Abstract
The inhibitory effect of neutering on mammary gland tumor development in dogs has been well described. However, we observed that the effect of neutering on tumor malignancy may be altered by aging. Therefore, we characterized mammary tumors in aged dogs by analyzing the expression of cellular senescence markers. Expressions of p16, p38, p21, and p27 antibodies, which are senescence-associated markers, were assessed in canine mammary tumors of aged dogs via immunohistochemical analysis. In addition, correlations between those expressions were analyzed. Expression of p16 was negatively associated with strong nuclear p27 expression. Expression of p38 was observed in most of the mammary tumors examined, and negative p38 expression was related to positive p21 expression. Moreover, p21 expression was associated with p27 expression; negative p21 expression was associated with negative p27 expression, while positive p21 expression was associated with positive p27 expression. The results confirm that the p21- and p27-encoding genes have similar expression patterns in the mammary tumors of aged dogs. In the present study, we characterized the expression of cellular senescence markers in these tumors and elucidated the relationships among their expression patterns.
Keywords: aging, dogs, mammary neoplasms, neuter
Introduction
In both humans and dogs, mammary cancer is a heterogeneous disease whose pathogenicity is influenced by numerous factors such as hormonal influences, environmental factors, age, and hereditary predisposition [3,5]. In our previous study, we described age-related differences in canine malignant mammary gland tumors [12]. Malignant mammary gland tumors of aged dogs exhibited considerable differences in receptor expression pattern of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) and histological type. Accordingly, we focused on the effect of aging on the development of these tumors.
Menopause, which is an important factor to consider in studies of breast cancer in humans, is characterized by the cessation of ovarian sex hormone production; therefore, breast cancers that occur after the menopausal period are not considered to be influenced by typical cyclic hormonal factors. An important difference between humans and dogs is that there is no menopausal period in the latter. However, from the standpoint of cessation of ovarian hormone secretion, neutering in dogs may be considered comparable to menopause in human females; accordingly, mammary tumors in older, neutered dogs may be comparable to postmenopausal human breast tumors. The characterization of canine mammary tumors according to neuter status may be useful for studying the effects of ovarian sex hormones on the development of mammary gland tumors in aged dogs. Therefore, in this study, we aimed to characterize the pathophysiology of canine mammary tumors by ovarian hormone production status.
Cellular senescence has roles in tumor suppression and aging [2,7,15]. Therefore, we aimed to study the effects of cellular senescence in aging and tumorigenesis. Expressions of specific cellular senescence markers were evaluated in mammary tumors of aged dogs, and the relationships among those expressions were analyzed.
Materials and Methods
Sample selection
All samples used in the assessment of canine mammary tumors were obtained from the Department of Veterinary Pathology, Konkuk University Animal Teaching Hospital, Seoul, Korea. Primary selection was limited to canine mammary gland tumors diagnosed in 2013, and 188 samples were selected. Samples were classified by age (< 11 and ≥ 11 years), tumor malignancy, and neuter status. For immunohistochemical analysis, the aged group (≥ 11 years, 91 samples) was assessed. Samples excluded after initial screening were not replaced; consequently, 79 samples underwent immunohistochemical analysis.
Immunohistochemistry
Four-micrometer-thick sections of formalin-fixed paraffin-embedded tissues were fixed on slides and deparaffinized with xylene, followed by serial rehydration using graded ethanol. Slides were washed three times with phosphate buffered saline (PBS). A 3% hydrogen peroxide solution diluted with PBS was used to block endogenous peroxidase activity. Subsequently, antigen retrieval was performed via the microwave retrieval or enzyme retrieval methods, according to the primary antibody used.
Microwave retrieval (750 W, 60 Hz, 15 min) in pH 9.0 Tris-EDTA buffer was performed for anti-ER (ER88; Biogenex, USA), PR (PR10A9; Immunotech SAS, France), HER-2/neu (CB1L; Biogenex), and p16-INK4 (p16-INK4; Abbiotec, USA) staining, whereas pH 6.0 citric acid buffer was used for anti-p21 (C-19; Santa Cruz Biotechnology, USA), p27 (SPM348; Santa Cruz Biotechnology), and MAPK14/p38 (LS-C212; LifeSpan Biosciences, USA) staining of samples. After washing with PBS, slides were covered with 5% normal goat serum for 30 min for anti-ER and anti-p38 staining to block nonspecific binding. Primary antibody staining was performed. Samples were incubated at room temperature for 3 h for anti-ER (1:60) and HER-2/neu (1:100), for 2 h at room temperature for anti-p21 (1:500) and p27 (1:200), and overnight at 4℃ for anti-p16 (1:300) and p38 (1:300) antibody staining.
Washing in PBS was followed by two-step immunolabeling with secondary antibody-HRP conjugation for 40 min. Visualization was achieved by using DAB+ chromogen (Dako REAL EnVision kit; DAKO, Denmark). Finally, slides were washed with distilled water and counterstained with Gill's hematoxylin, and coverslips were applied.
Tumor classification
Tumors positive for hormone receptor expression are luminal-type tumors. Tumors with positive expression for ER or PR and negative expression for HER-2 were classified as luminal A type. Tumors with positive expression for ER or PR and positive expression for HER-2 were classified as luminal B type. Tumors that were hormone receptor-negative but HER-2 receptor expression-positive were classified as HER-2-overexpressing type, and tumors negative for the expression of all three receptors were classified as triple-negative.
Immunohistochemical analysis
Nuclear ER and PR expressions were considered positive if they were expressed at levels higher than 10% in tumor cells [10]. Evaluation of HER-2 expression was based on the Hercep test, and a complete plasma membrane expression greater than 10% was considered positive [13]. Expression of p16 was considered positive if strong nuclear and cytoplasmic expressions were observed. In the case of p38, only nuclear expression was observed; accordingly, strong nuclear p38 expression was evaluated as positive. Expression of p21 was evaluated as positive when strong nuclear and cytoplasmic expressions were observed. In the case of p27, strong nuclear and cytoplasmic expressions indicated positive expression. However, cases with only strong nuclear expression of p27 were observed; such cases were separately evaluated.
Statistical analysis
The Statistical Package for Social Science 17.0 software (SPSS, USA) was used for statistical analysis. Analysis of frequency, Pearson's chi-squared test, and Fisher's exact test were applied, and p values of less than 0.05 (p < 0.05) were considered to indicate statistical significance.
Results
Tumor classification by age, neuter status, and tumor malignancy
A total of 188 canine mammary gland tumor samples were analyzed in this study. Ninety-seven of the sampled dogs (51.6%) were aged < 11 years and 91 dogs (48.4%) were ≥ 11 years. Seventy-six of the sampled dogs (40.4%) were neutered and 112 dogs (59.6%) were intact. Forty-one tumor samples (45.1%) were benign and 50 tumor samples (54.9%) were diagnosed as malignant.
Tumor characteristics according to age
In the tumor group aged less than 11 years, the proportion of benign tumors was 50.5% (n = 49), and that of malignant tumors was 49.5% (n = 48). However, in the group aged 11 years or over, benign tumors (45.1%, n = 41) were relatively less common than malignant tumors (54.9%, n = 50).
Tumor occurrence in the two age groups was correlated with neuter status (p < 0.05). In the tumor group aged less than 11 years, tumor development was more common in intact females (69.1%, n = 67) than in neutered females (30.9%, n = 30). However, in the group aged 11 years or over, the tumor occurrence proportions were similar in the intact (49.5%, n = 45) and neutered (50.5%, n = 46) females.
Characteristics of tumor according to neuter status
In the intact group, the number of dogs with benign tumors (56/112, 50%) was the same as that of those with malignant tumors (56/112, 50%). However, in the neutered group, the number of dogs with benign tumors (34/76, 44.7%) was smaller than that of dogs with malignant tumors (42/76, 55.3%).
When only samples from dogs aged less than 11 years were considered, 33 dogs in the intact group had benign tumors (49.3%), while 34 were found to have malignant tumors (50.7%). Among the neutered dogs in the group aged less than 11 years, 16 had benign tumors (53.3%) and 14 had malignant tumors (46.7%) (panel A in Fig. 1).
Fig. 1. Correlation between tumor malignancy and neuter status, classified by age group: < 11 years (A) and ≥ 11 years (B).
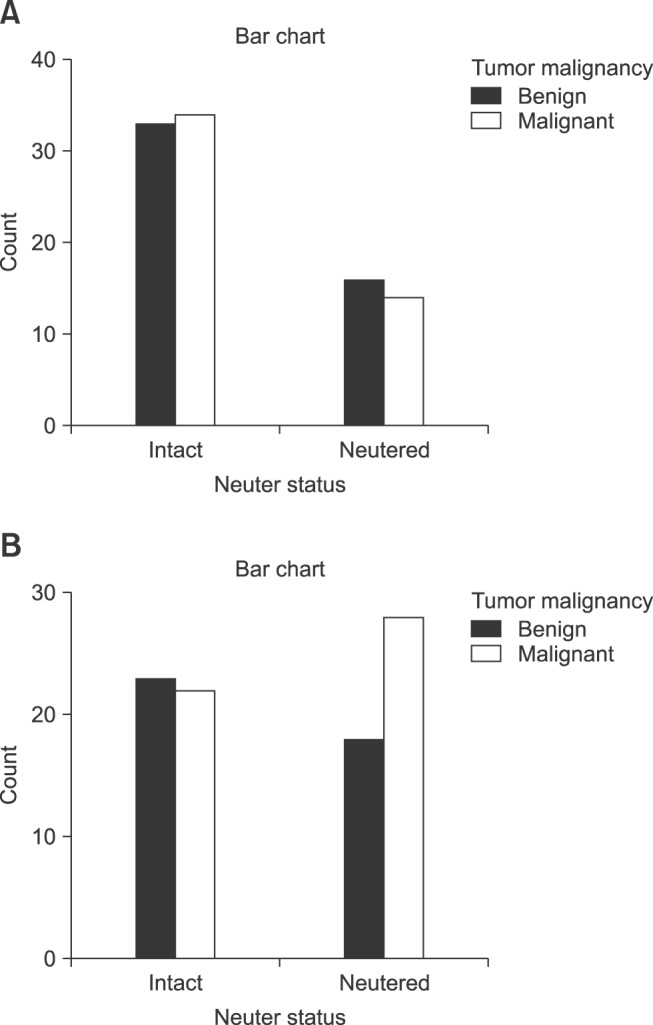
Further analysis of dogs aged 11 years and over revealed that 23 intact dogs possessed benign tumors (51.1%) and 22 intact dogs had malignant tumors (48.9%), whereas 39.1% (18/46) of neutered dogs in this age group had benign tumors and 54.9% (28/46) had malignant tumors (panel B in Fig. 1).
Immunohistochemical analysis
Sample selection
Seventy-nine well-preserved samples were selected from 91 aged dogs (> 11 years of age) for immunohistochemical analysis. Thirty-four (43.0%) of those samples were benign and 45 (57.0%) were malignant. Moreover, 38 (48.1%) of those samples were from neutered dogs and 41 (5.19%) were from intact dogs.
Immunohistochemical results and molecular phenotypes
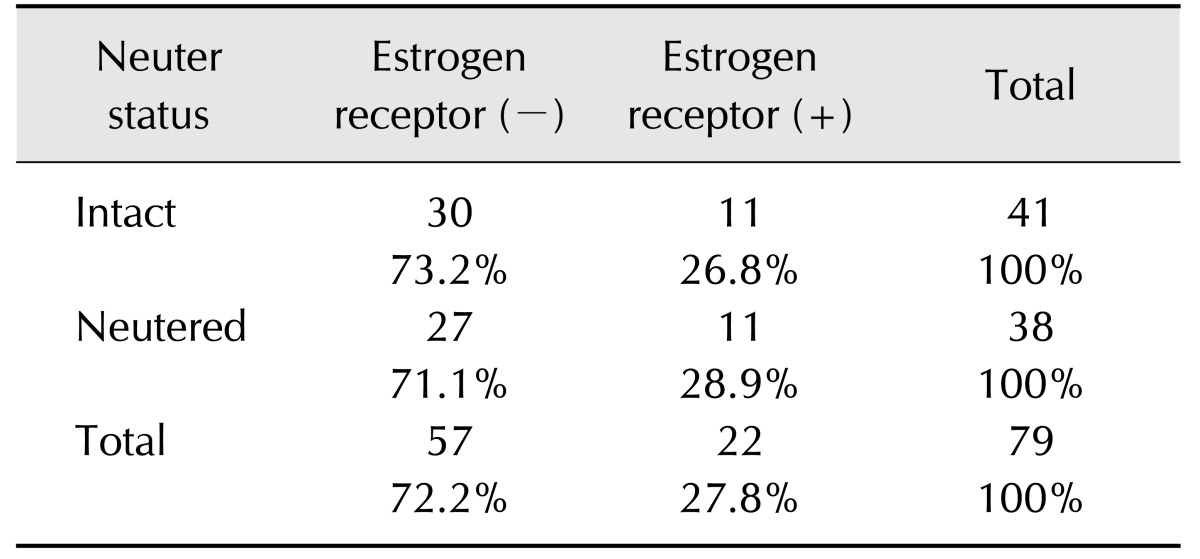
Immunohistochemical staining revealed that, among the 79 samples from aged dogs, 27.8% were ER-positive (n = 22; panel A in Fig. 2), 62.0% were PR-positive (n = 49; panel B in Fig. 2), and 78.5% were HER-2-positive (n = 62; panel C in Fig. 2) tumors. Molecular classification of the 79 tumors indicated that the luminal B type was the most prevalent at 53.2% (n = 42), followed by the HER-2-overexpressing (25.3%, n= 20) type and the luminal A (11.4%, n = 9) type; the triple-negative type was the least common (10.1%, n = 8). According to neuter status, the number of dogs with ER-negative tumors was high in both the intact group (30/41, 73.2%) and the neutered group (27/38, 71.1%) (Table 1).
Fig. 2. Immunohistochemical results. Positive expression of (A) estrogen receptor, (B) progesterone receptor, (C) human epidermal growth factor receptor-2, (D) p16, (E) p38, (F) p21, (G) p27, and (H) p27 (nuclei only) as evidenced by staining of canine mammary gland tumors. Peroxidase/DAB stain and Gill's hematoxylin counterstain. Scale bars = 90 µm.
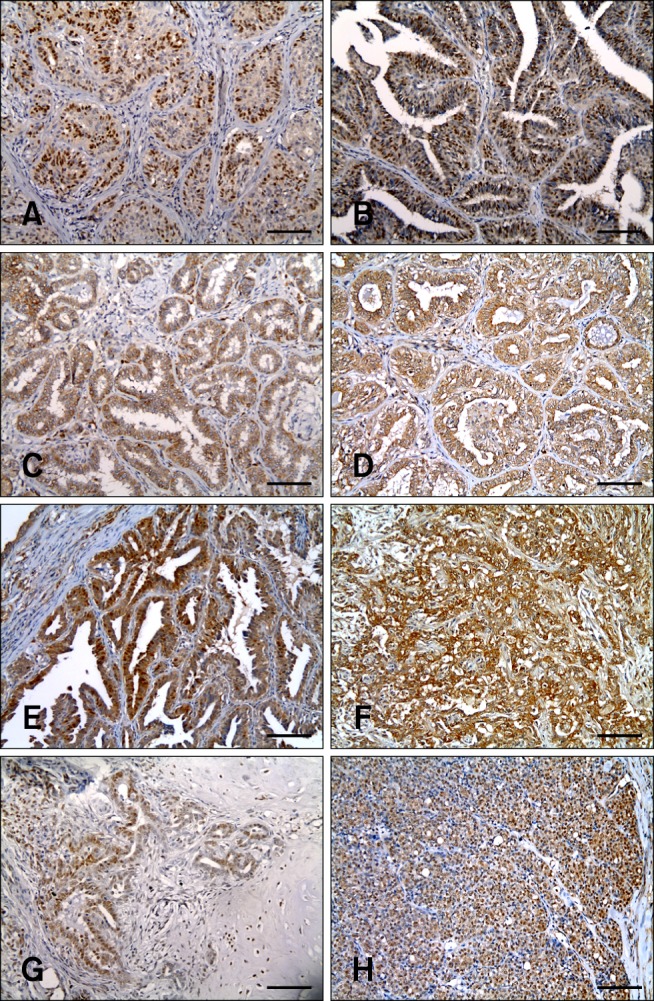
Table 1. Expression of estrogen receptor by neuter status.
Fifty-five (69.6%) samples were positive and 24 (30.4%) were negative for p16 staining (panel D in Fig. 2). Most tumors were p38-positive; 71 samples (89.9%) were p38-positive and 8 (10.1%) were p38-negative (panel E in Fig. 2). Thirty-six samples (45.6%) showed positive expression of p21, whereas 43 (54.4%) had negative expression of p21 (panel F in Fig. 2). Forty-two samples (53.2%) were p27-positive and 37 (46.8%) were p27-negative (panel G in Fig. 2). Further, when only nuclear expression was examined, 21 samples (26.6%) were found to be p27-positive, while 58 (73.4%) were p27-negative (panel H in Fig. 2).
Correlations between immunohistochemical results
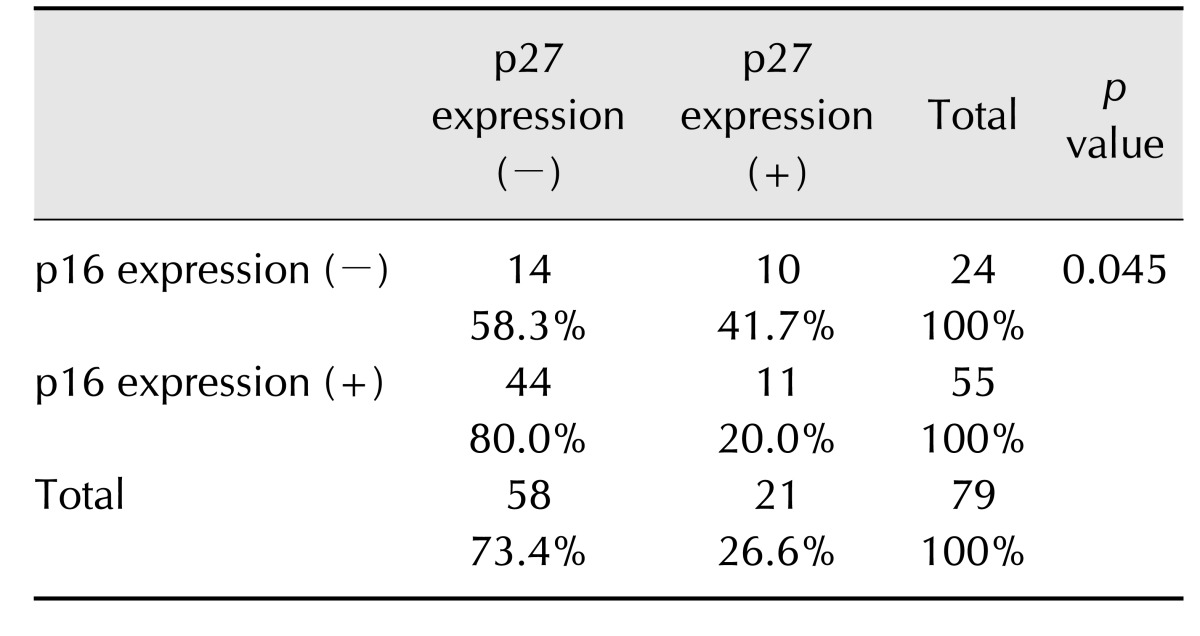
In neutered dogs, expression rates of p16 were higher in positive expression samples (78.9%, 30/38) than in negative expression samples (61.0%, 25/41). Moreover, p16 expression rates were higher in neutered females than in intact females. The number of dogs with p16-expressing tumors was higher in the neutered group (30/38, 78.9%) than in the intact group (25/41, 61.0%). Further, the expression of p16 was associated with strong nucleus expression of p27 (p < 0.05) (Table 2). When p16 expression was positive, nuclear p27 expression was predominantly negative (80%, 44/55).
Table 2. Correlation between p16 expression and p27 expression in nuclei.
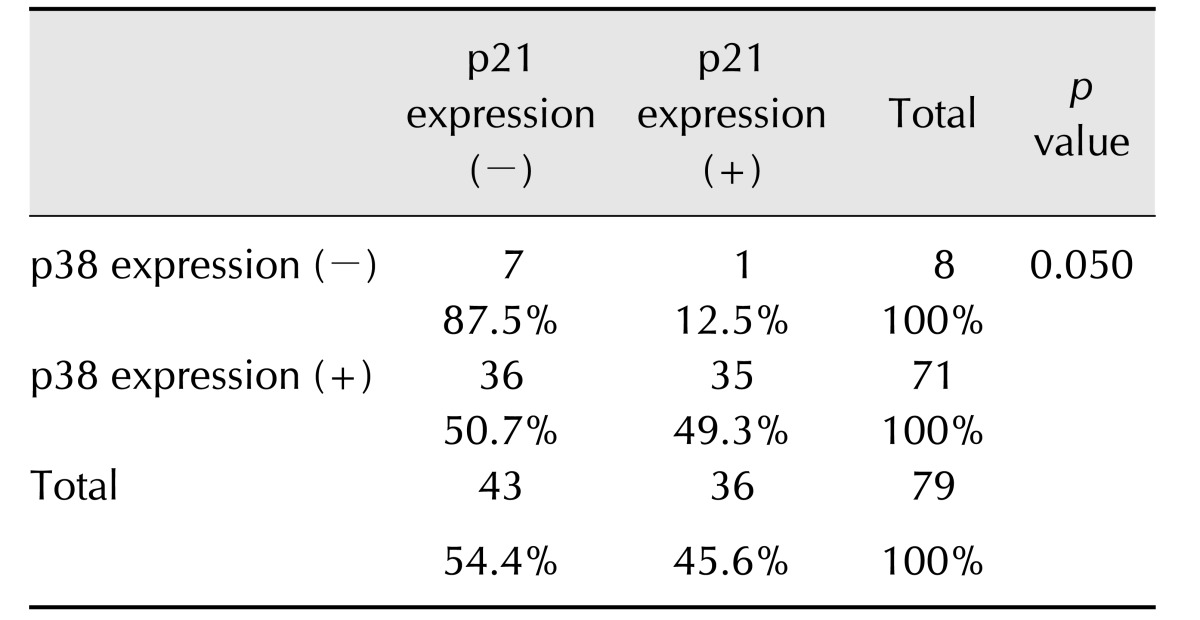
Expression of p38 was only associated with p21 expression (p < 0.05) (Table 3). Most of the tumors were p38-positive (89.9%, 71/79). Among the p38-positive tumors, there was no significant difference in expression of p21. However, most p38-negative tumors were also negative for p21 expression (87.5%, 7/8).
Table 3. Correlation between p38 expression and p21 expression.
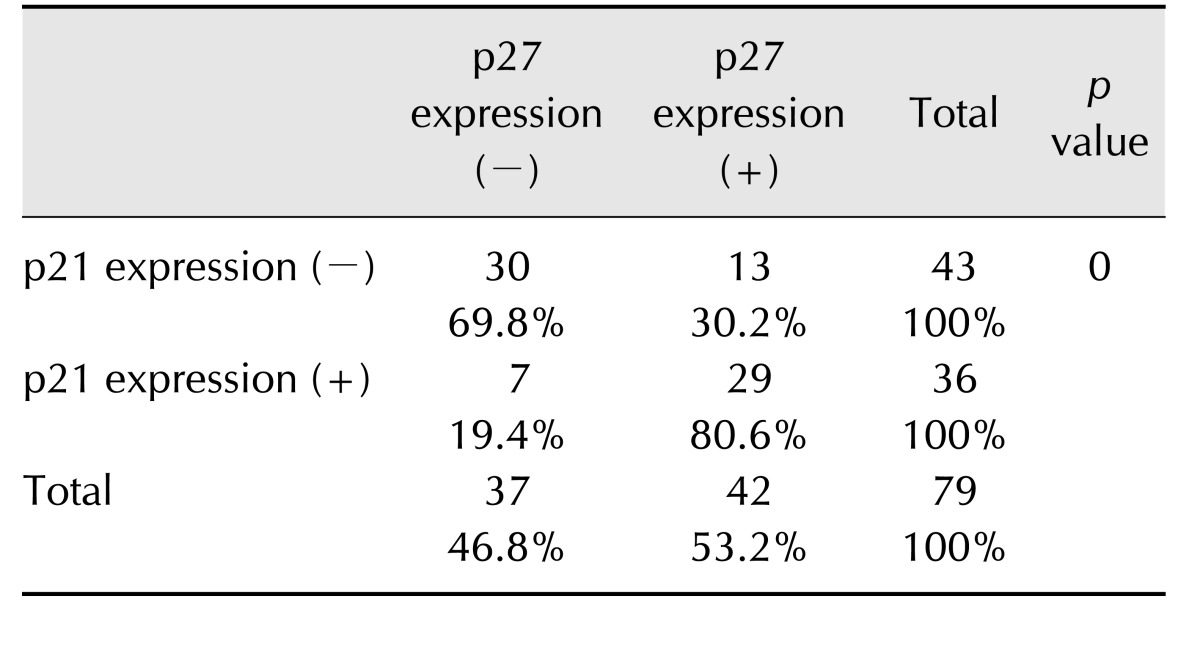
Expression of p21 exhibited a degree of dependence on tumor malignancy. p21-positive expression was observed more frequently in benign tumors (55.9%, 19/34) than in malignant tumors with most malignant tumors being p21-negative (62.2%, 28/45). Further, p21 expression was closely associated with p27 expression (p < 0.05) (Table 4). Tumors with p21-negative expression also exhibited p27-negative expression (69.8%, 30/43), whereas p21-positive tumors also tended to be p27-positive tumors (80.6%, 29/36).
Table 4. Correlation between p21 expression and p27 expression.
Expression of p27 was also observed to exhibit dependence on tumor malignancy. Benign tumors were associated with p27-positive expression (61.8%, 21/34), while malignant tumors were more commonly p27-negative (53.3%, 24/45). As noted in the preceding paragraph, p27 expression was associated with p21 expression (p < 0.05). In addition, strong nuclear expression of p27 was shown to be negatively associated with p16 expression (p < 0.05).
Discussion
Senescence is an endogenous barrier to malignant transformation. Cellular senescence-associated factors act as cell cycle regulators and tumor suppressors; therefore, their functional inactivation may lead to neoplastic transformation [7]. Senescence in tumors may be closely related with tumor malignancy or outcome [7]. Accordingly, cellular senescence has a critical role in the pathology of cancer [21] and aging [18,27]. Therefore, in the present work, we aimed to characterize the effect of aging on tumorigenesis by analyzing cellular senescence marker expression.
We investigated the characteristics of mammary tumors according to age and neuter status. Mammary cancer in neutered dogs may be comparable with postmenopausal breast cancer as, during the postmenopausal period, the mammary gland does not undergo cyclic hormonal influences; therefore, neutered dogs may be considered a model for human postmenopausal breast cancer. Humans typically undergo completion of menopause at the age of 60; accordingly, a 60-year-old human female may be compared with an 11-year-old dog [22]. On this basis, we grouped dogs as being older or younger than 11 years of age and investigated the characteristics of tumors according to cellular senescence marker expression and neuter status. We analyzed 188 mammary tumors by age group and neuter status and detected a relationship between age and tumor malignancy. The development of malignant canine mammary tumors was more frequent in dogs over 11 years old than in younger dogs. Therefore, our results support previous results showing that malignant mammary tumors are more prevalent in older dogs [12].
Analysis according to neuter status additionally revealed that mammary gland tumors were more common in intact dogs than in neutered dogs. However, in intact dogs, the relative proportions of benign and malignant tumors were the same, while in neutered dogs, malignant tumors were more frequent. Detailed analyses according to age revealed that, in dogs less than 11 years of age, the incidence of mammary tumors was not affected by neuter status. However, in the ≥ 11 years age group, the incidence of malignant mammary tumors was higher in neutered dogs than in intact dogs. In short, although the incidence of mammary tumors was relatively low in neutered dogs under 11 years of age, there was no significant difference between intact and neutered dogs over 11 years of age. The deprivation of ovarian sex hormones by ovariohysterectomy (OHE) effectively prevents the development of mammary tumors; this effect was noticeable in dogs under 11 years of age in the present study. However, the preventative effect of neutering on mammary tumors was attenuated in dogs older than 11 years. Therefore, we speculate that the higher incidence of mammary gland tumors in the latter may be attributed to factors other than ovarian sex hormones. In the present study, we attempted to identify such factors via immunohistochemical staining of cellular senescence markers in dogs older than 11 years.
Expression of p16 is considered a biomarker of aging [6,17]. As a cyclin-dependent kinase inhibitor, p16 prevents cell cycle progression and induces growth arrest or apoptosis [24]. Further, p16 is a well-known tumor suppressor [9,16], and p16 mutations are one of the most frequent genetic abnormalities in human tumors [23]. In the present study, we found that not all tumor cells expressed p16; moreover, p16 expression was likely to be negative in the intact group and positive in the neutered group. Further, tumor malignancy was higher in neutered dogs over 11 years old. Although we were unable to identify an association between p16 expression and tumor malignancy, the results of this study may be comparable to those in human studies showing that p16 expression is associated with high malignancy [1], high tumor grade, and estrogen deficiency [20].
In other studies, the loss of p16 and decreased levels of p27 have been reported to be of prognostic significance [4,8,26,29]. Although we did not evaluate expression levels in this study and were therefore unable to determine associations with tumor prognosis, we did observe a relationship between the expressions of p16 and p27 with p16 expression negatively associated with strong nuclear expression of p27 (p < 0.05). Nuclear expression of p27 was observed to be relatively low in mammary gland tumors of dogs over 11 years of age; moreover, positive expression of p16 was associated with negative p27 nuclear expression.
Both p21 and p27 also act as cyclin-dependent kinase inhibitors and have important roles as regulators of the cell cycle. These cell-cycle proteins are important in establishing senescence. In the present study, analysis of mammary tumors of dogs over the age of 11 years showed that negative expression of p21 was more frequent than positive p21 expression, and positive expression of p27 was slightly more than negative p27 expression. However, the differences were not statistically significant.
The expression patterns of p21 and p27 showed similar relationships with tumor malignancy. Most benign tumors exhibited positive p21 and p27 expression, whereas malignant tumors tended to show negative p21 and p27 expression. Additionally, close associations between p21 expression and p27 expression were detected (p < 0.05). Tumors with negative expression for p21 also showed negative expression for p27; similarly, p21-positive tumors were also p27-positive. In this study, the expressions of p21 and p27 were associated with tumor malignancy, which is contrary to results in studies of human tumors. In a previous study of human malignant tumors, p21 and p27 exhibited opposite expression patterns [18]. However, in studies of canine tumors, malignant tumors with metastatic potential were associated with negative expression of p21 and p27 [14]; those results are similar to the results of the present study.
Expression of p38, a member of the mitogen-activated protein kinase family that is activated by environmental stress and inflammatory cytokines [19], is reported to be associated with the onset of senescence [11,28]. Among the mammary gland tumors from dogs over 11 years of age, most showed positive p38 expression. However, no significant relationship between p38 expression and tumor malignancy or neuter status was detected. Among the senescence markers assessed in this study, only the expression of p21 was directly associated with that of p38. When the expression of p38 was negative, negative p21 expression was also observed.
The inhibitory effect of neutering on mammary gland tumors in dogs has been previously reported. However, we observed that aging increases the incidence of malignant mammary tumors in neutered dogs. Further, it has been previously shown that the proportion of malignant mammary tumors is higher in older dogs [12]. In this study, we found that neutered dogs account for the larger proportion of cases of malignant mammary gland tumors among older dogs.
The timing of performing OHE is reported to affect the development and prognosis of mammary gland tumors [25], implying that the presence of, or exposure to, ovarian sex hormones has an important role in the development of mammary tumors. In Korea, OHE in dogs is commonly performed after several heat cycles. The effect of OHE timing on the present results requires further investigation.
Our analysis of hormone receptor expression in mammary tumors of aged dogs revealed no correlation between neutering and ER receptor expression, and no direct significant association between neuter status and molecular phenotypes was detected. While tumor ER expression does not represent estrogen levels in the blood, it does imply that tumor development and growth is affected by estrogen. In tumors of dogs older than 11 years, the expression of ER receptors was decreased regardless of neutering status, and the incidence of malignant mammary tumors was higher in neutered dogs than in intact dogs. These results indicate that the inhibitory effect of neutering on mammary tumors decreases with aging. The results also suggest that, as observed in older human females, factors other than ovarian sex hormones have a major role in the development of mammary tumors in aged dogs.
In this study, we investigated the expression patterns of senescence-associated markers in dogs older than 11 years and identified correlations among the markers' expression patterns. The data were analyzed in the context of results reported in previous studies of humans and dogs. Further studies are needed to determine whether the differences observed are characteristic of only the mammary gland tumors of aged dogs, or whether they may also be observed in dogs below the age of 11 years.
Acknowledgments
This paper was supported by Konkuk University, Republic of Korea in 2016.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Bohn OL, Fuertes-Camilo M, Navarro L, Saldivar J, Sanchez-Sosa S. p16INK4a expression in basal-like breast carcinoma. Int J Clin Exp Pathol. 2010;3:600–607. [PMC free article] [PubMed] [Google Scholar]
- 2.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MCU, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 6.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102:1536–1546. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frizelle SP, Grim J, Zhou J, Gupta P, Curiel DT, Geradts J, Kratzke RA. Re-expression of p16INK4a in mesothelioma cells results in cell cycle arrest, cell death, tumor suppression and tumor regression. Oncogene. 1998;16:3087–3095. doi: 10.1038/sj.onc.1201870. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara-Igarashi A, Goto-Koshino Y, Mochizuki H, Sato M, Fujino Y, Ohno K, Tsujimoto H. Inhibition of p16 tumor suppressor gene expression via promoter hypermethylation in canine lymphoid tumor cells. Res Vet Sci. 2014;97:60–63. doi: 10.1016/j.rvsc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Gama A, Alves A, Schmitt F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. Virchows Arch. 2008;453:123–132. doi: 10.1007/s00428-008-0644-3. [DOI] [PubMed] [Google Scholar]
- 11.Jung MS, Jin DH, Chae HD, Kang S, Kim SC, Bang YJ, Choi TS, Choi K, Shin DY. Bcl-xL and E1B-19K proteins inhibit p53-induced irreversible growth arrest and senescence by preventing reactive oxygen species-dependent p38 activation. J Biol Chem. 2004;279:17765–17771. doi: 10.1074/jbc.M305015200. [DOI] [PubMed] [Google Scholar]
- 12.Kim HW, Lim HY, Shin JI, Seung BJ, Ju JH, Sur JH. Breed-and age-related differences in canine mammary tumors. Can J Vet Res. 2016;80:146–155. [PMC free article] [PubMed] [Google Scholar]
- 13.Kim NH, Lim HY, Im KS, Kim JH, Sur JH. Identification of triple-negative and basal-like canine mammary carcinomas using four basal markers. J Comp Pathol. 2013;148:298–306. doi: 10.1016/j.jcpa.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Klopfleisch R, Gruber AD. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res Vet Sci. 2009;87:91–96. doi: 10.1016/j.rvsc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Koenig A, Bianco S, Fosmire S, Wojcieszyn J, Modiano JF. Expression and significance of p53, Rb, p21/waf-1, p16/ink-4a, and PTEN tumor suppressors in canine melanoma. Vet Pathol. 2002;39:458–472. doi: 10.1354/vp.39-4-458. [DOI] [PubMed] [Google Scholar]
- 16.Kotake Y, Naemura M, Murasaki C, Inoue Y, Okamoto H. Transcriptional regulation of the p16 tumor suppressor gene. Anticancer Res. 2015;35:4397–4401. [PubMed] [Google Scholar]
- 17.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JC, Young PR. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 20.Milde-Langosch K, Bamberger AM, Rieck G, Kelp B, Löning T. Overexpression of the p16 cell cycle inhibitor in breast cancer is associated with a more malignant phenotype. Breast Cancer Res Treat. 2001;67:61–70. doi: 10.1023/a:1010623308275. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Mancera PA, Young ARJ, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 2014;14:547–558. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- 22.Queiroga FL, Raposo T, Carvalho MI, Prada J, Pires I. Canine mammary tumours as a model to study human breast cancer: most recent findings. In Vivo. 2011;25:455–465. [PubMed] [Google Scholar]
- 23.Serrano M. The tumor suppressor protein p16INK4a. Exp Cell Res. 1997;237:7–13. doi: 10.1006/excr.1997.3824. [DOI] [PubMed] [Google Scholar]
- 24.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 25.Sorenmo KU, Shofer FS, Goldschmidt MH. Effect of spaying and timing of spaying on survival of dogs with mammary carcinoma. J Vet Intern Med. 2000;14:266–270. doi: 10.1892/0891-6640(2000)014<0266:eosato>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Tsihlias J, Kapusta L, Slingerland J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu Rev Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- 27.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Sequential activation of the MEK-extracellular signalregulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SCC, Chan JKC, Lee KC, Hsiao WLW. Differential expression of p16/p21/p27 and cyclin D1/D3, and their relationships to cell proliferation, apoptosis, and tumour progression in invasive ductal carcinoma of the breast. J Pathol. 2001;194:35–42. doi: 10.1002/path.838. [DOI] [PubMed] [Google Scholar]