAGCAGCCA-3′), and the fragment was amplified with GSP2 (5′-GCACCATGTCAGTATTGAAGCGT-3′). The nested PCR was performed with GSP3 (5′-CTGA TCTACACTGGGGTTGTGCGG-3′). The purified LD PCR products were added to a reaction mixture containing 4 µL of BigDye terminator reaction mix (ABI Prism BigDye Terminator Cycle Sequencing Kit; Perkin-Elmer Applied Biosystem, USA) and 10 pmol of sense or antisense primer. Sequencing reactions were subjected to an initial denaturation at 96℃ for 1 min, followed by 25 cycles consisting of 96℃ for 10 sec, 50℃ for 5 sec, and 60℃ for 4 min. Each nucleotide was determined at least three times in each direction. The sequencing data were collected on an ABI Prism 3100 genetic analyzer (Perkin-Elmer Applied Biosystem) and fragments were assembled into a complete genome by using DNASTAR software (DNASTAR, USA). Nucleotide sequences of the full-length genome of the two Korean CVB isolates were compared with those of CVB/O, the reference strain [20]. For alignment and phylogenetic analysis, Clustal W (ver. 1.81) and the MegAlign program (DNASTAR, USA) were used [25].
Plaque assay
For viral titer determination, 10-fold serial dilutions (from 10−1 to 10−5) of viruses were prepared in duplicate and added to confluent monolayers of Vero cells in 12-well plates (Nunc, USA). The Vero cells were incubated with virus for 90 min at 37℃ with 5% CO2 to allow virus attachment and then washed with medium. The Vero cell monolayers were then overlaid with 2 mL of a mixture containing 2% carboxymethylcellulose (CMC) (Sigma-Aldrich, USA), 1.5% FBS, and DMEM and incubated for 2 days at 37℃ and 5% CO2. Plaque-forming units (PFUs) were counted after staining with Crystal Violet (Sigma-Aldrich).
Animal experiments
Inbred BALB/c male mice (AnNHsd strain; 4 weeks old and 15–17 g body weight) were purchased from KOATECH (Korea). All mice were rested for 7 days before inoculation. Prior to the present experiments, we tested suitable titers of CVB2 viruses. Before mice were inoculated with 2.5 × 104 PFU of CVB2, the general strain of mice was checked and evaluated. Immediately before inoculation, body weight was recorded. Mice (n = 102) were divided randomly into four groups and each group was inoculated intraperitoneally with 2.5 × 104 PFU of CVB2 in 0.5 mL of cell lysate suspended in DMEM. Negative control groups were inoculated with uninfected Vero cell lysate in DMEM. Mice were sacrificed at 7 and 13 days post-inoculation (pi), and heart, pancreas, liver, spleen, kidney, and lung were removed for virological and pathological studies. Organs were frozen in dry ice immediately and stored at −80℃ until homogenized. Another group was washed in PBS (Gibco, USA) and rapidly fixed in 10% phosphate-buffered formalin (Sigma-Aldrich) for histological analysis. Sections (3 µm thick) were formalin-fixed and stained with hematoxylin and eosin (H&E; Sigma-Aldrich) to determine the level of inflammation for evaluation by light microscopy. All animal studies were approved by the Institutional Animal Care and Use Committee of the Korean Ministry of Food and Drug Safety (IACUC No. 1001KFDA007).
Statistical analyses
Statistical analyses were performed with SPSS (ver. 15.0; IBM, USA). P values < 0.05 were considered statistically significant.
Results
Analysis of the CVB2 genome sequences
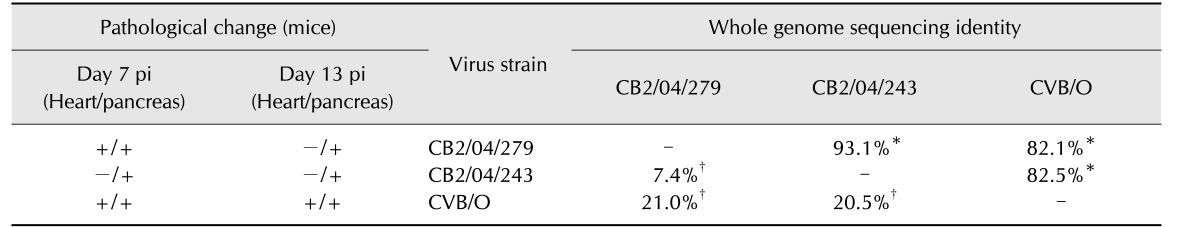
We previously compared the replication patterns of CB2/04/279 and CB2/04/243 strains in Vero cells cultured to determine whether they are biologically identical to the reference CVB/O strain. All three CVB2 strains were replicated to equivalent titers in Vero cells (data not shown). Nucleotide sequences of the full-length genomes of the two Korean CVB isolates were compared with those of the reference (CVB/O). The sequence of the two CVB2 isolates differed from the published sequence of the reference CVB/O strain. Variation between CB2/04/279 and CB2/04/243 full genome sequences was observed. The two Korean CVB2 isolates had a nucleotide sequence identity of 93.1%, whereas the Korean isolates had only 82.1% to 82.5% identity to CVB/O. In addition, the two Korean CVB2 isolates had a nucleotide sequence divergence of 7.4%, whereas the Korean isolates had sequence divergences of 20.5% and 21 % from CVB/O (Table 1).
Table 1. Summary of virology and pathology results.
To compare changes of identity, divergence of full genome sequence, and histology between CVB2 strains, the following was undertaken. Mice were inoculated intraperitoneally with 2.5 × 104 PFU/0.5 mL (PFU, plaque-forming unit) of a CVB2 strain to reveal histopathological changes in their heart and pancreas on days 7and 13 post-inoculation (pi). In addition, percentages of identity and divergence of homology of genome sequences between the CB2 reference strain (CVB/O) and the Korean isolates, which differed in clinical signs, were compared. The sequences of Korean isolate strains CB2/04/279 and CB2/04/243 had a high degree of homology in contrast, strains CB2/04/279 and CVB/O exhibited highly similar patterns of histological change in mice. Positive (+) and negative (−) of the histological lesion in mice group. *Percent nucleotide sequence identity between full genomes of two CB2 strains. †Percent nucleotide sequence divergence between full genomes of two CB2 strains.
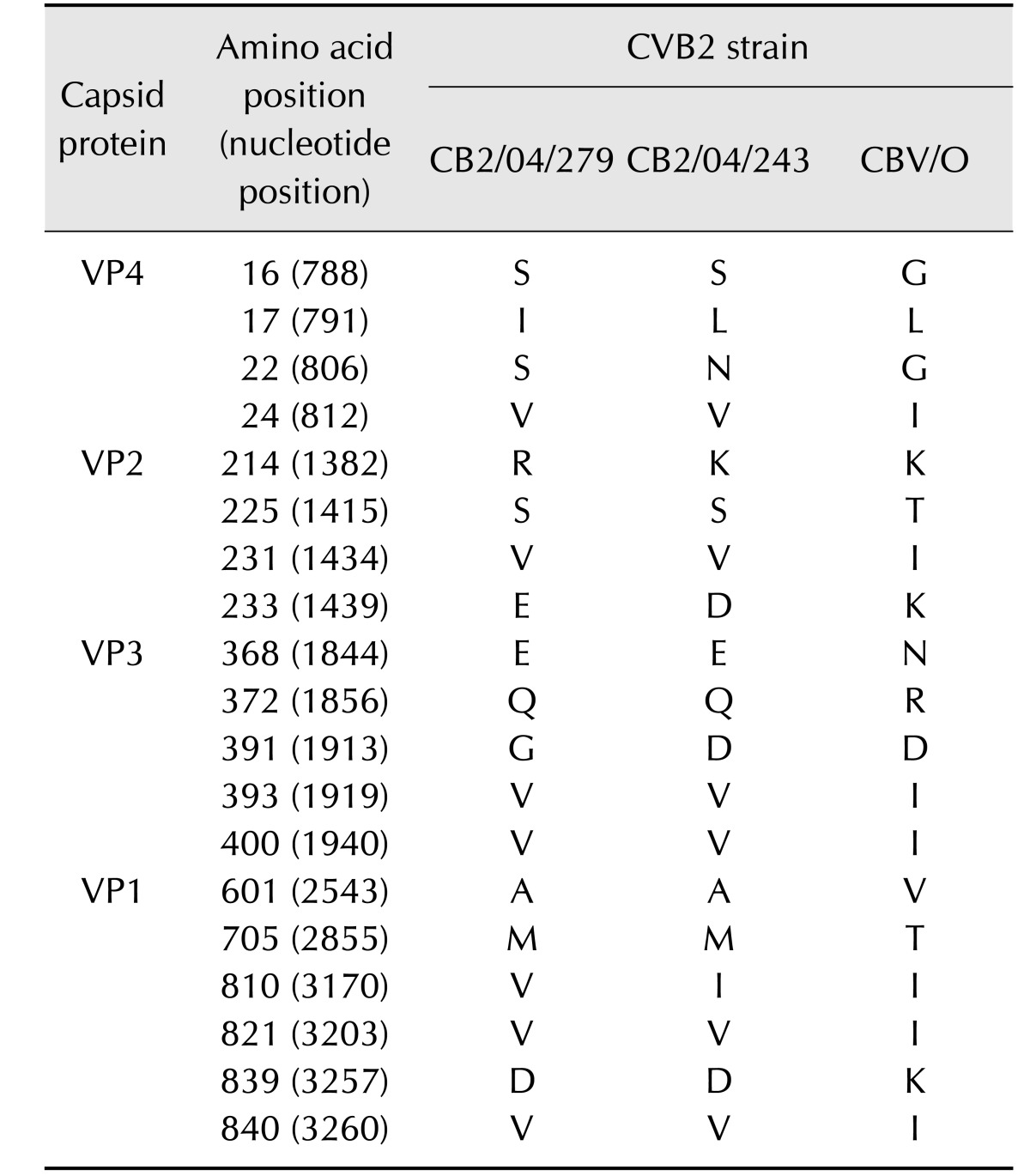
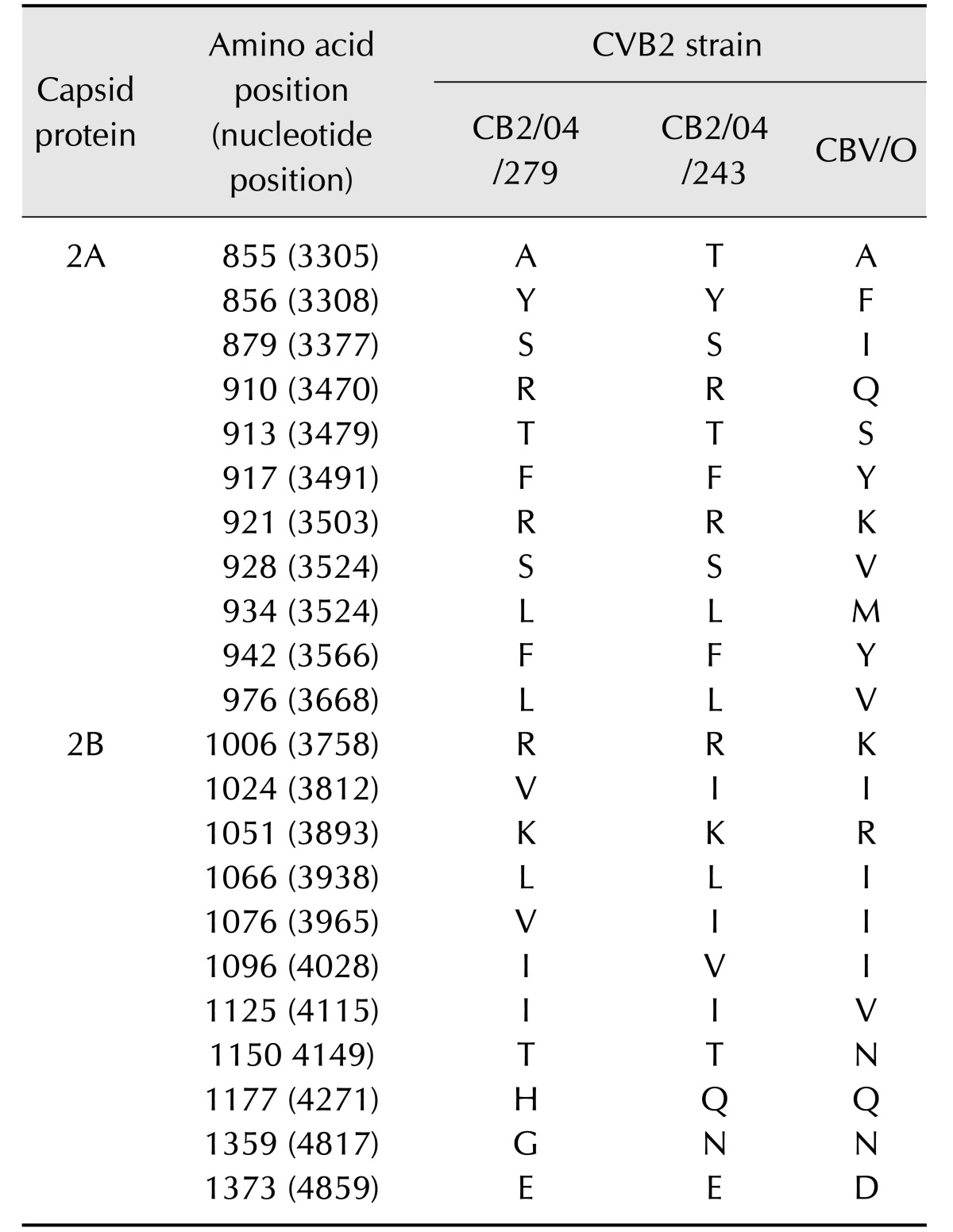
While there was 97.6% nucleotide sequence identity between the 5′ NTRs of CB2/04/279 and CB2/04/243, there was 85.8% to 86.7% identity between the 5′ NTRs of the two Korean isolates and CVB/O. Amino acid sequence alignments of the four capsid proteins VP1–VP4 (defined by nt 743–3296) of CB2/04/279 and CB2/04/243 showed high degrees of homology to the CVB/O strain. The two Korean CVB2 isolates had 99% amino acid identity. Moreover, there was 97% to 98% amino acid sequence identity between the two Korean isolates and CVB/O. Variation in amino acids among the three strains was observed in the well-conserved VP4 protein, which is not exposed on the surface (Table 2). One site (VP4 aa 22) out of the four variations in the VP1 region differed among the three strains. The three exposed capsid proteins (VP1–VP3) showed variations in 15 amino acids; four sites were within the VP2 ‘puff’ region, five sites were in the VP3 knob, one within the antigenic region in poliovirus and human rhinovirus 14, and variation in six sites was present in the VP1 region. Additionally, one site (aa 233, nt 1439) of the VP2 variations differed among the three strains. Amino acid sequences of nonstructural proteins (P2 and P3, defined by nt 3297–7306) of CB2/04/279 and CB2/04/243 were compared to that of CVB/O. The sequence variation was greater in the nonstructural protein-coding P2 (Table 3) and P3 (Table 4) regions than in the capsid protein-coding P1 region. Analysis of the amino acid sequences of the nonstructural proteins revealed that the three strains were 96.6% to 97.7% identical. Fifty-seven sites in CB2/04/279 and CB2/04/243 differed from those in CVB/O (data not shown). These variations occurred in protease 2A (11 sites), which is essential for virus replication; protein 2B (11 sites), which is critical for the membrane permeabilization function; protein 3A (14 sites), which is essential for membrane association; and protein 3D (21 sites), which regulates RNA-dependent RNA polymerase (data not shown). Alignment of the 3′ NTRs (105 nt long; nt 7307–7411) of CB2/04/279 and CB2/04/243 with CVB/O showed many sites with variation (data not shown). While there was 92.4% nucleotide sequence identity between the 3′ NTRs of CB2/04/279 and CB2/04/243, there was 84.8% to 86.7% nucleotide sequence identity between the 3′ NTR of the two Korean isolates and the CVB/O strain.
Table 2. Differences of amino acid of CVB2 Korean strains (CB2/04/279, CB2/04/243) and reference strain (CVB/O) in P1 (VP1–VP4).
Sites at which the sequences of CVB2 Korean strains differed from all aligned sequences are noted. Amino acids are numbered by position in individual proteins for GenBank accession No. AF085363.
Table 3. Differences of amino acid of CVB2 Korean strains (CB2/04/279, CB2/04/243) and reference strain (CVB/O) in P2 (2A–2C).
Sites at which the sequences of CVB2 Korean strains differed from the aligned sequences are noted. Amino acids are numbered by position in individual proteins for GenBank accession No. AF085363.
Table 4. Differences of amino acid of CVB2 Korean strains (CB2/04/279, CB2/04/243) and reference strain (CVB/O) in P3 (3A–3D).
Sites at which the sequences of CVB2 Korean strains differed from the aligned sequences are noted. Amino acids are numbered by position in individual proteins for GenBank accession No. AF085363.
Histopathological changes in selected organs
To investigate CVB2-inducing pathologic changes, mice were inoculated with 2.5 × 104 PFU of CVB2 intraperitoneally. At two times, days 7 and 13 pi, the mice were sacrificed. Notable morbidity was not detected in the inoculated mice. All mice survived throughout the duration of the study, and no death occurred in the control group. Organs, including heart, pancreas, spleen, kidney, liver, and lungs, were harvested and H&E stained for histopathological assessment.
Pancreas
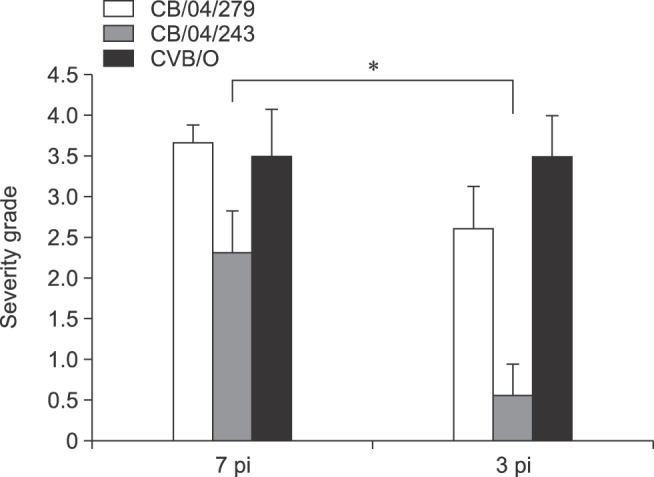
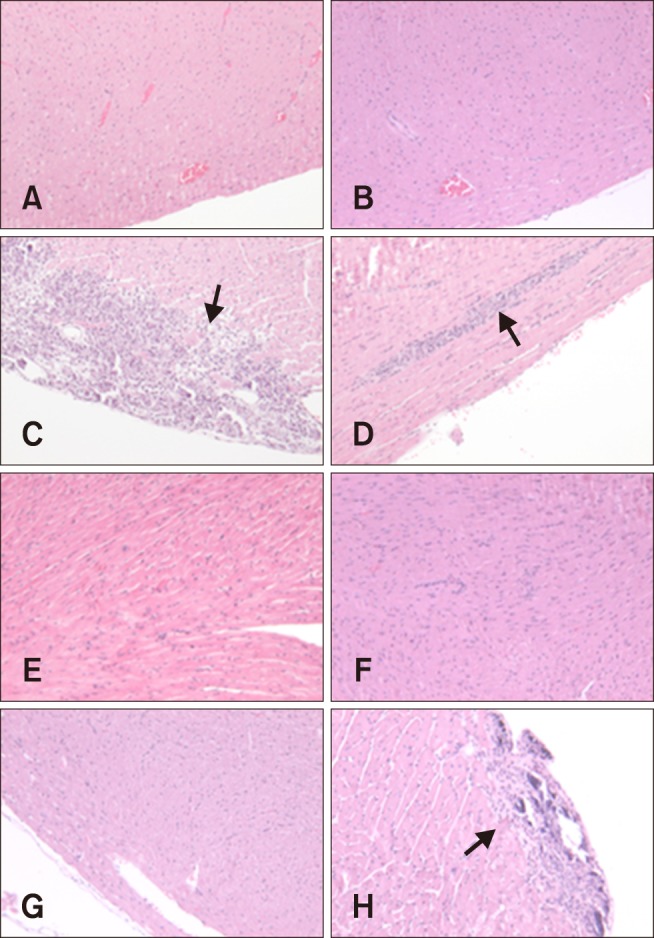
On day 7 pi, all mice inoculated with CB2/04/279 and CVB/O strains developed extensive inflammation in the exocrine pancreas but not in islets. However, 5 of the 6 inoculated CB2/04/243 mice exhibited mild inflammation in the exocrine pancreas. Active inflammatory changes characterized by diffuse lymphocytes and macrophage infiltration and mineralization were detected in the pancreas (Fig. 1). Destruction of acinar cells was observed, but the islets of Langerhans were intact. There was no evidence of pancreatic inflammation in the control group. On day 13 pi, inflammation was observed in the pancreas of the CB2/04/279 and CVB/O groups. The CB2/04/243 strain induced less pancreatic inflammation than the other strains. Pancreatic histological inflammation was almost the same on days 7 and 13 pi with the exception of the group inoculated with CB2/04/243. Histological sections were examined by independent readers, and pancreatic inflammation was assayed as the severity grade of lesions (Fig. 2). The severity of pancreatitis was marked at different times, and statistical analysis was conducted by using independent t-tests in order to determine whether or not mice inoculated with different virus strains show different levels of inflammation on days 7 and 13 pi. The severity of pancreatitis in the CB2/04/243 inoculated group was compared with those in the groups inoculated with CVB/O and CB2/04/279 strains on days 7 and 13 pi. The level of inflammation in the pancreas of mice inoculated with CB2/04/243 (p = 0.014) was significantly decreased on day 13 pi compared to that in CVB/O (p = 1.000) and CB2/04/279 (p = 0.085).
Fig. 1. Histopathological lesions of the pancreas from mice inoculated with CVB2 on days 7 (A, C, E, and G) and 13 (B, D, F, and H) post-inoculation (pi). Mice were mock-infected with saline (A and B), or inoculated with CB2/04/243 (C and D), CB2/04/279 (E and F), or CVB/O (G and H). Mice were sacrificed on days 7 and 13 pi and pancreatic tissues were fixed in formalin, paraffin embedded, cross-sectioned at a 3 µm thickness, and stained with hematoxylin and eosin. 200×.
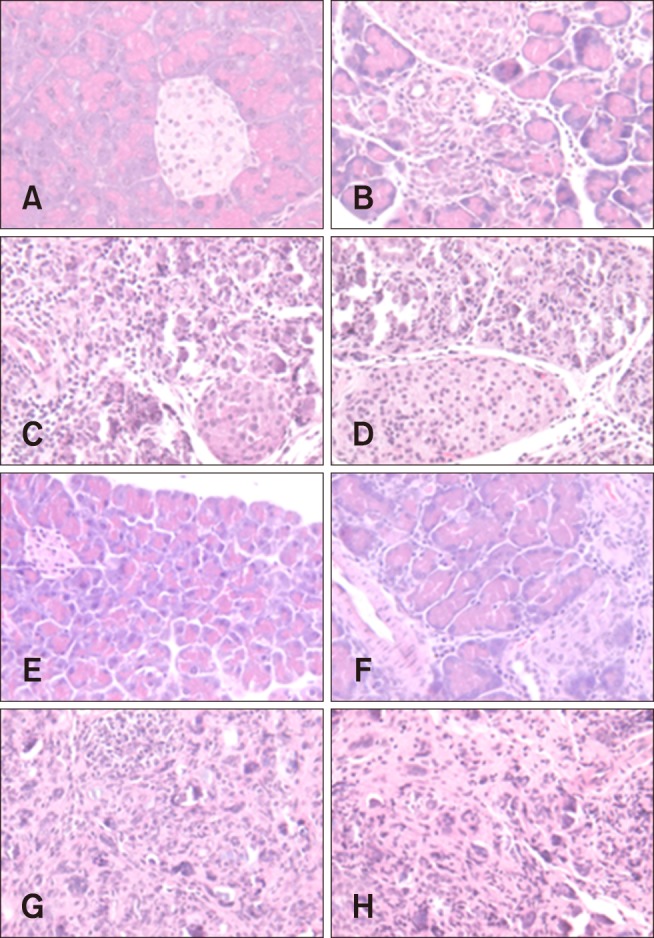
Fig. 2. Mice inoculated with CB2/04/243( ) were compared with those inoculated with CVB/O (■) and CB2/04/279 (□) for pancreatitis severity level on days 7 and 13 post-inoculation (pi). Average severity grades of lesions in animals: 1, minimal; 2, mild; 3, moderate; 4, marked. Severity grades among strains were compared by independent t-tests (n = 6 or 8 per group). *p < 0.05 considered statistically significant.
) were compared with those inoculated with CVB/O (■) and CB2/04/279 (□) for pancreatitis severity level on days 7 and 13 post-inoculation (pi). Average severity grades of lesions in animals: 1, minimal; 2, mild; 3, moderate; 4, marked. Severity grades among strains were compared by independent t-tests (n = 6 or 8 per group). *p < 0.05 considered statistically significant.
Hearts
On day 7 pi, myocarditis was observed in 33% (2/6) and 25% (2/8) of mice inoculated with CB2/04/279 and CVB/O, respectively. In particular, one mouse inoculated with CB2/04/279 had pericarditis, which is an inflammation of the membrane covering the heart (Fig. 3). However, myocarditis was not observed in any mouse inoculated with CB2/04/243. On day 13 pi, myocarditis was observed in only one mouse of the eight (12%) inoculated with CVB/O, whereas there were no histopathological changes in any mouse inoculated with CB2/04/279 or CB2/04/243. Moreover, calcium was deposited in the inflammatory lesions of mice inoculated with CVB/O. Myocarditis was not observed in the absence of pancreatitis. There was no evidence of myocardium inflammation in the control group.
Fig. 3. Histopathological lesions of the hearts from mice inoculated with CVB2 on days 7 (A, C, E, and G) and 13 (B, D, F, and H) post-inoculation (pi). Mice were mock-infected with saline (A and B), or inoculated with CB2/04/243 (C and D), CB2/04/279 (E and F), or CVB/O (G and H). Mice were euthanized 7 and 13 days pi. Arrows indicate area of inflammation in heart. Hearts were fixed in formalin, paraffin embedded, cross-sectioned at a 3 µm thickness, and stained with hematoxylin and eosin. 100×.
Other organs
Mice inoculated with CB2/04/279 showed kidney fibrosis on day 7 pi, and focal liver inflammation, extramedullary hematopoiesis (EMH) of the spleen, and hemorrhaging in the lungs on day 13 pi. One mouse inoculated with CB2/04/243 and CVB/O had focal inflammation in the liver on day 7 pi. One mouse inoculated with CB2/04/243 showed EMH in spleen and hemorrhaging in lung on day 13 pi. One mouse inoculated with CVB/O had focal inflammation in liver, EMH in spleen, and hemorrhaging in lung on day 13 pi. CVB2 RNA was detected in the heart, pancreas, and spleen on days 7 and 13 pi, and infectious viruses were isolated on days 7 and 13 pi (data not shown).
Discussion
CVB2 is a human pathogen that causes a broad spectrum of disease ranging from summer grippe to far more serious conditions such as myocarditis and meningitis [15]. The various clinical symptoms of CVB2 seem to be based on genetic diversity, as they are rapidly evolving viruses [12,19]. Despite the well-known virology of CVB3, there is little information on CVB2. In particular, the disease mechanism, relationship of clinical symptoms and virulent phenotypes, and further tissue tropism in murine models of CVB2 have not been previously established. In 2004 in Korea, a CVB2 strain was isolated from a stool specimen from a patient with acute myocarditis associated with heart failure (CB2/04/279), as well, another CVB2 strain was isolated from a throat swab specimen of a Korean patient with aseptic meningitis associated with fever (CB2/04/243). In this study, determination of their serotypes was carried out by undertaking neutralization testing with an enterovirus antiserum set. The genotypes were determined by sequencing the VP1 region.
To investigate the molecular and virological characteristics of the two Korean clinical isolates, sequence data and histological findings were analyzed. The sequences of the two Korean isolates, strains CB2/04/279 and CB2/04/243, had a high degree of homology, despite having different clinical manifestations. Although the CVB/O reference strain was isolated in the early 1950s from a patient with different clinical symptoms, CB2/04/279 and CVB/O showed a highly similar pattern of severity of histological changes in mice. Patterns of amino acid variation between CB2/04/243 and CB2/04/279 were almost identical. Therefore, sequences which were common to CB2/04/279 and CVB/O but not to CB2/04/243 were identified. These sequences were located in the nonstructural region, particularly in 3A and 3D proteins within P3 [26]. This might imply that the sequence diversity of the amino acids of CB2/04/243 might affect its clinical manifestations and tissue tropism. The 5′ NTR and 3′ NTR regions of the Korean isolates and the CVB/O strain had highly diverse sequences. The variations in these regions might reflect evolutionary changes that have occurred over time. The variations in the structural genes could be the result of differentiation of the capsid proteins that were exposed to the environment and the immune response. However, there is a small possibility that the structural gene variations (amino acid 22 and amino acid 233 of VP4 and VP2, respectively) affect the clinical manifestations and tissue tropism associated with the CVB2 inoculations [17,18,24]. Although the two Korean isolates are not CVB3 strains, the secondary structure of the 5′ NTR, a region implicated in affecting the expression of cardiovirulent phenotypes in CVB3, in the two CVB2 strains was analyzed. Two studies mapped to a short RNA sequence within the 5′ NTR, termed domain II or stem-loop II (SLII) in the murine myocarditic phenotype of the clinical isolates of CVB3 [7,8]. Although it is unclear whether the predicted secondary structure of SLII may determine the virulence of the CVB2 strains, the secondary structures of the 5′ NTR of these strains were analyzed. The SLII sequences of the two Korean isolated strains, CB2/04/279 and CB2/04/243, were highly similar, and the predicted secondary structures were also very similar. However, the CVB/O strain had a different secondary structure in its SLII region. Therefore, there was no significant correlation between the secondary structure of SLII and virulence in these CVB2 strains. However, all SLII structures demonstrated structural conservation in the apical stem-loop (nt 141–160). Additionally, this conserved stem-loop was demonstrated in the SLII of CVB3. Sequence variations were found within the nonstructural regions of CB2/04/279 and CB2/04/243. It is possible that the severity of inflammation in the pancreas may be unrelated to viral replication and tissue tropism and hence pathogenicity in mice. This possibility needs to be confirmed by undertaking further research on CVB2 virulence. Additionally, further experiments are needed to determine whether the patterns of pathologic damage to the pancreas and heart could be detected in other mouse strains such as A/J (H2a) and C3H/HeJ.
In the present study, a mouse model for CVB2 strains isolated in Korea was established. The study results offer basic and crucial information for use in further studies on CVB2, including research into tissue tropism and the interaction between virus and host.
Acknowledgments
We thank June-Woo Lee and Hye-Sook Jeong from the National Institute of Health in Korea. Further supports were also provided by the Research Institute of Veterinary Science, and the BK21 Program for Veterinary Science, College of Veterinary Medicine, Seoul National University, Republic of Korea.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Ahn J, Chio J, Joo CH, Seo I, Kim D, Yoon SY, Kim YK, Lee H. Susceptibility of mouse primary cortical neuronal cells to coxsackievirus B. J Gen Virol. 2004;85:1555–1564. doi: 10.1099/vir.0.19695-0. [DOI] [PubMed] [Google Scholar]
- 2.Baboonian C, Treasure T. Meta-analysis of the association of enteroviruses with human heart disease. Heart. 1997;78:539–543. doi: 10.1136/hrt.78.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badorff C, Knowlton KU. Dystrophin disruption in enterovirus-induced myocarditis and dilated cardiomyopathy: from bench to bedside. Med Microbiol Immunol. 2004;193:121–126. doi: 10.1007/s00430-003-0189-7. [DOI] [PubMed] [Google Scholar]
- 4.Bowles NE, Richardson PJ, Olsen EGJ, Archard LC. Detection of coxsackie-B-virus-specific RNA sequences in myocardial biopsies samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;1:1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- 5.Chiou CC, Liu WT, Chen SJ, Soong WJ, Wu KG, Tang RB, Hwang B. Coxsackievirus B1 infection in infants less than 2 months of age. Am J Perinatol. 1998;15:155–159. doi: 10.1055/s-2007-993917. [DOI] [PubMed] [Google Scholar]
- 6.Dalldorf G, Melnick JL, Curnen EC. The coxsackie virus group. In: Rivers TM, Horsfall FL Jr, editors. Viral and Rickettsial Infections of Man. 3rd ed. Philadelphia: Lippinocott; 1959. pp. 519–546. [Google Scholar]
- 7.Dunn JJ, Bradrick SS, Chapman NM, Tracy S, Romero JR. The stem loop II within the 5′ nontranslated region of clinical coxsackievirus B3 genomes determines cardiovirulence phenotype in a murine model. J Infect Dis. 2003;187:1552–1561. doi: 10.1086/374877. [DOI] [PubMed] [Google Scholar]
- 8.Dunn JJ, Chapman NM, Tracy S, Romero JR. Genomic determinants of cardiovirulence in coxsackievirus B3 clinical isolates: localization to the 5′ nontranslated region. J Virol. 2000;74:4787–4794. doi: 10.1128/jvi.74.10.4787-4794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin DE, Hardwick JM. Perspective: virus infections and the death of neurons. Trends Microbiol. 1999;7:155–160. doi: 10.1016/s0966-842x(99)01470-5. [DOI] [PubMed] [Google Scholar]
- 10.Jenista JA, Powell KR, Menegus MA. Epidemiology of neonatal enterovirus infection. J Pediatr. 1984;104:685–690. doi: 10.1016/s0022-3476(84)80944-0. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RT. Viral Infections of the Nervous System. Philadelphia: Lippincott-Raven; 1998. Meningitis, encephalitis, and poliomyelitis; pp. 87–132. [Google Scholar]
- 12.Kim KS, Hufnagel G, Chapman NM, Tracy S. The group B coxsackieviruses and myocarditis. Rev Med Virol. 2001;11:355–368. doi: 10.1002/rmv.326. [DOI] [PubMed] [Google Scholar]
- 13.Kim KS, Tracy S, Tapprich W, Bailey J, Lee CK, Kim K, Barry WH, Chapman NM. 5′-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol. 2005;79:7024–7041. doi: 10.1128/JVI.79.11.7024-7041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liapounova NA, Mouquet F, Ennezat PV. Acute myocardial infarction spurred by myopericarditis in a young female patient: coxsackie B2 to blame. Acta Cardiol. 2011;66:79–81. doi: 10.1080/ac.66.1.2064971. [DOI] [PubMed] [Google Scholar]
- 15.Melnick JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3th ed. Philadelphia: Lippincott-Raven; 1996. pp. 655–712. [Google Scholar]
- 16.Melnick JL, Ledinko N, Kaplan AS, Kraft LM. Ohio strains of virus pathogenic for infant mice (coxsackie group), simultaneous occurrence with poliomyelitis virus in patients with “summer grippe”. J Exp Med. 1950;91:185–195. doi: 10.1084/jem.91.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muckelbauer JK, Kremer M, Minor I, Diana G, Dutko FJ, Groarke J, Pevear DC, Rossmann MG. The structure of coxsackievirus B3 at 3.5 Å resolution. Structure. 1995;3:653–667. doi: 10.1016/s0969-2126(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 18.Minor PD, Ferguson M, Evans DMA, Almond JW, Icenogle JP. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 1986;67:1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- 19.Polacek C, Lindberg AM. Genetic characterization of the coxsackievirus B2 3′ untranslated region. J Gen Virol. 2001;82:1339–1348. doi: 10.1099/0022-1317-82-6-1339. [DOI] [PubMed] [Google Scholar]
- 20.Polacek C, Lundgren A, Andersson A, Lindberg AM. Genomic and phylogenetic characterization of coxsackievirus B2 prototype strain Ohio-1. Virus Res. 1999;59:229–238. doi: 10.1016/s0168-1702(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 21.Rueckert RR. Picornaviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3th ed. Philadelphia: Lippincott-Raven; 1996. pp. 609–654. [Google Scholar]
- 22.Sawyer MH. Enterovirus infections: diagnosis and treatment. Pediatr Infect Dis J. 1999;18:1033–1040. doi: 10.1097/00006454-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol. 2004;193:127–131. doi: 10.1007/s00430-003-0193-y. [DOI] [PubMed] [Google Scholar]
- 24.Sherry B, Mosser AG, Colonno RJ, Rueckert RR. Use of monoclonal antibodies to identify four neutralizing immunogens on a commom cold picornavirus, human rhinovirus 14. J Virol. 1986;57:246–257. doi: 10.1128/jvi.57.1.246-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Towner JS, Ho TV, Semler BL. Determinants of membrane association for poliovirus protein 3AB. J Biol Chem. 1996;271:26810–26818. doi: 10.1074/jbc.271.43.26810. [DOI] [PubMed] [Google Scholar]
- 27.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101:425–484. [PMC free article] [PubMed] [Google Scholar]