Abstract
Critical illness can be associated with transient alterations in circulating thyroid hormone concentrations, indicating the presence of non-thyroidal illness (NTI). NTI is well described in humans, but there are few reports on its occurrence and prognostic significance in dogs. This retrospective study assessed the occurrence of NTI in a population of dogs with systemic inflammatory response syndrome (SIRS) and investigated its association with disease severity (APPLEfast scores). A total of 41 SIRS dogs were included and were divided by SIRS origin (non-septic SIRS, n = 10; septic SIRS, n = 41) and final outcome (survivors, n = 37; non-survivors, n = 4). Healthy, age-matched dogs (n = 15) were included as controls. Serum thyroid hormone levels including total T3, free T3, total T4, and reverse T3 were measured upon admission. Compared to controls, there were significant changes in serum thyroid hormone concentrations in SIRS dogs, suggesting the presence of NTI. Septic SIRS dogs had higher APPLEfast scores and lower serum thyroid hormones concentrations than those in non-septic SIRS and control dogs. In conclusion, NTI was frequent in dogs with SIRS and may be associated with the presence of sepsis or high illness severity.
Keywords: canine, euthyroid sick syndrome, systemic inflammatory response syndrome, thyroid hormones
Introduction
Critical illness can be associated with dysfunction in multiple organs and remarkable endocrine and metabolic changes [6,25]. Alterations in the circulating levels of thyroid hormones have been widely documented in human medicine and may affect 60% to 70% of critically ill patients with various diseases [6]. This condition is typically characterized by a reduction in the concentration of serum total triiodothyronine (TT3) and a concurrent rise of serum reverse-T3 (rT3) levels; as well, low serum total thyroxine (TT4), free thyroxine (fT4), and, occasionally, thyrotropin (TSH) concentrations are reported with severe and protracted illness [1,4,27]. These are usually transient abnormalities in otherwise euthyroid patients and are commonly recognized under the name of non-thyroidal illness (NTI) [4,27]. The pathogenesis of NTI seems to be multifactorial and mainly attributed to a reduced peripheral deiodination of TT4 to TT3, increased deiodination of TT3 to diiodothyronine, reduced binding of thyroid hormones to transport proteins and nuclear receptors, and impaired intracellular uptake; behind these mechanisms the roles of protracted fasting, hypoxia, ischemia-reperfusion injury, and inflammatory cytokines have been investigated [4].
Thyroid hormones are important for homeostasis and adaptation to stress and pathological conditions, and several studies in critically ill human patients have linked the presence of NTI with poor outcomes and disease severity [1,4]. There is evidence that an acute fall in circulating thyroid hormone concentrations during acute critical illness could represent an adaptive response to reduce energy expenditure and protein breakdown; in contrast, low TT3 and TT4 serum levels during a prolonged or chronic phase of critical illness could be maladaptive [4]. Consensus on therapeutic implications of the above-mentioned abnormalities is currently lacking [4].
There are few reports regarding NTI in veterinary critical care. The syndrome has been documented in some acute conditions in dogs, but its prognostic significance remains unclear [20,24,25]. In a population of puppies with parvoviral enteritis, non-survivors had significantly lower concentrations of serum TT4 during hospitalization [24]. In addition, in a group of dogs with naturally occurring infection by Babesia canis rossi, lower values of serum TT4 and fT4 were documented in non-survivors [26]. Alterations in TT4, fT4, and TSH concentrations were demonstrated upon admission in dogs with systemic inflammatory response syndrome (SIRS) and sepsis, but no relationship to outcome was identified [20]. Derangement of the thyroid axis was documented in chronic inflammatory conditions and during heterogeneous non-thyroidal diseases [13,15,23]. Finally, significant abnormalities in thyroid function test results have been reported in healthy euthyroid dogs during anesthesia or surgical procedures [28].
The aim of the present retrospective study was to assess the prognostic significance of serum thyroid hormones, including free T3 (fT3), TT3, rT3, and TT4, in a population of dogs with SIRS. We hypothesized that lower serum thyroid hormones concentrations were associated with disease severity (APPLEfast scores) and mortality (survival at hospital discharge).
Materials and Methods
This study involved a retrospective analysis of a population of dogs affected by SIRS associated with acute pancreatitis, parvoviral enteritis, or septic peritonitis that was prospectively enrolled in a previous study performed at our Veterinary Teaching Hospital (VTH) between February 2012 and January 2014. The study was approved by the local Scientific Ethical Committee for Animal Testing (ID 22/79/2014).
Dogs were included in the study if they exhibited two or more of the following criteria: body temperature < 38.1℃ or > 39.2℃; heart rate > 120/min; respiratory rate > 20/min; WBC count < 6,000/µL or > 16,000/µL, percentage of band cells > 3% of the total WBC count, or a serum C-reactive protein (CRP) concentration >1.68 mg/dL [5,10]. At least one aliquot of serum collected at the time of hospital admission and stored frozen at −80℃ was obtained from each dog. Dogs were excluded if thyroid hormones or drugs capable of suppressing the thyroid axis (e.g., glucocorticoids, anti-inflammatory drugs, anticonvulsants, and sulphonamides) had been administered in the month prior to hospital admission. Age-matched dogs (n = 15), presented at the VTH for routine screening and prophylaxis, were included as healthy controls based on their anamnestic, physical, and clinicopathological data.
The study population of SIRS dogs was divided in groups according to the origin of SIRS. Specifically, the non-septic SIRS group included dogs affected by acute pancreatitis, while the septic SIRS group included dogs with parvoviral enteritis and septic peritonitis.
Acute pancreatitis was diagnosed by the presence of consistent clinical signs, characteristic ultrasonographic findings (i.e., hypoechoic and/or enlarged pancreas, hyperechoic mesentery, peritoneal effusion), and a positive canine pancreatic lipase immunoreactivity (cPLI) test result (Canine SNAP cPL; IDEXX Laboratories, USA) [14,22]. Clinical diagnosis of parvoviral enteritis was confirmed by a positive real-time polymerase chain reaction for a fecal sample. Sequencing of the VP2 gene was performed to identify antigenic variants of canine parvovirus (CPV) and evaluate their potential associations with disease severity [2]. Septic peritonitis was diagnosed based on cytological or bacteriological evidence of bacterial abdominal infection. The APPLEfast score [11], calculated at the time of hospital admission in order to assess disease severity, and the length of hospital stay were recorded and included as analysis variables. SIRS dogs were also classified as survivors (survived to hospital discharge) or non-survivors (died despite medical treatment or humanely euthanized by the clinical investigators due to moribund conditions or end-stage disease). Dogs that were euthanized for financial reasons were excluded from the study.
Hematological and chemistry profiles, including CRP and albumin concentrations, obtained upon hospital admission were reviewed in all enrolled dogs. Complete blood count was determined by an automated cell counter (ADVIA 2120 Hematology System; Siemens Healthcare Diagnostics, USA). CRP (CRP OSR6147; Beckman Coulter, Germany) level was measured by using an immunoturbidimetric assay that had been previously validated by our group for dog serum samples [8]. All analyses were performed by using an automated chemistry analyzer (OYMPUS AU 400, Olympus Optical, Germany). Serum thyroid hormone levels were measured at the end of the study period in a single batch assay of serum collected upon admission and stored frozen at −80℃. The TT3 and TT4 levels were measured by performing radioimmunoassays (RIA) as previously described [17,19]. For analytical purposes, RIA results below the detection limit of the assay (< 0.4 nmol/L for TT3 and < 3.0 nmol/L for TT4) were considered equal to 0.2 nmol/L and 1.5 nmol/L, respectively. The fT3 and rT3 levels were assayed by performing ultraperformance liquid chromatography coupled to tandem mass spectrometry operating in multiple reaction monitoring mode and electrospray ionization positive mode. All analytes were directly determined without the need of derivatization. The linearity of the analytical method was assessed over a wide range of concentrations (0.01–50 ng/mL). The recovery of both fT3 and rT3 was > 82%, with a coefficient of variation < 7%. The within-day and between-day precision ranges were 1.82% to 7.81% and 2.29% to 15.62%, respectively. All investigated variables were also measured in healthy control animals.
Statistical analysis
Normality was checked graphically and by applying the Kolmogorov-Smirnov test. Because of the presence of non-normal distributions for most variables, nonparametric testing was adopted for all analyses. Data were expressed by using standard descriptive statistics and are presented as median and range. The Mann-Whitney U test was used to evaluate differences between the overall population of SIRS and control dogs and for comparisons between survivor and non-survivor SIRS dogs, while a Kruskal-Wallis test was used to compare variables between different groups (controls, septic SIRS, and non-septic SIRS). If the Kruskal-Wallis test result was positive, a Conover test post hoc analysis for pairwise comparison of subgroups was performed. Test result p values < 0.05 were considered statistically significant. Correlation between variables was assessed by using Spearman's Rank correlation coefficient. All analyses were performed by using statistical software (MedCalc Software, Belgium).
Results
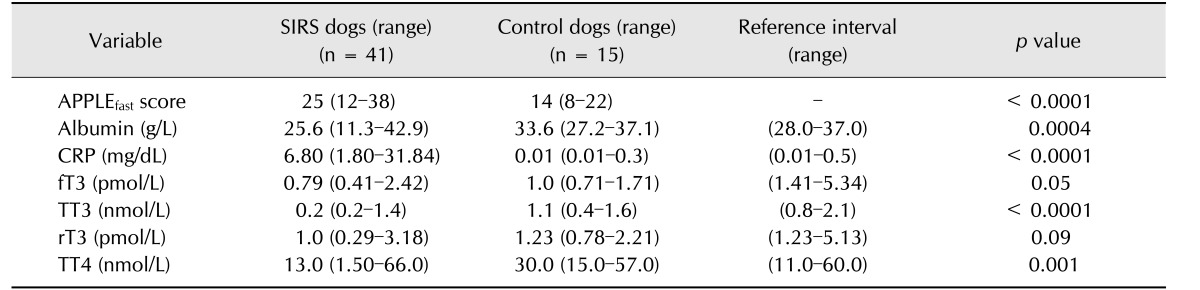
Forty-one patients met the inclusion criteria and were classified as SIRS dogs. Among the SIRS dogs, median age (8 months, range 2 months to 15 years) and median body weight (17.3 kg, range 3.9–40.2 kg) were not significantly different from those of the control dogs (median age 4.8 years, range 2 months to 8 years; median body weight 20.6 kg, range 4.7–38.0 kg). Overall, 20 dogs were male and 21 were female. Breed distribution of the study population was as follows: mixed breed dogs (18), Spanish Greyhound (4), Labrador Retriever (3), Spanish Mastiff (3), Standard Poodle (3), American Staffordshire Terrier (2), English Bulldog (2), Rottweiler (1), Weimaraner (1), American Pitbull Terrier (1), Manchester Terrier (1), Great Dane (1), and Bernese Mountain Dog (1). Breed distribution of the control dogs was German Shepard Dog (3), mixed breed dogs (3), Flat Coated Retriever (2), Argentine Mastiff (2), Labrador Retriever (1), Whippet (1), Cocker Spaniel (1), Dogue de Bordeaux (1), and Great Dane (1). Thirty-seven of the 41 dogs were survivors, while 4 were non-survivors. All non-survivors were in the septic SIRS group and had a diagnosis of septic peritonitis. Median duration of hospitalization in SIRS dogs was 7 days (range 1–13 days). SIRS dogs had significantly higher APPLEfast score and serum CRP concentration and significantly lower TT3, TT4, and albumin levels compared to those in control dogs (Table 1).
Table 1. APPLEfast score, acute phase proteins, and thyroid hormone concentrations in dogs with systemic inflammatory response syndrome (SIRS) and control dogs.
CRP, C-reactive protein; fT3, free triiodothyronine; TT3, total triiodothyronine; rT3, reverse triiodothyronine; TT4, total thyroxine.
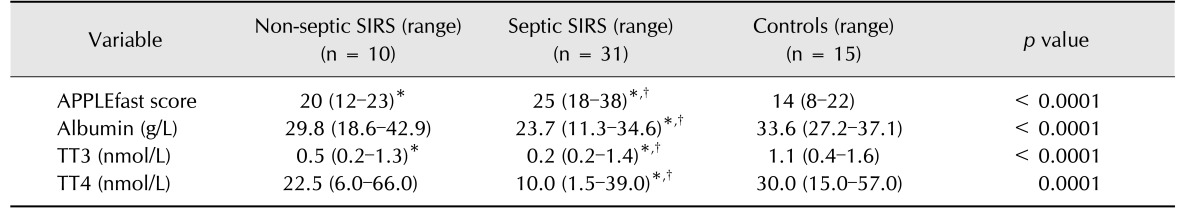
The overall population was divided in two groups according to the origin of SIRS and final diagnosis. Specifically, the non-septic SIRS group consisted of dogs diagnosed with acute pancreatitis (n = 10), while the septic SIRS group included dogs with septic peritonitis (n = 9) and parvoviral enteritis (n = 22). The CPV variants identified by sequencing of the VP2gene were CPV-2c (19/22) and CPV-2b (3/22).
Significantly different clinical and clinicopathological results between septic SIRS and non-septic SIRS dogs are summarized in Table 2. The septic SIRS dogs had a significantly higher APPLEfast score and significantly lower concentrations of serum albumin, TT3, and TT4 than those in non-septic SIRS and control dogs (Figs. 1 and 2). Non-survivors (n = 4) had significantly lower serum albumin (median 15.6 g/L, range 11.3–23.0 g/L) and TT4 concentrations (median 1.5 nmol/L, range 1.5–6.0 nmol/L) compared to survivors (n = 37; median 26.2 g/L, range 18.1–42.9 g/L; median 16 nmol/L, range 1.5–66.0 nmol/L, respectively). Among the survivors, there were no significant correlations between duration of hospital stay and serum thyroid hormone concentrations. Both serum TT4 and TT3 concentrations were negatively correlated with APPLEfast scores (r = −0.4, p < 0.01 and r = −0.3; p < 0.05, respectively). Serum TT4 and albumin concentrations were positively correlated (r = 0.56; p = 0.0001), while the correlation between TT3 and albumin was not significant (r = 0.3; p = 0.05).
Table 2. Variables with statistically different results between non-septic systemic inflammatory response syndrome (SIRS; pancreatitis, n = 10), septic SIRS (parvoviral enteritis, n = 22; septic peritonitis, n = 9) and control (n = 15) dogs.
TT3, total triiodothyronine; TT4, total thyroxine; rT3, reverse triiodothyronine. *Difference from controls; †Difference from non-septic SIRS.
Fig. 1. Box plots of serum total triiodothyronine (TT3) concentrations among dogs with non-septic systemic inflammatory response syndrome (SIRS), dogs with septic SIRS, and control dogs; the central box represents the values from the lower to upper quartile (25 to 75 percentile). The middle line represents the median. The vertical line extends from the minimum to the maximum value, excluding outside and far out values which are displayed as down-pointing triangles. Asterisk indicates significant (p < 0.05) differences among groups.
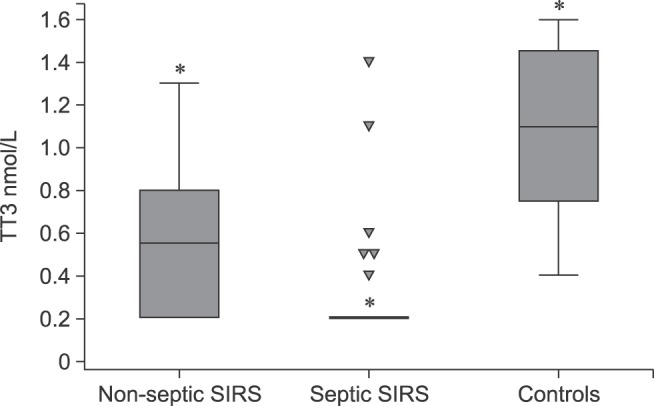
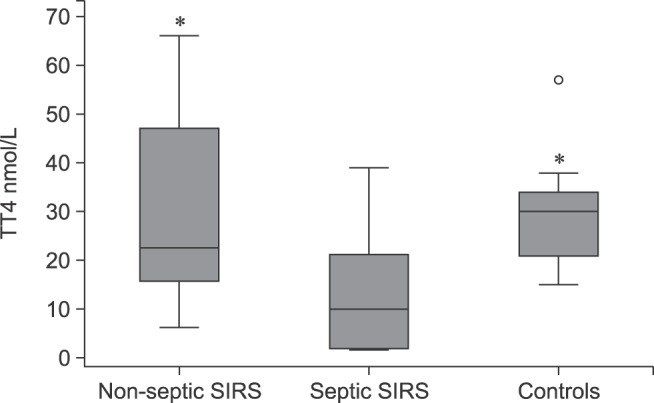
Fig. 2. Box plots of serum total thyroxine (TT4) concentrations among dogs with non-septic systemic inflammatory response syndrome (SIRS), dogs with septic SIRS, and control dogs; the central box represents the values from the lower to upper quartile (25 to 75 percentile). The middle line represents the median. The vertical line extends from the minimum to the maximum value, excluding outside and far out values which are displayed as open circles. Asterisk indicates significant (p < 0.05) difference from septic SIRS.
Discussion
The presence of NTI has been documented in different human and veterinary critical conditions including systemic inflammation [1,20]. In the current study, a panel of serum thyroid hormones was assayed in specific canine diseases: acute pancreatitis, parvoviral enteritis, and septic peritonitis. These diseases were included as they are representative and homogeneous spontaneous models of canine infectious and non-infectious SIRS. The high serum CRP concentration in the SIRS dogs confirmed the presence of systemic inflammation in our study population [5]. In order to stratify SIRS patients according to disease severity and mortality risk, as has been previously done [9,20], the dogs' APPLEfast scores were calculated. There was a significantly higher value APPLEfast score in the septic SIRS group than in the non-septic SIRS and control dogs. The reduction in serum TT4 value in the septic SIRS group is in agreement with previous veterinary reports investigating NTI in similar settings [20,24,25], and is a common finding in clinical studies on canine NTI [13,15].
Changes in serum TT3, fT3, and rT3 concentrations have been widely documented in critically ill human patients, but are less reported in veterinary literature [7,13,15,18]. Low levels of TT3 are the most common finding and had the strongest correlation with outcome in a retrospective evaluation of thyroid hormones among heterogeneous non-thyroidal diseases in dogs [15]. Similar results were obtained in a retrospective evaluation of thyroid hormones in critically ill dogs requiring intensive care therapy; low TT3 levels were frequently detected and associated with mortality [7]. In our study, a significant reduction in serum TT3 concentration in canine SIRS was observed, indicating its potential as a sensitive marker of NTI, as has been described in humans [27].
An increase in serum rT3 has been reported during NTI in humans [27]. A similar increase has been reported in healthy euthyroid dogs during general anesthesia and surgery [28], and in a small population of healthy dogs following endotoxin administration [18]; however, no similar results in canine species during spontaneous SIRS have been reported. In the present study, the median concentration of rT3 was not significantly different between SIRS and control dogs. This may indicate that rT3 variations may be less susceptible to NTI in spontaneous severe canine disease, at least with respect to TT3 abnormalities. It is also possible that rT3 variations are somehow influenced by the onset of the disease, and that serial monitoring of that hormone may reveal different changes in its concentrations. The relevance of the measurement of serum rT3 during canine NTI was apparently limited in this population of SIRS dogs, and that limited role needs to be examined in further studies.
Regarding serum fT3 concentrations, the difference between SIRS and control dogs was not significant, although lower values were detected in the SIRS group. This may indicate that, as observed in humans [27], serum fT3 values do not parallel changes in serum TT3 concentrations and are little affected by the presence of NTI in dogs, at least under acute inflammatory conditions. However, the performance and accuracy of the assay used in this study should be considered when interpreting our result.
The pathogenesis of NTI is incompletely described but is assumed to be multifactorial. The binding of thyroid hormones to circulating proteins and their metabolism at the tissue level are possibly involved. Circulating thyroid hormones are tightly bound to thyroid-binding proteins, including albumin. Such molecules are negative acute phase proteins and may decrease in acute critical illness. The high prevalence of hypoalbuminemia in SIRS dogs, particularly in septic SIRS, may account for the decreased thyroid hormones concentrations observed in our study population. This observation is partially supported by the moderate correlation between TT4 and albumin concentrations. However, other mechanisms in the fall of serum thyroid hormones, particularly for TT3, should be considered. However, such investigations were beyond the scope of the present study.
Dogs with septic SIRS had significantly lower serum thyroid hormones (TT3 and TT4) and higher APPLEfast scores than those in non-septic SIRS and control dogs. These results may suggest that the prevalence and the degree of NTI is strictly related to severity of illness. The negative correlation observed between thyroid hormones (TT3 and TT4) and the APPLEfast score may further support this statement. Derangement in serum thyroid hormone levels have been previously demonstrated in a cohort of dogs with SIRS; however, no relationship with survival or with SIRS origin (infectious versus non-infectious) was reported [20]. Different analytical methods, case series compositions, and disease categories may have accounted for the different results observed in our study. The presence of NTI has been associated with a negative outcome in different canine diseases [15,24,25,26], and low TT3 levels were correlated with mortality in critically ill dogs and in canine heterogeneous non-thyroidal diseases [7,15]. In addition, low TT4 concentrations were significantly associated with a negative outcome in puppies with parvoviral enteritis at 24 and 48 hours after admission [24].
In our study, significantly lower TT4 values were found in non-survivor dogs with SIRS. In contrast, there was no difference detected between survivors and non-survivors among the other serum thyroid hormones assessed in this study. However, our survival analysis was limited by the low number of non-survivors in our population; the prognostic significance of thyroid hormones in terms of outcome prediction in canine SIRS should be addressed by further studies.
There are some limitations to be considered before interpreting the results of the current study. The retrospective nature of the study limited the measurement of thyroid hormones in multiple standardized time points, and partially restricted the availability of serum samples for evaluation of a more extended thyroid panel (e.g., to also include fT4 and TSH). However, only dogs diagnosed with selected causes of SIRS and with complete clinical and clinicopathological data were included in the study, allowing improved completeness of data available for analysis upon admission. Concerning the method of subgrouping our patients, we decided to include both dogs with parvoviral enteritis and septic peritonitis in the septic SIRS category. Despite both diseases being considered reproducible models of abdominal sepsis [3,16], a potential age-related difference in clinical and clinicopathological variables among disease groups, including controls, could be a major concern. Specifically, younger dogs with parvoviral enteritis may have partially influenced concentrations of some of the variables investigated (e.g., serum albumin). However, statistical tests performed to compare the different groups divided according to final diagnosis (acute pancreatitis, parvoviral enteritis, septic peritonitis, and controls) produced similar results without adding any other significant information (data not shown). The predominance of variant CPV-2c in our population did not allow comparative analysis of variants in dogs affected by CPV. In addition, breed and sex-related differences have been reported to affect thyroid hormone concentrations in healthy dogs [12,21]. Although sex distribution was homogeneous in our population, and only medium-large breed dogs were included, no breed- or sex-matched controls were considered, which may have partially biased the results. It is theoretically possible that some of the SIRS dogs included in the study may have had concurrent hypothyroidism despite the low prevalence of this disease and the lack of historical and clinical features consistent with its presence. Although the authors consider the occurrence of hypothyroidism unlikely in this population, the additional measurement of TSH and fT4 would have better ruled out this hypothesis and completed the thyroidal evaluation in our SIRS dogs. Finally, the data generated from the current study refer to specific categories of canine SIRS and should not be overinterpreted or extended to different diseases or more chronic situations.
In conclusion, our study confirms a wide frequency of serum thyroid hormones alterations can indicate the presence of NTI in a cohort of dogs with SIRS. Serum concentrations of TT3 and TT4 might be considered useful and reproducible markers of NTI during acute inflammatory states in dogs. Thyroid hormones abnormalities were more severe in septic than in non-septic SIRS dogs, and they were positively correlated with APPLEfast scores. The results suggest the presence of extensive thyroid axis impairment in SIRS dogs with severe illness. Whether the presence of NTI should be considered as an adaptive response to a critical disease or the consequence of endocrine system dysfunction and failure remains a topic of debate; as well, there is uncertainty about the need for therapeutic strategies with hormone supplementation. Further prospective, large-scale studies investigating the pathogenesis and the prognostic role of NTI in canine SIRS are warranted.
Acknowledgments
This study was presented in part as preliminary results at the 14th European Veterinary Emergency and Critical Care Congress; June 11-14, 2015; Lyon, France.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, Falagas ME. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol. 2011;164:147–155. doi: 10.1530/EJE-10-0695. [DOI] [PubMed] [Google Scholar]
- 2.Battilani M, Balboni A, Ustulin M, Giunti M, Scagliarini A, Prosperi S. Genetic complexity and multiple infections with more Parvovirus species in naturally infected cats. Vet Res. 2011;42:43. doi: 10.1186/1297-9716-42-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley AM, Mayhew PD, Culp WTN, Otto CM. Alterations in the hemostatic profiles of dogs with naturally occurring septic peritonitis. J Vet Emerg Crit Care (San Antonio) 2013;23:14–22. doi: 10.1111/vec.12013. [DOI] [PubMed] [Google Scholar]
- 4.Boonen E, Van den Berghe G. Endocrine responses to critical illness: novel insights and therapeutic implications. J Clin Endocrinol Metab. 2014;99:1569–1582. doi: 10.1210/jc.2013-4115. [DOI] [PubMed] [Google Scholar]
- 5.Christensen MB, Langhorn R, Goddard A, Andreasen EB, Moidal E, Tvarijonaviciute A, Kirpensteijn J, Jakobsen S, Persson F, Kjelgaard-Hansen M. Comparison of serum amyloid A and C-reactive protein as diagnostic markers of systemic inflammation in dogs. Can Vet J. 2014;55:161–168. [PMC free article] [PubMed] [Google Scholar]
- 6.Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011;10:117–124. doi: 10.14310/horm.2002.1301. [DOI] [PubMed] [Google Scholar]
- 7.Elliot DA, King LG, Zerbe CA. Thyroid hormone concentrations in critically ill canine intensive care patients. J Vet Emerg Crit Care. 1995;5:17–23. [Google Scholar]
- 8.Gentilini F, Mancini D, Dondi F, Ingrà L, Turba ME, Forni M, FamigliBergamini P. Validation of a human immunoturbidimetric assay for measuring canine C-reactive protein. Abstracts of European Society of Veterinary Clinical Pathology (ESVCP) Special Program; In conjunction with the European College of Veterinary Internal Medicine-Companion Animals (ECVIM-CA) 15th Congress; 14-16 September 2006; Amsterdam, The Netherlands. [Google Scholar]
- 9.Giunti M, Troia R, Bergamini PF, Dondi F. Prospective evaluation of the acute patient physiologic and laboratory evaluation score and an extended clinicopathological profile in dogs with systemic inflammatory response syndrome. J Vet Emerg Crit Care (San Antonio) 2015;25:226–233. doi: 10.1111/vec.12257. [DOI] [PubMed] [Google Scholar]
- 10.Hauptman JG, Walshaw R, Olivier NB. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet Surg. 1997;26:393–397. doi: 10.1111/j.1532-950x.1997.tb01699.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayes G, Mathews K, Doig G, Kruth S, Boston S, Nykamp S, Poljak Z, Dewey C. The acute patient physiologic and laboratory evaluation (APPLE) score: a severity of illness stratification system for hospitalized dogs. J Vet Intern Med. 2010;24:1034–1047. doi: 10.1111/j.1939-1676.2010.0552.x. [DOI] [PubMed] [Google Scholar]
- 12.Hegstad-Davies RL, Torres SMF, Sharkey LC, Gresch SC, Muñoz-Zanzi CA, Davies PR. Breed-specific reference intervals for assessing thyroid function in seven dog breeds. J Vet Diagn Invest. 2015;27:716–727. doi: 10.1177/1040638715606953. [DOI] [PubMed] [Google Scholar]
- 13.Kantrowitz LB, Peterson ME, Melián C, Nichols R. Serum total thyroxine, total triiodothyronine, free thyroxine and thyrotropin concentrations in dogs with non-thyroidal disease. J Am Vet Med Assoc. 2001;219:765–769. doi: 10.2460/javma.2001.219.765. [DOI] [PubMed] [Google Scholar]
- 14.McCord K, Morely PS, Armstrong J, Simpson K, Rishniw M, Forman MA, Biller D, Parnell N, Arnell K, Hills S, Avgeris S, Gittelman H, Moore M, Hitt M, Oswald G, Marks S, Burney D, Twedt D. A multi-institutional study evaluating the diagnostic utility of the Spec cPL and SNAP cPL in clinical acute pancreatitis in 84 dogs. J Vet Intern Med. 2012;26:888–896. doi: 10.1111/j.1939-1676.2012.00951.x. [DOI] [PubMed] [Google Scholar]
- 15.Mooney CT, Shiel RE, Dixon RM. Thyroid hormone abnormalities and outcome in dogs with non-thyroidal illness. J Small Anim Pract. 2008;49:11–16. doi: 10.1111/j.1748-5827.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 16.Otto CM, Drobatz KJ, Soter C. Endotoxemia and tumor necrosis factor activity in dogs with naturally occurring parvoviral enteritis. J Vet Intern Med. 1997;11:65–70. doi: 10.1111/j.1939-1676.1997.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 17.Panciera DL, MacEwen EG, Atkins CE, Bosu WT, Refsal KR, Nachreiner RF. Thyroid function tests in euthyroid dogs treated with L-thyroxine. Am J Vet Res. 1990;51:22–26. [PubMed] [Google Scholar]
- 18.Panciera DL, Ritchey JW, Ward DL. Endotoxin-induced non-thyroidal illness in dogs. Am J Vet Res. 2003;64:229–234. doi: 10.2460/ajvr.2003.64.229. [DOI] [PubMed] [Google Scholar]
- 19.Paradis M, Sauvé F, Charest J, Refsal KR, Moreau M, Dupuis J. Effects of moderate to severe osteoarthritis on canine thyroid function. Can Vet J. 2003;44:407–412. [PMC free article] [PubMed] [Google Scholar]
- 20.Pashmakova MB, Bishop MA, Steiner JM, Suchodoiski JS, Barr JW. Evaluation of serum thyroid hormones in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care (San Antonio) 2014;24:264–271. doi: 10.1111/vec.12172. [DOI] [PubMed] [Google Scholar]
- 21.Reimers TJ, Lawler DF, Sutaria PM, Correa MT, Erb HN. Effects of age, sex, and body size on serum concentrations of thyroid and adrenocortical hormones in dog. Am J Vet Res. 1990;51:454–457. [PubMed] [Google Scholar]
- 22.Ruaux CG. Diagnostic approach to acute pancreatitis. Clin Tech Small Anim Pract. 2003;18:245–249. doi: 10.1016/S1096-2867(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 23.Saridomichelakis MN, Xenoulis PG, Chatzis MK, Kasabalis D, Steiner JM, Suchodoiski JS, Petanides T. Thyroid function in 36 dogs with leishmaniosis due to Leishmania infantum before and during treatment with allopurinol with or without meglumine antimonate. Vet Parasitol. 2013;197:22–28. doi: 10.1016/j.vetpar.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Schoeman JP, Goddard A, Herrtage ME. Serum cortisol and thyroxine concentrations as predictors of death in critically ill puppies with parvoviral diarrhea. J Am Vet Med Assoc. 2007;231:1534–1539. doi: 10.2460/javma.231.10.1534. [DOI] [PubMed] [Google Scholar]
- 25.Schoeman JP, Herrtage ME. Serum thyrotropin, thyroxine and free thyroxine concentrations as predictors of mortality in critically ill puppies with parvovirus infection: a model for human paediatric critical illness? Microbes Infect. 2008;10:203–207. doi: 10.1016/j.micinf.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Schoeman JP, Rees P, Herrtage ME. Endocrine predictors of mortality in canine babesiosis caused by Babesia canis rossi. Vet Parasitol. 2007;148:75–82. doi: 10.1016/j.vetpar.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 28.Wood MA, Panciera DL, Berry SH, Monroe WE, Refsal KR. Influence of isoflurane general anesthesia or anesthesia and surgery on thyroid function tests in dogs. J Vet Intern Med. 2009;23:7–15. doi: 10.1111/j.1939-1676.2008.00216.x. [DOI] [PubMed] [Google Scholar]