Abstract
A 12-year-old castrated Toy Poodle was referred to the Kangwon National University Animal Hospital with an oligotrophic nonunion fracture in the distal 1/3 of the left radius and an intact ulna. After fixation by a locking plate and screws, adipose-derived mesenchymal stem-cell sheets expressing bone morphogenetic protein 7 (BMP-7) were transplanted to the fracture site to enhance the healing activity. The fracture was healed at 9 weeks after surgery. In the present case, the mesenchymal stem-cell sheets expressing BMP-7 promoted bone regeneration and healing in a nonunion fracture.
Keywords: adipose-derived mesenchymal stromal cells, bone morphogenetic protein 7, dogs, ununited fractures
The term nonunion refers to a fracture that has no possibility of healing without further surgical intervention [4]. In a nonunion bone fracture, the progression of fracture healing has ceased and there is movement at the fracture site [9]. Common causes of a nonunion condition include inadequate coaptation or fixation, poor reduction or apposition of bone fragments, infection, impaired blood supply, and loss of bone or bone fragments [7]. Factors such as age, high-dose corticosteroid therapy, and systemic disease may also negatively affect the rate of bone healing. For the successful treatment of a nonunion bone fracture, adequate mechanical stability and enhancement of biological activities, such as through the use of a cancellous bone graft, at the fracture site are recommended [4,7,9].
Bone autografting is the safest and most effective grafting procedure because it involves the use of a patient's own osteogenic cells (which enhance osteogenesis) and proteins (which enhance osteoinduction) and provides a framework into which the new bone can grow (i.e., osteoconduction) [5]. However, bone autografts are limited in quantity and harvesting them requires additional surgical intervention, which frequently results in pain and complications [4]. Because of these limitations, biosynthetic bone graft substitutes and bone-tissue-engineering techniques are being investigated [2].
Tissue-engineering strategies are an attractive and promising approach to the repair of bone fractures. Strategies involving the use of degradable biomaterial scaffolds, stem cells, and biologically active molecules such as growth factors have been extensively studied [3,10,15]. Mesenchymal stem cells (MSCs) can be isolated from various tissues. Among these tissues, adipose tissue-derived MSCs (Ad-MSCs) are a promising source of cells for transplantation in tissue-engineered bone. Ad-MSCs have been shown to induce bone formation and fracture repair in numerous animal studies. Moreover, Ad-MSCs can be easily transfected with exogenous genes, which can induce a significant increase in their osteogenic differentiation potential [10].
Bone morphogenetic proteins (BMPs) are growth factors that can be affected by various determinants. BMPs belong to the transforming growth factor β superfamily and are responsible for the cascade of developmental processes in which progenitor cells differentiate into osteoblasts, leading to osteogenesis [6,8,12]. This unique feature of BMPs has been successfully used in bone repair [14]. Additional elements necessary for bone regeneration include interactions among cells, growth factors, and extracellular matrices (ECMs) [15]. Therefore, a tissue-engineering strategy for developing biodegradable scaffolds to optimize artificial growth and differentiation factor delivery and for enhancing attachment, proliferation, and migration of osteoprogenitors to imitate the process of natural bone repair was devised [15].
Previous studies have shown that BMP-modified Ad-MSCs induce vertebral defect regeneration in rodents [6]. BMP-overexpressing MSCs not only secrete BMPs to promote osteogenesis but also can differentiate into osteogenic cells [8]. However, transplanted single-cell suspensions do not attach, survive, or proliferate in target tissues [11]. Cell sheets are beneficial for cell transplantation because cell–cell junctions and endogenous ECMs are preserved in such sheets [1]. This ensures homeostasis in the cellular microenvironment, thereby allowing sustained long-term delivery of growth factors and cytokines that promote tissue repair [8,11]. This case report documents the clinical application of Ad-MSCs overexpressing BMP-7 (BMP-7-MSC) sheets in a fracture site of a dog with a nonunion fracture in the distal radius.
A 12-year-old castrated Toy Poodle was referred to Kangwon National University Animal Hospital with a nonunion fracture in the distal 1/3 of the left radius and an intact ulna. The owner stated that the dog had been injured 7 months previously. Clinical history of the dog included surgical correction of the radius bone fracture on the left forelimb with external skeletal fixation for 5 months; however, fracture healing through external fixation was unsuccessful. Despite the external skeletal fixator being in place for lengthy period, the fracture did not heal. There was no infection at the pin tract and no significant motion at the fracture site. Therefore, the external skeletal fixator was removed in order to apply a new treatment method. In a local animal hospital, a bandage was placed on the area to protect the fracture site and provide temporary support after removal of the external skeletal fixator.
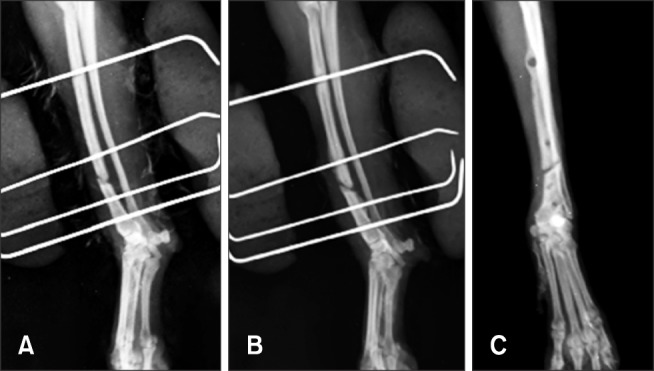
At our hospital, the dog's clinical signs included non-weightbearing lameness and pain in the left forelimb. Instability at the fracture site was assessed by manual palpation under anesthesia after bandage removal, and significant instability in the fracture region was not detected. The fracture line was persistently visible on the radiographic images, which revealed a transverse fracture of the diaphysis of the radius (Fig. 1). Complete blood count and serum biochemistry tests yielded normal results. Electrolyte levels were also within the normal range.
Fig. 1. Radiographic images acquired 1 month (A), 4 months (B), and 5 months (C) after surgery with external skeletal fixation.
The dog was premedicated with 20 mg/kg cefazolin, 4 mg/kg tramadol, and 0.1 mg/kg midazolam preoperatively via intravenous injections. Anesthesia was induced by injecting 4 mg/kg propofol intravenously. After tracheal intubation, anesthesia was maintained by using isoflurane, and 100% pure oxygen was supplied. A craniomedial incision was made on the left forelimb. The fracture site was repaired by applying a 2.0 mm, 8-hole locking plate and four 2.0 mm locking screws. The suspected fracture line was placed in the center of the plate through intraoperative needle palpation. We decided not to resect the fibrous tissue to avoid disturbance of mechanical stability at the fracture site and to preserve the fracture region. After fixation, four BMP-7-MSC sheets were transplanted circumferentially to the fracture site.
The BMP-7-MSC sheets were produced and provided by the Department of Veterinary Surgery, College of Veterinary Medicine, Seoul National University, Korea. Briefly, MSCs derived from canine hip adipose tissue were isolated and characterized [13]. The canine BMP-7 gene was cloned and used to construct a lentiviral vector encoding BMP-7 and green fluorescent protein cDNA downstream of the elongation factor-1 alpha promoter. Twenty-four hours before transfection, 4 × 106 HEK293T cells were seeded in a 100-mm-diameter culture dish. The following day, a lentiviral packaging mix (System Biosciences, USA) and lentiviral transgene plasmids were transfected to create the lentivirus particles, which were collected and transduced into Ad-MSCs at passage one. After the BMP-7-MSCs reached 90% confluence, stable cells were selected by applying puromycin (3 µg/mL; Thermo Fisher Scientific, USA). The BMP-7-MSCs were then subcultured, and passage three cells were used in the following steps. The BMP-7-MSC sheets were prepared as previously described [1]. The BMP-7-MSCs were seeded at a density of 1 × 104 cells/cm2 in a 100-mm-diameter culture dish and cultured in growth medium containing 82 µg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich, USA) for 10 days.
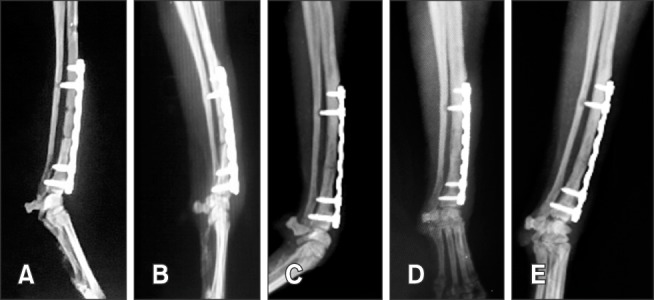
Postoperatively, 20 mg/kg cefazolin and 4 mg/kg tramadol were administered, and a fentanyl patch was applied. The surgical site healed without complications. A modified Robert Jones bandage was applied for 7 days. Radiographic images were acquired at 2, 4, 7, 9, and 16 weeks after surgery. The fracture line was not visible at 9 weeks after surgery (Fig. 2). Lameness was absent at 6 weeks after surgery.
Fig. 2. Postoperative radiography shows the absence of the fracture line at 9 weeks after surgical application of a locking plate and screws and Ad-MSC sheets overexpressing BMP-7. Radiographic images acquired 2 weeks (A), 4 weeks (B), 7 weeks (C), 9 weeks (D), and 16 weeks after surgery (E).
Fractures are classified as delayed or nonunion if the fracture has not achieved radiographic union within the expected time. Typically, fractures that fail to heal after a treatment of 6 to 8 months are considered nonunion fractures. Nonunion fractures account for approximately 3.4% of all bone fractures in dogs [7]. In nonunion fractures, bone regeneration is difficult because fracture-healing progression has ceased [9]. In the present case, we hypothesized that the reason for the nonunion in this case was impairment of the blood supply resulting from the original trauma or surgical intervention. Lack of significant instability at the fracture site was assessed by manual palpation. Each bone segment in the fracture area was bridged by fibrous tissue, leading to partial stability. In general, small breed dogs have implications for healing of distal radius-ulna fractures. Reduced vascularity associated with patient size and fracture location can influence healing. Therefore, small breed dogs are known to be predisposed to developing nonunion in distal diaphyseal radius-ulna fractures.
The current standard for treating nonunion fractures involves the application of bone grafts, despite considerable research into developing new therapies. This widely used bone graft strategy generally produces positive clinical results but has many drawbacks such as serious complications at the donor site, limited bone supply, increased number of surgical sites, reduced bone formation by the graft, potential infection risk, and host immune responses. Hence, approaches to bone healing that use bone morphogenetic molecules, proteins, and cells have been examined as alternatives to conventional bone grafting. Several factors that increase bone formation, such as MSCs, vascular endothelial growth factor, platelet-derived growth factor, and BMPs, are currently being used for fracture treatment. Among the above-mentioned factors, the osteogenic function of BMPs and the possibility of osteoblastic function of MSCs have been investigated [7]. Transplantation of Ad-MSCs to nonunion fracture sites have been reported to have positive results [11,13].
The injection of single-cell stem-cell suspensions can result in uneven cell distribution and weak cell adhesion, which can ultimately result in cell death. Thus, cell-sheet technology has been developed to enhance the regenerative capacity of tissue-engineered products [11]. Cell sheets maintain intact cell-cell junctions and the ECM, which provides cells a good structural support to maintain the integrity of the transplant. Cell sheets have been widely used in clinical situations, and have shown good biocompatibility and osteogenic potential [1,8]. In the present case, we used Ad-MSC sheets as a carrier for the local delivery of BMPs and concluded that such sheets are an effective treatment for nonunion fractures.
Therapies combining MSCs and growth factors have been investigated as effective approaches to enhancing bone repair. Of the assessed growth factors, BMPs, as potent osteoinductive factors, have been extensively studied. BMPs have been used to treat delayed or nonunion fractures and cases in which healing was expected to be compromised [12]. The present case showed that the local application of Ad-MSC-BMP-7 sheets had a positive effect on bone healing in the dog's nonunion fracture. Overexpression of BMP-7 can affect the osteogenic differentiation of endogenous and exogenous MSCs. In addition, the donor stem cells, while differentiating into osteoblasts, may function as a BMP delivery vehicle.
Although numerous treatment methods have been applied at nonunion sites, the combined use of stem cells and BMPs seems to have good potential [15]. Despite multiple positive animal experiments and successful application in humans, reports on the clinical use of stem cell and BMP combinations in veterinary cases are rare. The major obstacle to more common use of technologies such as MSC–BMP combinations in veterinary fracture therapy is their prohibitively high cost, but one would hope that improvements in the production of these cells and factors will result in cost reductions over time; perhaps resulting in commercially available BMP-7-overexpressing Ad-MSC sheets.
The potential adverse effects of the use of BMPs include excess bone formation if too high a dose is used or the dissipation of protein from the graft site and stimulation of malignancies if it is used near tumors or their metastases [12]. In the present case, complications associated with the use of BMP were not observed. However, further studies will be needed to determine, if the present results are typical of treatments with MSC sheets overexpressing BMPs.
In the present case, the dog was diagnosed with an oligotrophic nonunion left radial fracture. Callus was absent or minimal, and the fracture gap was simply bridged by fibrous tissue. Despite the lack of significant instability at the fracture site, the radial bone had not healed during a 7-month period. We considered the observed amount of mechanical instability was insufficient to delay healing. Generally, biologically active nonunion sites have sufficient biologic activity but inadequate mechanical stability. In the present case, despite the absence of significant instability at the fracture and the presence of an intact ulna, fracture healing had been delayed. On that basis, and because the dog was 12-years-old, we posited that the fracture had poor biological activity. Therefore, we choose this outside-the-box, combination Ad-MSC–BMP-7 treatment to enhance the biological activity at the fracture.
We applied BMP-7-MSC sheets to encourage bone regeneration in, and healing of, the dog's nonunion fracture; the result was a successfully healed bone within a 9-week period. Therefore, BMP-7-MSC sheets can provide a viable alternative to bone grafts in the management of nonunion fractures.
In conclusion, we successfully treated a nonunion fracture that was difficult to repair because of an insufficiency of active biologic factors. Based on our results, the application of Ad-MSC sheets overexpressing BMP-7 in the treatment of nonunion fractures could be efficacious in veterinary medicine.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01051759), Republic of Korea.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Akahane M, Nakamura A, Ohgushi H, Shigematsu H, Dohi Y, Takakura Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J Tissue Eng Regen Med. 2008;2:196–201. doi: 10.1002/term.81. [DOI] [PubMed] [Google Scholar]
- 2.Aliabadi A, Esfandiari A, Farahm M, Mahjoor A, Mojaver S. Evaluation of the effects of bovine demineralized bone matrix and coralline hydroxyapatite on radial fracture healing in rabbit. J Cell Anim Biol. 2012;6:109–114. [Google Scholar]
- 3.Bruder SP, Fox BS. Tissue engineering of bone: cell based strategies. Clin Orthop Relat Res. 1999;367 Suppl:S68–S83. doi: 10.1097/00003086-199910001-00008. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, Gerbhard F. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. doi: 10.1016/j.bone.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Goulet JA, Senunas LE, DeSilva GL, Greenfield MLVH. Autogenous iliac crest bone graft: complications and functional assessment. Clin Orthop Relat Res. 1997;(339):76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15:583–594. doi: 10.1089/scd.2006.15.583. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Shim K, Yoon T, Kang S. Clinical application of bone forming peptide-1 for nonunion fracture healing in a dog with Cushing's disease: a case report. Vet Med (Praha) 2015;60:527–531. [Google Scholar]
- 8.Kim Y, Lee SH, Kang B, Kim WH, Yun H, Kweon O. Comparison of osteogenesis between adipose-derived mesenchymal stem cells and their sheets on poly-ε-caprolactone/β-tricalcium phosphate composite scaffolds in canine bone defects. Stem Cells Int. 2016;2016:8414715. doi: 10.1155/2016/8414715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HB, Chung YS, Heo SY, Kim NS. Augmentation of bone healing of nonunion fracture using stem cell based tissue engineering in a dog: a case report. Vet Med (Praha) 2009;54:198–203. [Google Scholar]
- 10.Li J, Zhao Q, Wang E, Zhang C, Wang G, Yuan Q. Transplantation of Cbfa1-overexpressing adipose stem cells together with vascularized periosteal flaps repair segmental bone defects. J Surg Res. 2012;176:e13–e20. doi: 10.1016/j.jss.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T, Tanaka Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46:418–424. doi: 10.1016/j.bone.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Pinel CB, Pluhar GE. Clinical application of recombinant human bone morphogenetic protein in cats and dogs: a review of 13 cases. Can Vet J. 2012;53:767–774. [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10:273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verstraete FJM, Arzi B, Huey DJ, Cissell DD, Athanasiou KA. Regenerating mandibular bone using rhBMP-2: part 2– Treatment of chronic, defect non-union fractures. Vet Surg. 2015;44:410–416. doi: 10.1111/j.1532-950X.2014.12122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waese EY, Kandel RR, Stanford WL. Application of stem cells in bone repair. Skeletal Radiol. 2008;37:601–608. doi: 10.1007/s00256-007-0438-8. [DOI] [PubMed] [Google Scholar]


