Abstract
Background
Eosinophils are immunomodulatory leukocytes that contribute to the pathogenesis of Th2 driven asthma and allergic lung diseases.
Objective
Our goal was to identify unique properties of eosinophils recruited to the lungs and airways of mice in response to challenge with asthma-associated fungal allergens.
Methods
Mice were challenged intranasally on days 0, 3 and 6 with a filtrate of Alternaria alternata. Recruited eosinophils were enumerated in bronchoalveolar lavage fluid. Eosinophils were also isolated from lungs of mice sensitized and challenged with Aspergillus fumigatus and evaluated ex vivo in tissue culture.
Results
Eosinophils persist in the airways for several weeks in response to brief provocation with A. alternata in wild-type, Gm-csf- and eotaxin-1-gene-deleted mice, while eosinophils are recruited but do not persist in the absence of IL-13. Eosinophils isolated from the lungs A. alternata-challenged mice are cytokine-enriched compared to those from IL5tg mice, including 800-fold higher levels of eotaxin-1. Furthermore, eosinophils from the lungs and spleen of fungal-challenged wild-type are capable of prolonged survival ex vivo, in contrast to eosinophils from both un-treated and fungal-allergen challenged IL5tg mice, which undergo rapid demise in the absence of exogenous cytokine support. TNFα (but not IL5, IL-3, eotaxin-1 or GM-CSF) was detected in supernatants of ex vivo eosinophil cultures from the lungs of fungal-allergen challenged wild-type mice. However, neither TNFα gene-deletion nor anti-TNFα neutralizing antibodies had any impact sustained eosinophil survival ex vivo.
Conclusion and Clinical Relevance
Eosinophils are phenotypically and functionally heterogeneous. As shown here, eosinophils from fungal-allergen challenged wild-type mice maintain a distinct cytokine profile, and, unlike eosinophils isolated from IL5tg mice, they survive ex vivo in the absence of exogenous pro-survival cytokine support. As treatments for asthma currently in development focus on limiting eosinophil viability via strategic cytokine blockade, the molecular mechanisms underlying differential survival merit further investigation.
Introduction
Eosinophils are immunomodulatory leukocytes with complex roles in health in disease that have not been fully characterized [1, 2]. For example, eosinophils have long been linked to the asthma and airways dysfunction, although their role in promoting disease was initially difficult to establish [3]. The recent reconsideration of asthma, and its reclassification as a set of intersecting phenotypes or endotypes, has at the same time served to clarify the role of eosinophils in disease pathogenesis [4]. Notably, not all asthma is eosinophil-driven; however, individuals with severe eosinophilic asthma, distinguished by the relative abundance of eosinophils (>2%) in the airways and peripheral blood, respond symptomatically to anti-eosinophil (ie., anti-IL5) therapy [5].
Mouse models of allergic airways disease have been used extensively to explore specific features of the human asthmatic response (reviewed in [6]). One of the most popular models features the inert antigen, ovalbumin, introduced via an intraperitoneal sensitization and intranasal challenge strategy. Ovalbumin sensitization and challenge typically results in pronounced eosinophil recruitment to the lungs and airways in association with remodeling and airways hyper-responsiveness (reviewed in [7]). Other asthma models feature eosinophil recruitment and activation in response to chemoattractant and/or eosinophil-activating cytokines [8 – 11]. In recent years, it has become clear that clinically relevant information may result from the use of environmental allergens and airway challenge via more physiologic means. As such, current models utilize intranasal provocation strategies that feature antigens and extracts from pollens, cockroach, house dust mites, and fungi (reviewed in [12 – 14]).
In this study, we examined the responses of wild-type and gene-deleted mice to a brief period of repetitive stimulation with a filtrate of the fungus, Alternaria alternata. A saprophyte of the Family Pleosporaceae, A. alternata is primarily an outdoor allergen, found in the soil and aerosolized seasonally. A. alternata has also been identified indoors, notably in homes with moisture or insect infestation [15]. For reasons that are not fully understood, repetitive sensitization to A. alternata is among the major risk factors for developing asthma and other allergic manifestations [16]. Sixteen independent A. alternata allergens have been identified, at least nine of which share cross-reactive epitopes with allergens from other fungal species [16, 17].
Several distinct mouse models of allergic airways inflammation have been developed featuring A. alternata spores and filtrates [18, 19]. Among recent findings, Kim and colleagues [20] found that a single intranasal inoculum of A. alternata amplified eosinophil recruitment secondary to primary sensitization to rye grass antigens. Similarly, Kita and colleagues [21, 22] reported that eosinophilic inflammation in response to A. alternata challenge was largely due to activation of innate type 2 lymphoid cells (ILC2s) and that allergen-dependent reactive eosinophil hematopoiesis was likewise related to the actions of the epithelial cytokine and alarmin, IL-33. Recently, Valladao and colleagues [23] reported that mice unable to mount a Th2 response (ie, IL-4, IL-13 or Stat6 gene-deleted mice) respond to A. alternata sensitization and challenge by recruiting neutrophils (as opposed to eosinophils) to the airways.
In this study, our intent was to identify the unique features of eosinophils recruited to the lungs and airways in response to challenge with fungal antigens. We found that eosinophils were recruited to and maintained in lung tissue in the absence of GM-CSF, a cytokine previously considered to be critical for eosinophil survival in response to this provocation. Furthermore, eosinophils isolated from the lungs of fungal-allergen challenged wild-type mice are intrinsically different from eosinophils isolated from the lungs of interleukin-5 transgenic mice, as they are not only cytokine-enriched, they release TNFα, and they survive for prolonged periods ex vivo in the absence of exogenous cytokine support.
Methods
Mice
Wild-type BALB/c and C57BL/6 mice (8 – 10 weeks old, male and female) were from Charles River Laboratories, Frederick, MD. Rag1−/− mice on the BALB/c background were from the Jackson Laboratory (stock 003145); colonies of eotaxin-1−/− [24] and Gm-csf−/− [25] mice were maintained in the 14BS vivarium, and IL-5 transgenic (tg; [26]), IL-13−/− [27] and Tnfα−/− [28] mice were maintained by the NIAID/Taconic consortium. Studies were carried out on age and gender matched mice. The National Institute of Allergy and Infectious Diseases Division of Intramural Research Animal Care and Use Committee, as part of the National Institutes of Health Intramural Research Program, approved all the experimental procedures as per protocol LAD 8E.
Allergen challenge and evaluation of cells and cytokines in the airways
Mice under isoflurane anesthesia were inoculated intranasally with a reconstituted filtrate of A. alternata (Greer Allergy Immunotherapy; 10 mg/mL, 50 μg/mouse in 50 μL phosphate buffered saline (PBS) with 0.1% bovine serum albumin (BSA)) on days 0, 3 and 6 as shown in Fig. 1A. At time points indicated, mice were sacrificed and subjected to bronchoalveolar lavage (BAL) with PBS with 0.1% BSA, twice each with 0.8 mL. Cytospins were prepared and stained with modified Giemsa (Diff-Quik; ThermoScientific); the fraction of eosinophils was determined by visual inspection and scoring of minimum of 100 stained cells/mouse. Cytokine levels in BAL fluid were initially screened by Proteome profiler cytokine array kit (ARY006; R&D Systems) performed as per manufacturer’s instructions. The array includes capture antibodies (Abs) which are spotted in duplicate on nitrocellulose membranes. Eosinophil lysates were incubated with biotinylated detection antibodies for 1 hr at room temperature. The sample/antibody mixture is then incubated with the nitrocellulose membrane at 4°C overnight with rocking. Membranes are washed and then incubated with 800CW streptavidin, which binds to the membrane-bound capture Ab/sample/biotinylated detection Ab complex for 30 minutes followed by washing and then scanned on a LiCor Odyssey CLx to generate outcomes as mean pixel density. Cytokines of interest were confirmed by Quantikine or DuoSet ELISA (R&D Systems). In some experiments, mice were sensitized with an extract of A. fumigatus (Af, 20 μg per mouse) emulsified with aluminum/magnesium hydroxide (ImjectAlum, ThermoFisher) on days 0 and 7, followed by intranasal inoculation with Af (25 μg/mouse in PBS with 0.1% BSA) on days 12, 13 and 14; eosinophils were isolated as described below on day 17.
Figure 1. Eosinophils are recruited to and are sustained in the airways after intranasal challenge with allergens from the fungus, A. alternata.
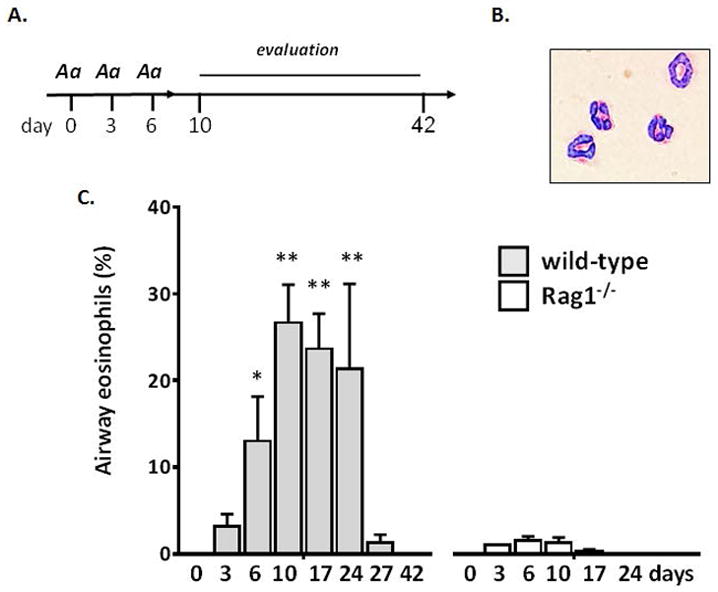
A. Basic protocol: mice are inoculated intranasally with a filtrate of the fungus, Alternaria alternata (Aa) on days 0, 3 and 6 (50 μg/mouse in 50 μL per inoculation) followed by evaluation at multiple time points thereafter. B. Airway eosinophils recovered from wild-type mice have typical morphology, including a ring-shaped nucleus and red granules when stained with modified Giemsa, original magnification, 40X. C. Airway eosinophils (% of total leukocytes) detected at days indicated after Aa challenge of wild-type (BALB/c) and D. lymphocyte-deficient Rag1−/− mice (BALB/c); n = 3 – 7 mice per point, **p < 0.01, *p < 0.05 vs. % eosinophils at day 3.
Flow cytometry, FACS, and analysis of eosinophil contents
Eosinophils in BAL fluid from mice challenged with A. alternata or from IL5tg mice were counted on a hemocytometer, and viability determined by trypan blue exclusion; cells were collected by centrifugation and frozen (90% fetal calf serum with 10% DMSO) at 106 cells/mL prior to analysis. Single cell suspensions were prepared from whole lung tissue of mice described [29]; upon thawing, cells were washed, stained for viability (live-dead) and eosinophils were isolated as CD45+SiglecF+Gr1−CD11c−MHCII− cells as components of the full myeloid panel as shown in Suppl. Fig. 1. Freshly isolated eosinophils identified in this manner were isolated by fluorescence-activated cell sorting (FACS), rinsed with PBS and re-suspended in lysis buffer (1% Igepal CA-630, 20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 2mM EDTA, and protease inhibitors (10 ug Aprotinin, 10 ug/m Leupeptin, and 10 ug.ml Pepstatin)) at 107 cells/mL. Lysates were rocked gently for 30 min at 4°C. Supernatants were clarified by centrifugation (14,000g × 5 min) and final protein concentration was determined by BCA assay. Cytokine contents were determined using 185 μg of total protein (= 2.7 × 106 cells) from each sample to probe a Cytokine profiler (ARY006; R&D Systems) as per manufacturer’s instructions as described above. Eotaxin-1 levels were confirmed by DuoSet ELISA assay (R&D systems).
Eosinophil survival ex vivo
Eosinophils were isolated from lungs of IL5tg mice, from lungs of mice challenged with A. alternata, and lungs of mice sensitized and challenged with A. fumigatus as described above as follows: After perfusion in situ via the right ventricle with PBS with 500 mM EDTA, the lungs were removed from the body cavity, minced and incubated for 90 min at 37°C RPMI with 5% fetal calf serum with DNase I (20 mg/mL; Sigma-Aldrich) and Collagenase D (40 mg/mL; Sigma-Aldrich). Red blood cells were lysed with sterile distilled H2O, and eosinophils were isolated by negative selection by removal of lymphocytes and neutrophils via magnetic bead separation methods (anti-CD45R, anti-CD90.2, and anti-Ly6B.2 conjugated beads) using a LS column (Miltenyi); purity > 90% eosinophils was determined by Diff-Quik stained cytospin preparations. Single cell suspensions were prepared from spleens of IL5tg and fungal-allergen challenged mice as previously described [30]; eosinophils were isolated to > 95% purity by magnetic bead separation methods using anti-CD45R and anti-CD90.2 conjugated beads and LS columns as described above. Isolated eosinophils from all sources were plated in growth medium (RPMI + 20% FCS) in 24 well plates at 106 cells/mL either with or without recombinant mouse IL-5 (5 ng/mL; R&D Systems), IL-13 (20 ng/mL; R&D Systems), goat anti-mouse TNFα (0.3 μg/mL, R&D Systems #AF-410-NA) or control Ig (R&D Systems, #AB-108C). Cells were evaluated on days indicated and viability determined by trypan blue exclusion. Cytokine levels in cell-free supernatants were determined by Quantikine or DuoSet ELISA (R&D Systems) as per manufacturer’s instructions.
Statistics
All quantitative findings were from at least two replicate datasets. Flow plots shown are representative of typical results. Data were analyzed via appropriate algorithms (Mann-Whitney u-test, Student’s t -test, ANOVA) utilizing GraphPad PRISM.
Results
Eosinophils persist in the airways in the absence of ongoing allergen provocation
As shown in Fig. 1A, BALB/c mice were challenged three times (days 0, 3, and 6) via intranasal inoculation with a filtrate of the fungus, A. alternata (Aa). Few to no eosinophils were detected immediately after the first and second inoculations (days 0 and 3). Prominent eosinophil recruitment to the airways was observed only after the third inoculation (day 6; 13 ± 5.2 % total leukocytes; [Fig. 1B and 1C]). Eosinophils were detected in the airways on day 10 (27 ± 4.4%) and remained through 24 (34 ± 9.8%) in the absence of any further allergen provocation. In experiments performed in recombinase-deficient Rag1−/− mice, eosinophil recruitment and persistence in the airways in response to Aa inhalation-only challenge was shown to be lymphocyte dependent even in the absence of a distinct sensitization period [Fig. 1C]. These results are consistent with those reported by Valladao and colleagues [23] who also found that eosinophil recruitment was blunted in response to A. alternata in Rag1−/− mice, in a protocol that included full sensitization as well as challenge with A. alternata. At the same time, our results are not fully consistent with those of Bartemes and colleagues [21], who reported that eosinophil recruitment in response to Aa provocation at early time points was dependent on IL-33 and the actions of type 2 innate lymphoid cells (ILC2s) alone. While we did detect IL-33 in the airways in response to challenge with Aa, levels over background were reached during the brief interval after the first intranasal inoculation only [Supplemental Fig. 2].
Interleukin-13 contributes to eosinophil persistence in the airways of Aa challenged mice
Cytokines in bronchoalveolar lavage (BAL) fluid were evaluated in samples from control mice and from mice subjected to Aa challenge on days 0, 3, and 6 (as in Fig. 1A) and evaluated on days 7, 10 and 17 [Table 1]. Among the canonical eosinophil pro-survival cytokines, peak levels of IL-5 (348 ± 95 pg/mL) were detected at day 7; no IL-3 and no GM-CSF were detected. Other cytokines that have been implicated in promoting eosinophil survival include eotaxin-1, detected at 170 ± 69 pg/mL at day 7, while no IL-23 nor IL-27 were detected. Aa challenge also resulted in prominent expression of IL-13 (3240 ± 593 pg/mL at day 7), with elevated levels persisting through day 17; by contrast, IL-4 remained below detectable limits throughout.
Table 1.
Cytokines detected in BAL fluid (pg/mL ± SE) of mice challenged on days 0, 3, and 6 as in Fig. 1A and evaluated at days 0 (prior to inoculation) 7, 10, and 17 by ELISA as indicated;
| Cytokinesin BAL | Limit of detection | Day 0 | Day 7 | Day 10 | Day 17 | ||||
|---|---|---|---|---|---|---|---|---|---|
| pg/mL | pg/mL | + SE | pg/mL | + SE | pg/mL | + SE | pg/mL | + SE | |
| IL-3 | 7.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IL-5 | 31.2 | 65.4 | 7.8 | 348* | 95 | 139 | 69 | 37 | 5.4 |
| GM-CSF | 7.8 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 |
| CCL11 | 7.8 | 18.4 | 4.5 | 170* | 69 | 9 | 4.5 | 9.3 | 4.0 |
| IL-4 | 15.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IL-13 | 62.5 | 0 | - | 3240** | 593 | 551* | 46 | 0 | - |
| IL-23 | 62.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IL-27 | 15.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IL-10 | 31.2 | 77.6 | 17.2 | 1770** | 596 | 139 | 104 | 37 | 16 |
| IL-1Ra | 156† | 65.3 | 10.8 | 3620** | 765 | 1000** | 181 | 329* | 66 |
n = 3 mice per time point,
p < 0.01,
p < 0.05 vs. levels detected for individual cytokines at day 0.
Limit reported by manufacturer; linear range was extended experimentally.
We also detected relatively high levels of anti-inflammatory cytokines IL-10 and the IL-1R-antagonist (IL-1Ra/IL1F3), the latter remaining above baseline levels through day 17. IL-10 is produced by eosinophils [31, 32] but there are no published reports describing of any direct impact of this mediator on eosinophil survival. Likewise, IL-1Ra has no known direct effects on eosinophils, although Hallsworth and colleagues [33] reported that this factor limited production of eosinophil pro-survival cytokines from airway smooth muscle cells.
In order to explore the impact of individual cytokine mediators on eosinophil persistence in the airways, we challenged specific gene-deleted mice with Aa as in Fig. 1A. As shown in Fig. 2A, wild-type C57BL/6 mice respond to Aa challenge as do BALB/c mice with eosinophil recruitment and persistence in the airways; Gm-csf deficiency had no impact on this response. Neutrophils detected in the airways of these mice, present at day 0 and unrelated to Aa provocation, most likely relate to strain-specific defects in alveolar macrophages [25]. Similarly, the extent of eosinophil recruitment to and persistence in the airways of eotaxin-1 gene-deleted mice was indistinguishable from that observed in the wild-type BALB/c [Fig. 2B]. By contrast to the responses observed in C57BL/6 mice, intranasal inoculation with Aa in BALB/c mice elicits prominent recruitment of neutrophils as well as eosinophils at these time points. As shown in Fig. 2C, mice devoid of IL-13 remained capable of recruiting eosinophils to the airways in response to Aa challenge, but eosinophils disappeared rapidly; very few remained by day 17.
Figure 2. Persistence of eosinophils in the airways in response to fungal allergen challenge requires IL-13.
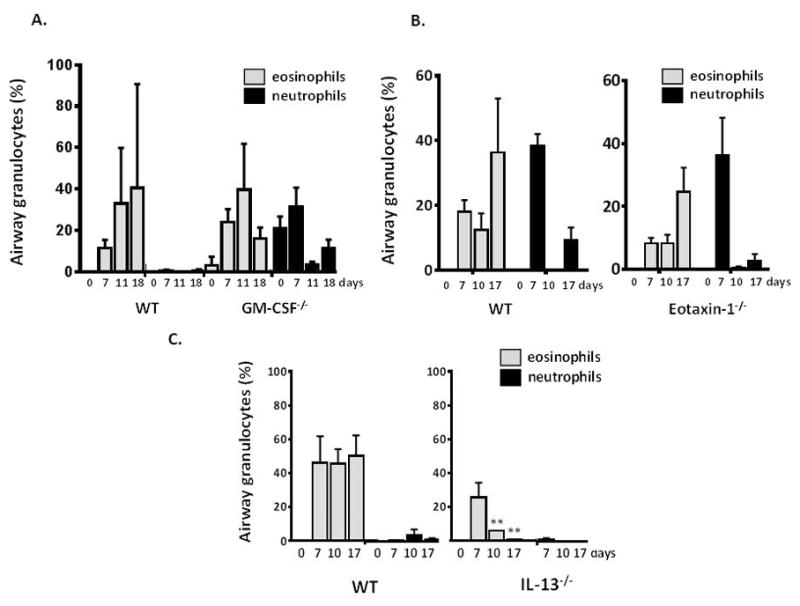
A. Airway granulocytes (% of total leukocytes) at days indicated after challenge with Aa as above; wild type (C57BL/6) vs. Gm-csf−/− mice. B. wild-type (BALB/c) vs. eotaxin-1−/− mice, C. wild-type (C57BL/6) vs. IL-13−/− mice; n = 3 – 6 mice per time point, **p < 0.01, *p < 0.05, vs. % eosinophils at day 7.
Eosinophils isolated from the lungs of Aa challenged mice remain viable ex vivo in the absence of pro-survival cytokines
Eosinophils isolated from the lungs of Aa challenged mice, IL5tg mice, and mice sensitized and challenged with A. fumigatus (Af) were placed in culture medium (RPMI with 20% FCS) either with recombinant mouse IL5 (5 ng/mL) or without additional cytokines. As shown in Fig. 3A, all eosinophils were sustained in culture in the presence of IL5. However, eosinophils from the lungs of IL5tg mice underwent rapid demise in the absence of exogenous IL5, and were minimally viable (3.1 ± 3.3%) after 2 days in culture. By contrast, a substantial fraction of the eosinophils (64 ± 11%) from the lungs of Aa-challenged mice remained viable at day 2 and remained similarly viable (57 ± 14%) after 4 days in culture without added IL5. Analogous results were obtained from eosinophils isolated from spleens of IL5tg mice [Fig. 3B], and lungs and spleens of wild-type mice subjected to Af sensitization and challenge [Fig. 3A and 3B]. Interestingly, although IL-13 gene-deletion led to diminished survival of eosinophils recruited to the airways in vivo, recombinant IL-13 (20 ng/mL) had no impact on survival of eosinophils ex vivo [Fig. 3C and 3D].
Figure 3. Eosinophils recruited to the lungs in response to fungal allergens do not depend on exogenous cytokine support for survival.
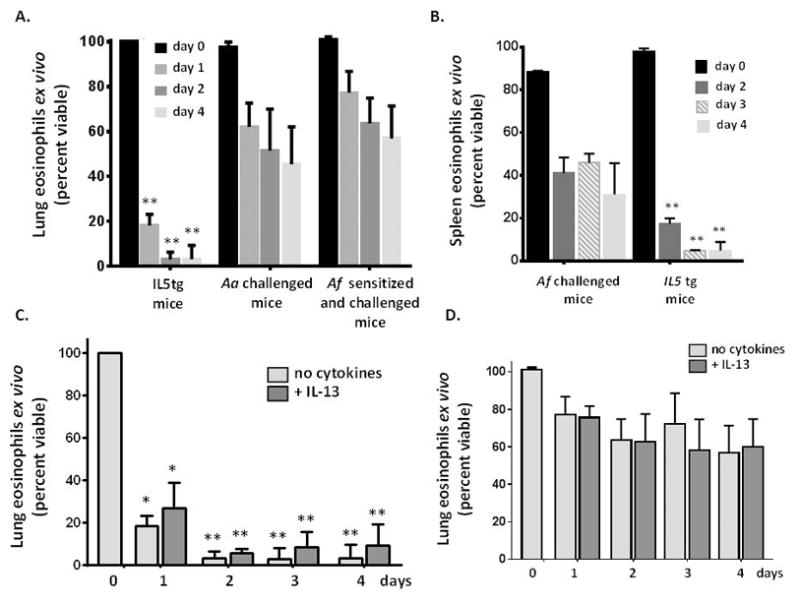
A. Survival of eosinophils isolated from lungs of IL5tg mice vs. eosinophils isolated from lungs of mice challenged with Aa (as in Fig. 1A) or sensitized and challenged with A. fumigatus (Af, see Methods); eosinophils were cultured in medium ex vivo without additional cytokines; n = 4 – 5 mice per time point, **p < 0.001, 2-way ANOVA. B. Survival of eosinophils isolated from the spleens of mice sensitized and challenged with A. fumigatus as in A. vs. spleens of IL5tg mice cultured ex vivo in medium without additional cytokines; n = 3 – 4 mice per time point, **p < 0.001, 2-way ANOVA. C. Survival of eosinophils isolated from lungs of IL5tg mice or D. isolated from the lungs of mice sensitized and challenged with Af, and cultured in medium with or without IL-13 (20 ng/mL); n = 5 mice per time point, *p < 0.05, **p < 0.001, 2-way ANOVA.
Eosinophils from the lungs of fungal-allergen challenged mice are phenotypically distinct from those from the lungs of IL5tg mice
Eosinophils store cytokines in their cytoplasmic granules; these mediators are released in response to endogenous or exogenous provocation [32, 33]. As shown in Table 2, eosinophils isolated from the lungs of fungal-allergen challenged mice maintain a complex cytokine profile, and are more enriched overall in pro-survival, proinflammatory and anti-inflammatory mediators than are eosinophils isolated from IL5tg mice. For example, eotaxin-1 was detected at high concentration in eosinophils from the lungs of fungal challenged mice, confirmed by ELISA at 787 ± 210 pg/107 eosinophils, while little to no eotaxin-1 was detected in lung eosinophils from IL5tg mice (1.3 ± 1.3 pg/107 cells). Interestingly, the only mediator that was significantly more prominent in eosinophils isolated from IL5tg mice was IL-16, a chemoattractant for cells that express CD4, including lymphocytes, monocytes, dendritic cells; IL-16 is also a strong chemoattractant for eosinophils themselves [34].
Table 2. Cytokine contents from lung eosinophils.
Cytokine profiling of lysates of eosinophils isolated from lungs of mice challenged with A. alternata vs. eosinophils from IL5tg mice;
| Mean pixel density | |||
|---|---|---|---|
| Cytokine | A. alternata | IL5-tg | Ratio** |
| IL-2 | 889 | 1a | 889 |
| IL1-Ra | 234737 | 149118 | 1.57 |
| IL1-beta | 2508 | 3061 | 0.82 |
| IL1-alpha | 4309 | 3650 | 1.18 |
| IFN-gamma | 2840 | 1248 | 2.28 |
| Eotaxin-1 | 978 | 1a | 978b |
| CCL1 | 1696 | 77 | 22.0 |
| GM-CSF | 2310 | 905 | 2.55 |
| G-CSF | 2076 | 530 | 3.92 |
| C5-C5a | 28487 | 4135 | 6.89 |
| CXCL13 | 3307 | 1560 | 2.12 |
| IL-27 | 1438 | 1105 | 1.30 |
| IL-23 | 3395 | 25 | 136 |
| IL-17 | 1898 | 1429 | 1.33 |
| IL-16 | 2419 | 23265 | 0.10 |
| IL-12p70 | 568 | 1a | 568 |
| IL-13 | 2177 | 1088 | 2.0 |
| IL-7 | 2022 | 1294 | 1.56 |
| IL-6 | 2011 | 1029 | 1.95 |
| IL-5 | 756 | 1a | 756 |
| IL-4 | 1874 | 454 | 4.13 |
| IL-3 | 2729 | 528 | 5.17 |
| CXCL12 | 3014 | 2162 | 1.39 |
| CCL5 | 3170 | 2132 | 1.49 |
| CXCL2 | 18673 | 9720 | 1.92 |
| CCL4 | 967 | 326 | 2.97 |
| CCL3 | 10571 | 7359 | 1.44 |
| CXCL9 | 2460 | 1674 | 1.45 |
| CCL12 | 1096 | 102 | 10.7 |
| CCL2 | 2177 | 549 | 3.97 |
| M-CSF | 6586 | 8433 | 0.78 |
| KC | 8053 | 1a | 8050 |
| I-TAC | 2335 | 255 | 9.16 |
| IP10 | 2389 | 410 | 5.83 |
| TREM-1 | 9031 | 4860 | 1.86 |
| TNF-alpha | 4915 | 2895 | 1.70 |
| TIMP-1 | 4722 | 2267 | 2.08 |
| CCL17 | 448 | 1a | 448 |
n = 5 mice (pooled lysates) per group as described in the Methods;
background signal,
ratio confirmed by DuoSet ELISA at 787 ± 210 pg eotaxin-1 per 107 eosinophils from A. alternata challenged mice vs. 1.3 ± 1.3 pg eotaxin-1 per 1 × 107 eosinophils from IL5tg mice, n = 3 – 4 mice per group,
p < 0.001.
Given the results in Table 2, we evaluated culture supernatants of eosinophils isolated from the lungs of fungal-challenged mice vs. those from IL5tg mice for immuno-reactive cytokines capable of sustaining eosinophil viability ex vivo. Of the canonical pro-survival cytokines, we detected no immuno-reactive IL5, IL3, eotaxin-1, IL-13 or GM-CSF at levels above medium control any of the cell culture supernatants [Supp. Table 1]. By contrast, immuno-reactive TNFα, a factor that can support eosinophil survival at low concentrations [35, 36] was detected at in culture supernatants of eosinophils isolated from lungs of fungal-allergen challenged mice only, at 35 ± 6 pg/mL and 18 ± 2 pg/mL at t = 24 and 72 hrs, respectively [Fig. 4A]. However, we found that survival of eosinophils isolated from the lungs of fungal-challenged TNFα gene-deleted mice was indistinguishable from wild-type [Fig. 4B], and addition of anti-TNFα neutralizing antibodies to the fungal-derived ex vivo eosinophil cultures had no impact on survival [Fig. 4C]. Additionally, eosinophils were detected in the airways of TNFα gene-deleted mice in response to fungal challenge, and persisted to an extent indistinguishable from wild-type [Suppl. Fig. 3]. These results indicate that, while release of TNFα is a distinguishing feature of eosinophils isolated from the lungs of fungal-allergen challenged wild-type mice, this cytokine does not play a singular role in maintaining survival of these cultures ex vivo.
Figure 4. TNFα is detected in cultures of eosinophils from lungs of fungal-challenged mice, but this cytokine is not critical for ex vivo survival.
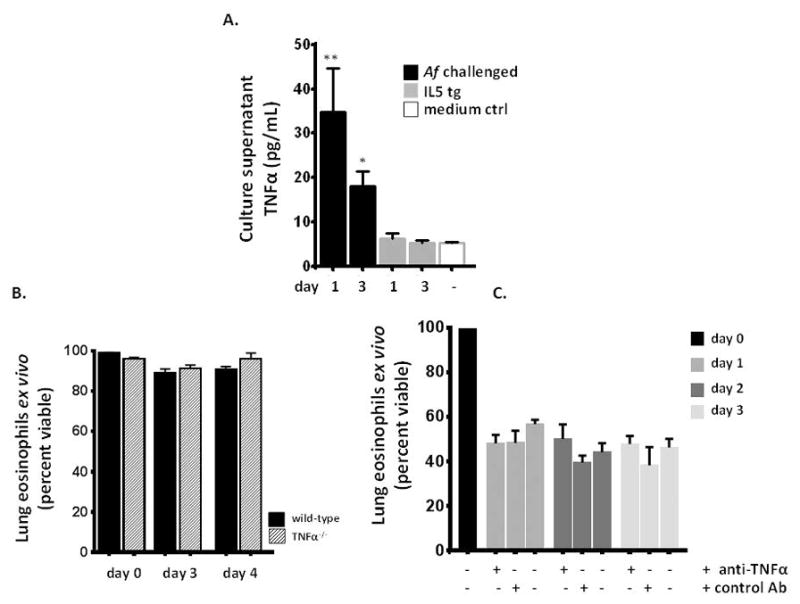
A. Immuno-reactive TNFα (but not IL5, IL-3, IL-13, eotaxin-1 or GM-CSF, see Suppl. Table 1) was detected in culture supernatants of eosinophils (106 cells/mL) from lungs of Af sensitized and challenged, but not IL5tg mice; n = 3 – 6 per point, **p < 0.01, *p < 0.05 vs. medium alone. Lower limit reported by manufacturer at 31.2 pg/mL; linear range was extended experimentally. B. Addition of anti-TNFα neutralizing antibody had no impact on sustained survival, n = 3 mice per point. C. Lung eosinophils isolated from TNFα gene-deleted mice were similarly capable of sustained survival, notably more robust on the C57BL/6 background; n = 5 – 10 mice per point.
Finally, we asked if IL5tg mice might respond as do the wild-type to fungal allergen challenge. We found that eosinophils isolated from the lungs of IL5tg mice subjected to Af sensitization and challenge responded as did the eosinophils from unchallenged IL5tg mice; the eosinophils isolated from the lungs of these mice underwent rapid demise ex vivo in the absence of exogenous IL5 [Fig. 5].
Figure 5. Eosinophils from the lungs of fungal-challenged IL5tg mice cannot sustain survival ex vivo.
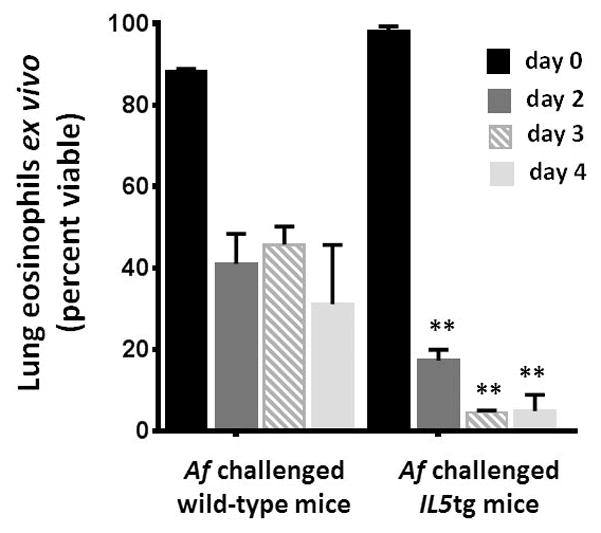
Survival of eosinophils isolated from lungs of fungal-challenged wild-type vs. fungal-challenged IL5tg mice vs. eosinophils isolated from lungs of mice challenged with Aa (as in Fig. 1A) or sensitized and challenged with A. fumigatus (Af, see Methods); eosinophils were cultured in medium ex vivo without additional cytokines; n = 4 – 5 mice per time point, **p < 0.001, 2-way ANOVA.
Discussion
In this study, we examined the responses of wild-type, gene-deleted, and IL5tg mice to challenge with Alternaria alternata (Aa) and Aspergillus fumigatus (Af), both prominent environmental allergens associated with the pathogenesis of human asthma. Our specific intent was to explore unique properties of eosinophils recruited to the lungs in response to fungal antigen allergen challenge.
As such, we were initially surprised to find that eosinophils persist in the airways for nearly a month after only a brief period of repetitive intranasal challenge with Aa. Among the cytokines implicated in promoting eosinophil survival, GM-CSF has been considered as providing critical support for eosinophil survival upon recruitment from blood into the tissues [12, 37]. In our study, we detected no GM-CSF in BAL fluid of mice treated with Aa (limit of detection, 7.8 pg/mL) and Gm-csf gene-deletion had no impact on eosinophil recruitment or prolonged survival in response to Aa challenge. Of additional interest, Gm-csf gene-deleted mice sustain a developmental block and have no mature, functional alveolar macrophages (AMs) [25]; this finding suggests that AMs are likewise not singularly crucial in promoting eosinophil recruitment or persistence in the lungs and airways. Similarly, eotaxin-1 gene deletion had no impact on eosinophil recruitment or persistence in response to Aa challenge. Rothenberg and colleagues [24] reported similar findings in mice subjected to the standard ovalbumin sensitization and challenge protocol.
By contrast, mice devoid of IL-13 were capable of recruiting eosinophils in response to Aa, although eosinophils did not persist in the airways of these mice, and levels returned to baseline by day 17. IL-13 is a well-characterized Th2 cytokine and a prominent secretory mediator from CD4+helper T lymphocytes. IL-13 is also generated by a large assortment of structural cells and numerous leukocyte lineages, including eosinophils themselves. Likewise, IL-13 has numerous and varied targets, and transduces signals via the IL-13Rα1/IL-4Rα complex on macrophages, fibroblasts, and epithelial cells [38]. Myrtek and colleagues [39] identified interleukin-13RαR1 on human peripheral blood eosinophils and characterized IL-13-dependent responses. By contrast, findings from Heller and colleagues [40] suggest that IL-13 may have no direct impact on the responses of isolated mouse eosinophils, a finding consistent with our observations here. Nonetheless, both human and mouse model data support a prominent and complex role for IL-13 in the pathogenesis of asthma, including recruitment of eosinophils to the airways [39]; monoclonal antibodies directed against IL-13 and its receptor are currently in development as asthma therapies [41].
Interestingly, Valladao et al. [23] reported that Aa sensitization and challenge in IL-13 gene-deleted mice led to neutrophil, as opposed to eosinophil recruitment to the lungs and airways. Although we detect neutrophils, together with eosinophils, in Gm-csf gene-deleted mice at baseline and in Aa-challenged mice on the BALB/c background [see Fig. 2], we found no neutrophil recruitment to the airways of Aa-challenged IL-13 gene-deleted mice (on the C57BL/6 background), perhaps because our model did not include a formal sensitization phase. It is also possible that variations in the vivarium facilities (ie…high vs. low barriers to pathogens) may result is divergent responses of this nature.
We also determined that a significant fraction of the eosinophils isolated from lungs and spleen of fungal-allergen challenged mice survive ex vivo in the absence of exogenous cytokine support, while eosinophils isolated from IL5tg mice alone, and IL5tg mice subjected to fungal allergen-challenge, all undergo rapid demise unless supplemented with IL5. Our findings build on those from Sedgwick et al. [42] who first reported prolonged survival responses from bronchoalveolar lavage (BAL) and peripheral blood eosinophils, specifically those isolated from allergic rhinitis patients subjected to segmental bronchopulmonary challenge with with ragweed antigen. Although no GM-CSF was detected in culture medium, ex vivo survival of human BAL eosinophils was reduced by addition of anti-GM-CSF antibodies [43].
Our findings are notable for several reasons. First, while IL5 is detected at high concentrations in the airways in response to fungal-allergen challenge (Table 1, at 348 ± 95 pg/mL) these observations highlight the fact that exposure to an environment enriched in IL5 alone, as in IL5tg mice, clearly drives eosinophils along a distinct developmental pathway. Eosinophils isolated from the lungs of IL5tg mice are not only phenotypically distinct (Table 2), they respond differently to cytokine withdrawal and are thus functionally distinct from eosinophils isolated from fungal-allergen challenged wild-type mice.
Taken together, these findings in their entirety suggest that eosinophils have substantial plasticity, and can adapt and change in response to signals in the environment. While eosinophils isolated from the peripheral blood of naïve human subjects certainly undergo apoptosis in the absence of pro-survival cytokines [44], a significant fraction of eosinophils isolated from peripheral blood and BAL of allergen-challenged human subjects can survive for 72 hours ex vivo without exogenous cytokine support [42, 43]. We have replicated these findings in part in wild-type mice, and found that a substantial fraction of eosinophils from the lungs and spleen of fungal-allergen challenged mice survive without addition of pro-survival cytokines, and, comparable to what has been reported for human BAL eosinophils [43], no IL5, IL3 or GMCSF was detected in the culture medium. In mice, a full understanding of eosinophil survival and responses ex vivo has been complicated by the fact that major source of these cells has been IL5tg mice [26, 45] and IL5-driven in vitro culture systems [46]; limited attention has been paid to intrinsic differences between responses of eosinophils accumulating in response to IL5 alone vs. those that develop and that are recruited to the lungs and airways in response to a more complex cytokine-enriched microenvironment.
In summary, we have elucidated several unanticipated properties of eosinophils recruited to the lungs in response to respiratory challenge with asthma-associated fungal allergens. First, we report eosinophil persistence in the lungs for up to a month after a brief period of repetitive challenge, a finding dependent not on GM-CSF, but on the Th2 cytokine, IL-13. Equally important, eosinophils recruited the lungs of fungal allergen-challenged mice are not phenotypically or functionally equivalent to those isolated from IL5tg mice. Specifically, eosinophils isolated from the lungs of fungal-allergen challenged mice are cytokine enriched and can survive ex vivo in the absence of exogenous cytokine support, in profound contrast to eosinophils isolated from the lungs of IL5tg mice, which undergo rapid demise under these conditions. As much of next generation asthma therapy is focused on limiting eosinophil recruitment and viability via strategic cytokine blockade, the molecular basis of persistence in tissue and differential survival is certainly worthy of further investigation.
Supplementary Material
Acknowledgments
We dedicate this manuscript to the memory of Dr. Jamie Lee, our colleague, friend and forever the Eosinophil-osopher-In-Chief. Work in our laboratory is supported by the NIAID Division of Intramural Research (ZIA-AI000941 to HFR).
Footnotes
Conflict of Interest Statement
The authors (W. E. Geslewitz, C. M. Percopo and H. F. Rosenberg) declare no conflicts of interest with respect to the execution and presentation of the work herein.
Author Contributions
WEG designed and performed experiments, and edited the manuscript.
CMP designed and performed experiments, and edited the manuscript.
HFR designed the study and wrote the first and subsequent drafts of the manuscript.
References
- 1.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nature Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563– 75. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr TF, Berdnikovs S, Simon HU, Bochner BS, Rosenwasser LJ. Eosinophilic bioactivities in severe asthma. World Allergy Org J. 2016;9:2. doi: 10.1186/s40413-016-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med. 2015;192:660– 8. doi: 10.1164/rccm.201504-0763PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keen ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651– 9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 6.Maltby S, Tay HL, Yang M, Foster PS. Mouse models of severe asthma: understanding the mechanisms of steroid resistance, tissue remodeling and disease exacerbation. Respirology. 2017;22:874–85. doi: 10.1111/resp.13052. [DOI] [PubMed] [Google Scholar]
- 7.Kumar RK, Herbert C, Foster PS. The “classical” ovalbumin challenge model of asthma in mice. Curr Drug Targets. 2008;9:485–94. doi: 10.2174/138945008784533561. [DOI] [PubMed] [Google Scholar]
- 8.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O’Neill KR, Colbert DC, Colby TV, Shen H, Blackburn MR, Irvin CC, Lee JJ, Lee NA. Co-expression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–89. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 9.Rankin JA, Picarella DE, Geba GP, Temann UA, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett J, Flavell RA. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA. 1996;93:7821–5. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Hoselton SA, Dorsam GP, Schuh JM. Eosinophils in fungus-associated allergic pulmonary disease. Front Pharmacol. 2013;4:8. doi: 10.3389/fphar.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar RK, Herbert C, Foster PS. Mouse models of acute exacerbations of allergic asthma. Respirology. 2016;21:842–9. doi: 10.1111/resp.12760. [DOI] [PubMed] [Google Scholar]
- 14.Kita H. ILC2s and fungal allergy. Allergol Int. 2015;64:219–26. doi: 10.1016/j.alit.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salo PM, Arbes SJ, Jr, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121:678–84. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kustrzeba-Wojcicka I, Siwak E, Terlecki G, Wolanczyk-Medrala A, Medrala W. Alternaria alternata and its allergens: a comprehensive review. Clin Rev Allerg Immunol. 2014;47:354–65. doi: 10.1007/s12016-014-8447-6. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel MF, Postigo I, Tomaz CT, Martinez J. Alternaria alternata allergens: markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ International. 2016;89–90:71–80. doi: 10.1016/j.envint.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Havaux X, Zeine A, Dits A, Denis O. A new mouse model of lung allergy induced by the spores of Alternaria alternata and Cladosporium herbarum molds. Clin Exp Immunol. 2015;139:179–88. doi: 10.1111/j.1365-2249.2004.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denis O, van den Brule S, Heymans J, Havaux X, Rochard C, Huaux F, Huygen K. Chronic intranasal administration of mould spores or extracts to unsensitized mice leads to lung allergic inflammation, hyperreactivity and remodelling. Immunology. 2007;122:268–78. doi: 10.1111/j.1365-2567.2007.02636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92–115. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, Kita H. IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy. 2016;71:977–88. doi: 10.1111/all.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valladao AC, Frevert CW, Koch LK, Campbell DJ, Ziegler SF. STAT6 regulates the development of eosinophilic versus neutrophilic asthma in response to Alternaria alternata. J Immunol. 2016;197:4541–51. doi: 10.4049/jimmunol.1600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg ME, MaClean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–90. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickerson GR, Bachurski CJ, Mark EL, Whitsett JA, Mulligan RC. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713– 6. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 26.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–31. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 28.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Percopo CM, Brenner TA, Ma M, Kraemer LS, Hakeem RM, Lee JJ, Rosenberg HF. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol. 2017;101:321–8. doi: 10.1189/jlb.3A0416-166R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer KD, Garcia-Crespo KE, Killoran KE, Rosenberg HF. Antigen profiles for the quantitative assessment of eosinophils in mouse tissues by flow cytometry. J Immunol Methods. 2011;369:91–7. doi: 10.1016/j.jim.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, Lee JJ, Appleton JA. Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol. 2014;193:4178–87. doi: 10.4049/jimmunol.1400852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Helmers RA, Lacy P, Moqbel R, Lee NA. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–84. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallsworth MP, Soh CP, Twort CH, Lee TH, Hirst SJ. Cultured human airway smooth muscle cells stimulated by interleukin-1beta enhance eosinophil survival. Am J Respir Cell Mol Biol. 1998;19:910–9. doi: 10.1165/ajrcmb.19.6.3275. [DOI] [PubMed] [Google Scholar]
- 34.Ferland C, Flamand N, Davoine F, Chakir J, Laviolette M. IL-16 activates plasminogen-plasmin system and promotes human eosinophil migration in extracellular matrix via CCR3-chemokine-mediated signaling and by modulating CD4 eosinophil expression. J Immunol. 2004;173:4417–24. doi: 10.4049/jimmunol.173.7.4417. [DOI] [PubMed] [Google Scholar]
- 35.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils and basophils. Trends Immunol. 2013;34:398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Esnault S, Malter JS. Granulocyte macrophage-colony-stimulating factor mRNA is stabilized in airway eosinophils and peripheral blood eosinophils activated by TNF-alpha plus fibronectin. J Immunol. 2001;266:4658–63. doi: 10.4049/jimmunol.166.7.4658. [DOI] [PubMed] [Google Scholar]
- 37.Esnault S, Malter JS. GM-CSF regulation in eosinophils. Arch Immunol et Therap Experimentalis. 2002;50:121–30. [PubMed] [Google Scholar]
- 38.May RD, Fung M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine. 2015;75:89–116. doi: 10.1016/j.cyto.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Myrtek D, Knoll M, Matthiesen T, Krause S, Lohrmann J, Schillinger D, Idzko M, Virchow JC, Friedrich K, Luttmann W. Expression of interleukin-13 receptor alpha 1-subunit on peripheral blood eosinophils is regulated by cytokines. Immunology. 2004;112:597–604. doi: 10.1046/j.1365-2567.2004.01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heller NM, Gwinn WM, Donnelly RP, Constant SL, Keegan AD. IL-4 engagement of the type I IL-4 receptor complex enhances mouse eosinophil migration to eotaxin-1 in vitro. PLoS One. 2012;7:e39673. doi: 10.1371/journal.pone.0039673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol. 2016;170:122–31. doi: 10.1159/000447692. [DOI] [PubMed] [Google Scholar]
- 42.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710– 8. [PubMed] [Google Scholar]
- 43.Yamamoto H, Sedgwick JB, Vrtis RF, Busse WW. The effect of transendothelial migration on eosinophil function. Am J Respir Cell Mol Biol. 2000;23:379–88. doi: 10.1165/ajrcmb.23.3.3707. [DOI] [PubMed] [Google Scholar]
- 44.Percopo CM, Dyer KD, Killoran KE, Rosenberg HF. Isolation of human eosinophils: microbead method has no impact on IL-5 sustained viability. Exp Dermatol. 2010;19:467–9. doi: 10.1111/j.1600-0625.2009.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–44. [PubMed] [Google Scholar]
- 46.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–9. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


