Abstract
The continuing fascination with serotonin (5-HT) as a nervous system chemical messenger began with its discovery in the brains of mammals in 1953. Among the many reasons for this decades-long interest is that the small numbers of neurons that make 5-HT influence the excitability of neural circuits in nearly every region of the brain and spinal cord. A further reason is that 5-HT dysfunction has been linked to a range of psychiatric and neurological disorders many of which have a neurodevelopmental component. This has led to intense interest in understanding 5-HT neuron development with the aim of determining whether early alterations in their generation lead to brain disease susceptibility. Here, we present an overview of the neuroanatomical organization of vertebrate 5-HT neurons, their neurogenesis, and prodigious axonal architectures, which enables the expansive reach of 5-HT neuromodulation in the CNS. We review recent findings that have revealed the molecular basis for the tremendous diversity of 5-HT neuron subtypes, the impact of environmental factors on 5-HT neuron development, and how 5-HT axons are topographically organized through disparate signaling pathways. We summarize studies of the gene regulatory networks that control the differentiation, maturation and maintenance of 5-HT neurons. These studies show that the regulatory factors controlling acquisition of 5-HT-type transmitter identity continue to play critical roles in the functional maturation and the maintenance of 5-HT neurons. New insights are presented into how continuously expressed 5-HT regulatory factors control 5-HT neurons at different stages of life and how the regulatory networks themselves are maintained.
Graphical Abstract
Introduction
In all species harboring a nervous system, from invertebrates to mammals, a small percentage of brainstem neurons become specialized to use serotonin (5-hydroxytryptamine, 5-HT) as a neurotransmitter (1–4). Unlike glutamate or Gaba, the main excitatory and inhibitory neurotransmitters of the brain, the action of 5-HT is that of a neuromodulator, adjusting neuronal excitability, to increase or decrease cell excitability according to the 5-HT receptors engaged (5, 6). Moreover, 5-HT has long-term effects on cell function such as trophic effects on growth (7, 8). Although the pool of 5-HT that is made in the brain accounts for a small percentage of total body 5-HT synthesis, brain 5-HT has been implicated in a multitude of functions from basic homeostatic processes such as thermoregulation and breathing (9, 10), to more elaborate functions such as affect and mood control (11–16), memory (17) and reward (18). 5-HT also plays a key role in several complex social behaviors such as male dominance, aggressive behavior, and maternal care (19–27).
In mammals, all brain 5-HT neurons are generated in a part of the developing hindbrain called the rhombencephalon, which gives rise to the pons and medulla (Figure 1) but later extend also into the mesencephalon following developmental reorganizations such as migration and folding of the neural tube (28, 29). 5-HT neurons are organized in cell clusters mainly, but not exclusively, along the midline (30–32). The raphe nuclei refer to the midline cell clusters. From there, 5-HT axons radiate broadly to innervate the entire central nervous system from the olfactory bulb to the spinal cord (30). Together with their highly divergent anatomical organization, hindbrain 5-HT neurons show anatomical and physiological specializations: specific neuronal subsets deliver 5-HT to different brain regions, and have different molecular and physiological identities (4). This compartmentalized regional organization of hindbrain 5-HT neurons into distinctive neural circuits forms the basis of their diverse brain functions.
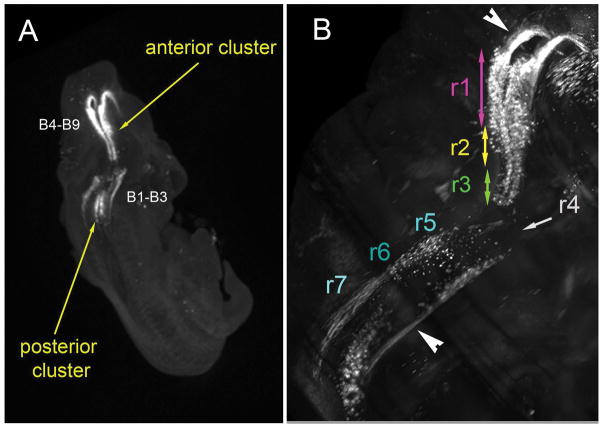
Figure 1. Anatomy of developing 5-HT neurons in the mouse brain.
3-D imaging of whole E12.5 mouse embryos. Tissue was fixed, immunostained in toto with 5-HT antisera and imaged with light sheet microscopy after tissue clarification. A) Dorsal view of the embryo, showing the anterior (B4–B9) and posterior (B1–B3) cell clusters. At this stage 5-HT neurons form two continuous parasagittal bands on either side of the midline. B) Higher magnification of the same embryo shows the 3D organization of cell bodies and the 5-HT efferent fiber tracts (arrowheads) that are directed both rostrally or caudally. The tentative position of earlier rhombomeric divisions (r1–r7) is indicated with different colors. Note the absence of 5-HT-labeled cells and axons in the brainstem segment corresponding to r4 at this embryonic stage.
Understanding the development of 5-HT brainstem neurons implies the dissection of developmental mechanisms that operate in parallel but also with interaction: a) mechanisms that specify 5-HT molecular identity and b) mechanisms that specify structural/regional identity and network wiring. These processes are controlled by intrinsic genetic programming and are also modulated by environmental factors. Thus, variants of developmental genes or environmental insults might impair the sequential developmental programs that instruct brain 5-HT neuron networks. These developmental variations could in turn underlie different susceptibility to neurological and psychiatric disorders. They may also underlie different responsiveness to pharmaceuticals targeting the 5-HT system (33–35).
In this review, we present a general overview of the neuroanatomical organization and the molecular diversity of vertebrate 5-HT neurons from a developmental perspective. We describe recent findings revealing the regulatory networks implicated in the differentiation, maturation, and maintenance of 5-HT neurons. We highlight some of the mechanisms through which 5-HT regulatory networks are thought to shape 5-HT neuron identity and how these networks are stably sustained into adulthood to preserve 5-HT function. Finally, we describe the signaling pathways that have been implicated in guiding 5-HT axons to their appropriate innervation zones and the potential of environmental factors to alter 5-HT neurotransmission through effects on 5-HT neuron morphology and migration.
Nomenclature and anatomical organization of the brain 5-HT neuron nuclei
From the early discovery of indolamine containing neurons in the rodent and human brain (31, 32) different 5-HT neuron clusters were identified in the hindbrain. The precise neuroanatomical boundaries of these cell clusters and their denominations have varied according to studies (summarized in Table 1). Some nomenclatures refer only to the 5-HT containing neurons (the B1–B9 cell groups, named from caudal B1 to rostral B9), whereas other nomenclatures refer to cytoarchitectonic structures that contain 5-HT neurons, such as the dorsal raphe (DRN) and median raphe (MRN) nuclei. Equivalences between these terms are valid to some extent but one must keep in mind that raphe nuclei contain a variable mix of 5-HT and non-5-HT neurons. For instance, the proportion of 5-HT neurons ranges from 50% in the DRN (36) to 21 % in the MRN (37) and less than 10 % in the paramedian raphe and the supra-lemniscal nuclei (37, 38).
Table 1.
Nomenclature and main projections of serotonin midbrain neurons
| Embryonic 5-HT Cluster | Rhombomere Alonso et al. 2013 Jensen et al. 2008 |
Common name (5-HT+non 5-HT) | Standard Abbreviation (5-HT+non 5-HT) Paxinos & Franklin 2008 |
Alpha-numeric Dahlstrom & Fuxe 1964 (5-HT) |
Main Projections |
|---|---|---|---|---|---|
| Anterior (rostral) Anterior |
R1-Sul R2-Sul R3-Sul |
Supra-lemniscal | SuL | B9 | Brainstem (raphe, locus coeruleus), basal ganglia, PFC |
| Is | Dorsal raphe | DR-R | B7r | substantia nigra, striatum, lat habenula | |
| DRD | B7d | Amygdala, piriform cortex, hypothal, dorsal hippocampus | |||
| DRV | B7v | Amygdala, piriform cortex, intralaminar thalamus, lateral habenula. | |||
| DRVL/LW | B7l | Sensory thalamus Sup colliculus |
|||
| R1 | DRC | B6 | SVZ, supra-ependymal plexus, preoptic area, septum, ventral hippocampus | ||
| Is R1 MnR R1 Mnc R2 |
Caudal Linear Median raphe N Pontine raphe N |
CLi MnR |
B8 | Mammillary, Hypothalamus Septum Hippocampus Limbic cortex medial habenula |
|
| R3 PnR R4 PnR |
PnR | B5 | Lateral septum, Hippocampus hypothalamus Raphe nuclei. dLTG |
||
| R5–R6 SGeR (Alonso et al.2013) R2–R3 (Jensen et al. 2008) |
Supragenual N | SGeR | B4 | Not known | |
| Posterior (caudal) | R5 R6 |
Raphe Magnus | RMg | B3 | All Brainstem-viscero-motor and chemosensitive nuclei (NTS, X, XI) |
| R7 | Raphe Pallidus | RPa | B2 | Spinal cord, sympathetic & parasympathetic preganglionic neurons | |
| R8 | Raphe obscurus | ROb | B1 | Brainstem Spinal cord |
Importantly, from a developmental point of view, 5-HT neurons can be separated into two main clusters, anterior (rostral) and posterior (caudal), with opposite polarity of their axonal projections. The anterior cluster neurons are the main source of 5-HT inputs to the forebrain and midbrain with additionally important projections in the hindbrain, including the cerebellum and the raphe neurons themselves (26, 39, 40). The posterior cluster is an important source of innervation of major brainstem nuclei and is the exclusive source of 5-HT innervation to the spinal cord. This primary division is best seen during early embryonic stages, (E11–E14 in rodents) (Figure 1) when 5-HT neurons form 2 paramedian bands on either side of the midline (32, 41–43).
Embryonic and fate mapping studies indicated that these primary anterior and posterior clusters are further segmented along the rostrocaudal axis according to rhombomeric (r) divisions (44). Rhombomeres are longitudinal compartments of the rhombencephalon from rostral (r1) to caudal (r8) where specific transcriptional codes (secreted morphogens and homeodomain-containing proteins) confer positional values. In this scheme, the rostral 5-HT cluster is generated from neural precursors in the isthmus, r1–r3, and the caudal cluster is generated from neural precursors of r5–r8 (44). An intervening gap between these two clusters corresponds to r4, where 5-HT identity is repressed and viscero-motoneurons are formed instead (45).
The final migration of 5-HT cell bodies and general morphogenetic remodeling of the hindbrain causes the 5-HT neurons of the rostral group to split into the B4 –B9 cell groups, and the caudal group to split into the B1–B3 cell groups (32, 42, 43). For instance, fate mapping of 5-HT progenitors generated in the isthmus and r1 showed that they gave rise to 5-HT neurons populating the DRN (B6, B7), B4, and parts of the MRN (B5, B8, B9) (44) (Table 1). 5-HT neurons from these cell groups then establish regionalized connections to different parts of the forebrain (39, 40) as summarized in Table 1. As can be seen from this table, different rhombomeres can contribute to a given raphe nucleus. In addition to the established divergent polarity of axons arising from the rostral and caudal 5-HT neuron clusters, there is a distinct topographic organization within the ascending forebrain projections as 5-HT axons originating from the DRN and MRN occupy complementary terminal territories in the forebrain (40, 46, 47). The DRN and MRN receive in turn a wide range of afferent inputs from the forebrain, that include the prefrontal cortex, central amygdala, bed nucleus of the stria terminalis, nucleus accumbens, preoptic area, hypothalamus, zona incerta and lateral habenula (48). As recently evaluated with transynaptic labeling strategy, these diverse brain areas establish monosynaptic glutamatergic, gabaergic or peptidergic inputs directly on the 5-HT (and gabaergic) raphe neurons (49, 50).
A rostral to caudal gradient of neurogenesis
Studies on the embryonic development of the raphe neurons have shown that 5-HT neurons are among the earliest generated neurons. They are produced at mid-gestation in rodents (embryonic day (E) 9.5–E12 in mice; E10.5–E13 in rats) and from the 5th to 7th week of gestation in humans (32, 42, 43, 51, 52). Immediately upon differentiation raphe neurons start producing 5-HT, facilitating their developmental analysis (Figure 2A, B). However, brain 5-HT synthesis does not begin to contribute to forebrain 5-HT levels until E16.5 and is instead supplied by the placenta prior to this stage (53).
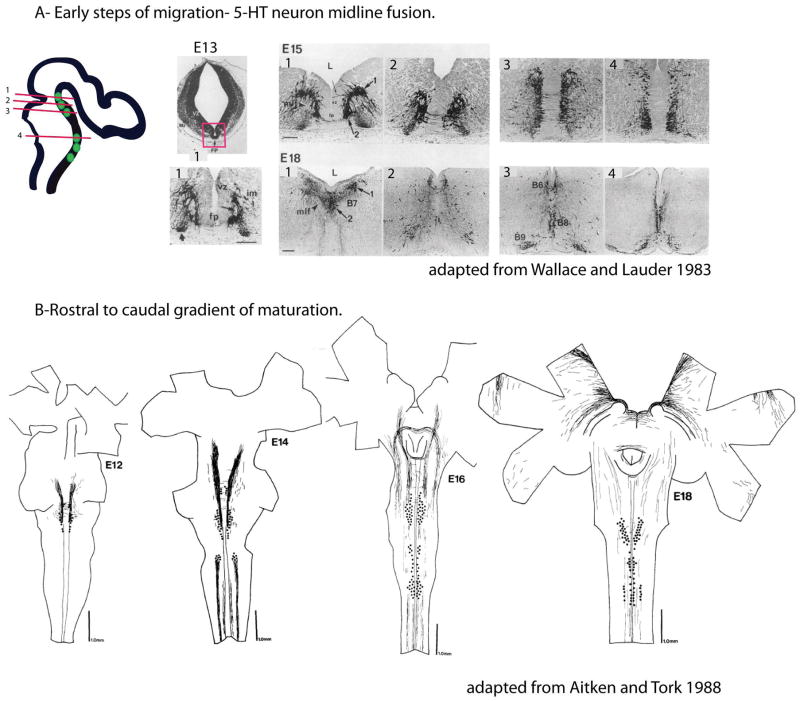
Figure 2. Maturation gradient of serotonin neurons.
A) Progressive midline fusion of the newborn 5-HT neurons in rat embryonic brains. The scheme indicates the different anteroposterior levels of sectioning in the hindbrain shown as red lines (1,2,3,4) that are illustrated in the micrographs. The position of the 5-HT neurons is indicated as green dots. Micrographs on the right show 5-HT immunostained sections at E13, E15 and E18 at these different rostral to caudal levels. Note that 5-HT cell groups are initially entirely separated by floor plate cells at E13. The floor plate starts to be invaded by outgrowing neurites at E15 at rostral levels (1, 2), midline fusion of the anterior cluster neurons begins at E18, while the fusion of the cells in the posterior cluster (3,4) is achieved postnatally. Pictures were taken from Wallace and Lauder, 1983. B) The rostral to caudal sequential appearance and maturation of 5-HT neurons is best seen on whole mounts of rat embryonic hindbrains immunostained with 5-HT. The drawings were taken from Aitken and Törk 1988 (41).
Differences in date of birth, together with axial position, could contribute to the specification of raphe neurons and account for the large transcriptional differences between the anterior and posterior cell groups reported in transcriptome analyses (54). 5-HT-labeled perikarya appear one day before in the anterior raphe cluster than in the posterior cluster (41–43) (Figure 2B). Other neurogenic gradients might also exist within the anterior and posterior raphe clusters. For instance, 5-HT progenitors originating from different sources have been described within the anterior raphe cluster. Part of the 5-HT progenitors are generated in the isthmus and secondarily migrate into r1 (28, 55) while the other r1–r3 progenitors originate from a ventral hindbrain progenitor pool called the p3 domain. Birthdating experiments in embryos showed that neurogenesis in r1 ceased approximately one day before r3 (56). This appears to match thymidine birthdating experiments in the midbrain suggesting that the peak of neurogenesis occurs earlier in the DRN than in the MRN (57). Maturation gradients were also noted within the posterior raphe cluster. A lateral group of 5-HT neurons was noted to emerge 2 days after the midline cell groups (41, 43) (Figure 2B), although it is unclear whether this reflects a late wave of 5-HT neurogenesis or a secondary migration of earlier born 5-HT neurons.
More recent high throughput molecular analyses showed that the rostrocaudal position of the 5-HT raphe neurons, as defined by rhombomeric origin, was the major component of the genetic variability amongst the 5-HT raphe neurons. This evidence was obtained using unbiased comparison of RNA-sequencing analyses from isolated 5-HT neurons sorted by position and by genetic fate-mapping of rhombomeres (58).
5-HT neuron molecular identity
5-HT neuron function derives from 5-HT neuron molecular identity. The identity of a 5-HT neuron is defined by its transcriptome: the collective of transcription products in a 5-HT neuron at a particular stage in life that encodes the stably expressed terminal effector proteins needed to build and maintain rich 5-HT connectivity, enable 5-HT neurotransmission and provide for their complex synaptic responsivity to environmental stimuli. It is now well established that 5-HT neurons do not possess a unitary transcriptome (54, 58–61). Indeed, intersectional and single cell analyses have identified tremendous diversity of 5-HT neuron transcriptomes with evidence in support of functional subtypes (26, 58–60, 62, 63). Moreover, unique 5-HT molecular identities may dictate highly selective axonal connectivity patterns of small 5-HT neuron subpopulations within specific raphe nuclei (64). Nevertheless, all 5-HT neurons do share a set of continuously expressed common characteristics with the most conspicuous being the capacity to biosynthetically make 5-HT (Figure 3A). The enzymes, tryptophan hydroxylase 2 (TPH2) and aromatic amino-acid decarboxylase (AADC), are the two terminal effectors directly catalyzing brain 5-HT synthesis. In addition, there are several enzymes needed for synthesis and recycling of BH4: the tetrahydrobiopterin cofactor obligatory for TPH2 enzyme catalysis. De novo BH4 synthesis depends on several enzymatic steps catalyzed by terminal effectors GTP cyclohydrolase I, (GTPCH, Gch1), 6-pyruvoyl-tetrahydropterin synthase (PTPS, Pts), and sepiapterin reductase (SR, Spr). Although it is an essential defining common feature of 5-HT neurons, some 5-HT neurons in the MRN express low levels of TPH2 (Figure 3B) (58).
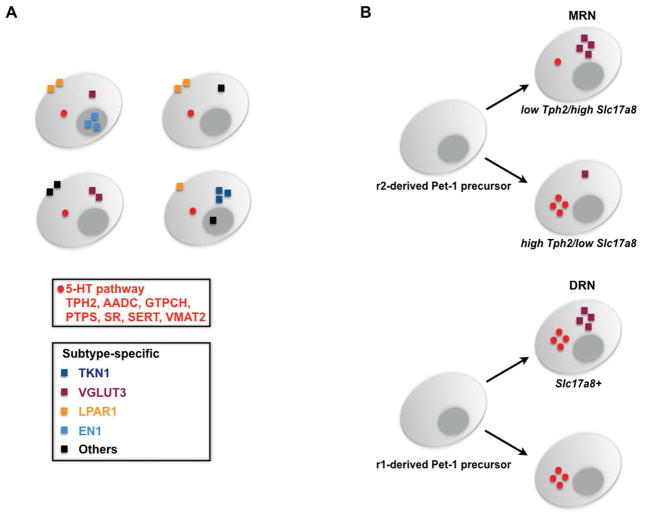
Figure 3. Diversity of 5-HT neuron identities.
A) The identities of 5-HT neurons comprise common and subtype-specific characteristics. Common characteristics include 5-HT pathway terminal effector proteins for 5-HT synthesis (Tryptophan hydroxylase 2, TPH2; Aromatic amino acid decarboxylase, AADC; Guanosine triphosphate cyclohydrolase, GTPCH; 6-pyruvoyl-tetrahydropterin synthase, PTPS; Sepiapterin reductase, SR), reuptake (Serotonin transporter, SERT, Slc6a4) and vesicular transport (Vesicular monoamine transporter 2, VMAT2, Slc18a2). Activation of the genes encoding these proteins endows newborn neurons with a 5-HT-type transmitter identity. Subtype-specific characteristics are expressed in subsets of 5-HT neurons and thus diversify 5-HT neuron molecular identity. Various peptides are well-established subtype-specific features of 5-HT neurons. A large number of other characteristics such as GPCRs, transporters, ion channels and TFs further diversify 5-HT neurons. Some examples are depicted: Vesicular glutamate transporter 3, VGLUT3 (Slc17a8); preprotachykinin, TKN1 (Substance P); lysophosphatidic acid 1 receptor, LPAR1; engrailed1 (homeodomain TF), EN1. Black squares indicate the numerous other 5-HT subtype specific proteins that diversify 5-HT neurons (see (58–60). B) Single cell RNAseq and single molecule FISH revealed that virtually all r2-derived Pet1+ precursors of the MRN give rise to 5-HT neurons but with negatively correlated levels of Tph2 and Slc17a8. Thus, two subtypes with either high Tph2/low Slc17a8 or low Tph2/high Slc17a8 expression are present in the r2-derived MRN. In contrast, Pet1+ precursors give rise to 5-HT neurons in the DRN with more uniform levels of TPH2; some of these 5-HT neurons express VGLUT3 (Slc17a8). See (58, 126).
In addition to 5-HT synthesis, 5-HT neurotransmission requires reuptake and vesicular transport activities. These functions are provided by expression of the high-affinity 5-HT transporter (SERT, Slc6a4) for 5-HT reuptake and the vesicular monoamine transporter 2, (VMAT2, Slc18a2) for loading of vesicles. Together, the common terminal effectors for 5-HT synthesis, reuptake and vesicular transport constitute a set of 5-HT pathway proteins whose activation confers 5-HT-type transmitter identity (Figure 3A). None of these terminal effectors is restricted to 5-HT neurons; even TPH2 is expressed in non-neuronal cells (65, 66). It is their co-expression together with expression of generic “neuronal” characteristics that defines 5-HT neuron-type identity. The 5-HT1a autoreceptor (Htr1a) was thought to be a common feature of 5-HT neurons in order to enable auto-inhibitory feedback on 5-HT neuron firing but recent studies presented evidence that some 5-HT neurons lack expression of this autoreceptor (67).
Subtype-specific identity features
As alluded to above, 5-HT neurons are heterogeneous (4) and are diversified by the restricted expression of a large number of different terminal effector and transcription factor genes (58–60). For example, a rich assortment of neuropeptides are expressed in subsets of human, rat, monkey, and cat 5-HT neurons, which is a well-documented example of how subtype-specific features diversify 5-HT neuron molecular identities (61, 63, 68, 69) (Figure 3A).
Peptide expression has been suggested to show species-specific differences based on an extensive Immunohistochemical study in the adult mouse raphe that failed to demonstrated peptide coexpression in DRN 5-HT neurons (70). However, a recent study of flow sorted neonatal DRN/MRN neurons indicated strong transcription of several peptide genes as evidenced by abundant RNAseq reads (71). The most abundantly transcribed genes in decreasing order were Sst (somatostatin), Penk, (proenkephalin), Pdyn (prodynorphin), Gal (galanin), and Nts (neurotensin). Single cell RNA-seq analyses of more mature 5-HT neurons revealed low reads for most peptide genes, however presence of galanin, and preprotachykinin transcripts helped define DRN/MRN subtype identities (58, 59). The discrepancies might be explained by lower peptide expression in mouse DRN 5-HT neurons, which escaped immunohistochemical detection. Another possibility is that peptides are more efficiently transported out of mouse 5-HT cell bodies to axon terminals or distal cell compartments. It will be interesting to determine whether peptidergic transcription in mouse 5-HT neurons is developmentally regulated and might vary in different physiological circumstances.
The vesicular glutamate transporter 3, (VGLUT3, Slc17a8) is another subtype specific feature through which 5-HT neurons in the DRN and MRN are diversified (72–74) (Figure 3A). As for peptides, estimates of VGLUT3/5-HT colocalization vary across the raphe nuclei, and shows species (rat/mice) differences (59, 75, 76). For example, in the ventromedial portion of the DRN and in the MRN of rats roughly 80% of 5-HT neurons are VGLUT3+ and a similar percentage of VGLUT3+ neurons are TPH2+. However, in the lateral regions of DRN (wings) the percentage of Tph2+ neurons that express VGLUT3 is about 5% (74). There is also a cluster of VGLUT3+/TPH2− neurons in the dorsomedial portion of the DRN (dmDRN, shell region) (74). The raphe distribution of VGLUT3+ neurons is similar in mice but with overall lower % colocalization with TPH2 (59). Optogenetically stimulated 5-HT neurons provides evidence that 5-HT and glutamate are co-released and that VGLUT3 is required for release of glutamate (77–79). One possibility is that a portion of DRN 5-HT neurons may be glutamatergic and co-release glutamate for fast synaptic transmission, which was first suggested in cell culture studies (80). In addition, VGLUT3 might facilitate filling of vesicles with 5-HT to increase 5-HT release and therefore it may function to enhance 5-HT transmission (81). VGLUT3+/5-HT+ neurons may be a specialized 5-HT subtype as these cells were found to have specific electrophysiological signatures in patch clamp analyses, with lower activation thresholds, suggesting that they may be recruited only by strong stimuli to boost serotonergic/glutamatergic signals (59). Further, in vivo recordings indicated that VGLUT3+/5-HT−, VGLUT3−/5-HT+, and VGLUT3+/5-HT+ neurons in the MRN possess significantly different firing rates, responses to sensory stimulation, and phase coupling to forebrain oscillations (82).
Maturation of 5-HT neurons
Newborn 5-HT neurons are in an immature state as they have yet to migrate to their adult raphe locations, develop axonal and dendritic structures, acquire adult firing characteristics and make connections with neuronal targets and afferents (83). The stage of 5-HT neuron maturation in rodents begins concomitant with the initiation of 5-HT synthesis and extends up to at least the third week of life (84–86). RNA-sequencing of flow sorted 5-HT neurons followed by hierarchical clustering revealed complex ascending and descending gene expression trajectories during the fetal to early postnatal phase of maturation (71). For example, expression of peptide genes, Sst, Penk, Pdyn and Nts was low at E11.5 when 5-HT-type transmitter identity is acquired but subsequently increased 2–3 orders of magnitude through the remaining fetal stage and into early postnatal life. Similarly, expression of the noradrenergic receptor gene, Adra1b; GABA receptor subunit genes, Gabra1, Gabra3, Gabra4, Gabra5; NMDA receptor subunit genes, Grin1, Grin2b and sodium channel genes, Scn1a, Scn1b, was not detected or was very low at the time 5-HT pathway genes are activated but exhibited progressive increases during the maturation stage (71). The up-regulation of these genes precedes the arrival of excitatory and inhibitory synaptic inputs to the DRN neurons, and the maturation of the electrophysiological firing properties of raphe neurons (85, 86). Expression of other genes, for example Slc17a8 and Gria4 encoding the GluR4 AMPA receptor subunit, was already robust at the time of 5-HT pathway gene activation and continued without significant change; other genes displayed modest increases or decreases. Groups of genes with descending expression trajectories were associated with gene ontology terms suggestive of earlier progenitor stage functions. These disparate expression trajectories suggest that maturation of 5-HT neurons results from the induction of specific genes, subsequent to acquisition of 5-HT-type transmitter identity, turning off of earlier stage genes, and adjusting expression levels of others.
Migration of 5-HT neurons shapes adult raphe nuclei and commences soon after they acquire 5HT-type transmitter identity. 5-HT neurons are born in the ventricular zone, close to the floor plate and then rapidly migrate out by a saltatory migration toward the pial surface. Videomicroscopic analyses at E10 and E11 showed that this migration results from soma translocation within a leading process (87). This study also provided evidence for an additional longitudinal migration along the rostral-caudal pial surface and suggested that the later born 5-HT neurons pile up behind the earlier born ones (87) although this would need to be confirmed by analyses over longer time periods.
Later migration events have been demonstrated by sequential developmental studies on fixed brain samples from different embryonic stages. This consists in a lateral to medial migration of 5-HT raphe neurons resulting in their progressive fusion along the midline. These movements coincide with the disappearance of the floor plate, and are preceded by 5-HT process ingrowth into the midline. The fusion follows a clear rostral to caudal temporal sequence in accordance with the time of generation of the 5-HT cell clusters (41, 43) (Figure 2A). Fusion is only completed at postnatal day 3 (P3) in the anterior cluster and by P6 in the posterior cluster (88). Overall these different cellular morphogenetic movements progressively shape the adult-like 5-HT raphe cell nuclei and are terminated by the end of the first postnatal week in rodents.
As soon as they are born 5-HT neurons are polarized and burgeon axons that contain 5-HT at particularly high concentrations in the growth cones suggesting that 5-HT can be released while neurons are still growing. 5-HT neurons initially form distinctive bundles of straight fibers. Their day-to-day progression has been well described in rat embryos (42, 43, 84). Ascending fiber tracts are visible at E12, running on both sides of the midline in the marginal zone; they reach the mesencephalic flexure by E14, the diencephalon by E15– E16, and the cerebral cortex by E17 (Figures 2B, 4). At that stage part of the 5-HT axons leave the main tract of the medial forebrain bundle (mfb) and start following other fascicles toward the habenula (fasciculus retroflexus), the hypothalamus (mamillotegmental tract), the amygdala (external capsule), the cerebral cortex and hippocampus (supracallosal stria and fornix) (Figure 4). In all these routes, raphe axons follow pre-existing fiber tracts rather than pioneering new pathways (42). 5-HT axons reach all the forebrain areas at the time of birth, but the terminal innervation takes another 3 full weeks to become established in different brain areas (Figure 4). Extensive axon terminal branching continues over a long postnatal period, with variations in the timing of invasion (42). For instance, the medial thalamus, septum and amygdala are among the earliest innervated areas, while substantia nigra, caudate nucleus and suprachiasmatic nucleus lag behind, as most of the terminal innervation occurs during the 2nd and 3rd postnatal weeks (Figure 4). These regional differences in timing of innervation, recently confirmed with precise quantitative analyses in mice (89), are not related to the time of arrival of axons but rather, seem to be correlated with the maturation of targets, suggesting that target derived factors promote fiber ingrowth (84, 90).
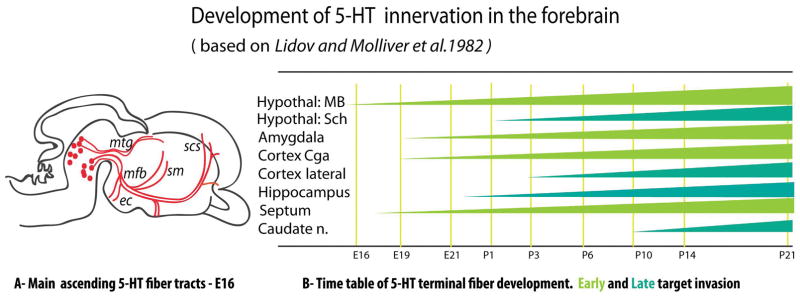
Figure 4. Development of 5-HT axons.
A-1, Scheme of E16 rat embryo, (adapted from 24). Ascending 5-HT axons grow rapidly between E12 and E16, following different preexisting axonal tracts such as the medial forebrain bundle (mfb), the mamillotegmental tract (mtg), the supracallosal striae (scs), external capsule (ec) and the stria medullaris (sm). Subsequent axon terminal branching and invasion of targets is a slower protracted process that continues late into early postnatal life. Axon guidance molecules such as WNT and SLIT1/SLIT2 have been implicated in directing this growth. A-2, The onset of 5-HT terminal Innervation in different brain structures shows marked regional differences. This is schematized as triangles with different shadings along the developmental stage E16 to P21. Light green = early target invasion; dark green = late target invasion. 5-HT fiber ingrowth has been found to be modulated by several factors, namely GAP43, STOP, 5-HT, protocadherin alpha, and ephrinAs.
Regional 5-HT axonal targeting appears to be selective from the outset with no evidence of exuberance or pruning. Although some early studies of 5-HT axon development had reported the existence of exuberant 5-HT raphe innervation in the primary somatosensory cortex (barrel cortex) and the visual thalamic nuclei (dLGN), these transient 5-HT innervation patterns were later found to be due to uptake of 5-HT by thalamic and retinal axons respectively and not to raphe 5-HT innervation; as both sensory thalamic neurons and retinal ganglion cells transiently express the 5-HT (Slc6a4) and vesicular monoamine (Slc18a2) transporters during an early postnatal period (7, 91).
A remarkable, feature of the 5-HT axons, which sets them apart from most other neuronal cell types in the adult central nervous system, is their capacity to regenerate after injury, and to show collateral sprouting in response to different types of neural damage. One of the most striking examples is the sprouting of 5-HT axons in response to lesions of the dopaminergic neurons in rodent or primate models of Parkinson’s disease (92–94). The other striking example is the growth of the descending 5-HT axons after a spinal cord injury. 5-HT axons are among the rare axons that are able to cross or at least penetrate a traumatic or ischemic scar (95, 96). This unusual response of serotonergic neurons after CNS injury could be due to a lack of axonal dieback of the axotomized 5-HT neurons and the fact that their growth is not inhibited by the chondroitin sulfate proteoglycans present in glial scars (97). However recent in vivo imaging data showed that cut 5-HT axons could also undergo an initial regression process followed by a regrowth (98).
Gene regulatory networks controlling 5-HT neuron identity
Early patterning events, reviewed in depth elsewhere (99–101), generate proliferating progenitors expressing the homeobox transcription factor (TF) gene, Nkx2-2. These Nkx2-2+ progenitors are tripotent and have competence to generate cranial motor neurons (MNs), as well as later born 5-HT neurons and oligodendrocyte precursors (OLPs). In young Nkx2-2+ progenitors expression of Phox2b ensures MN production while suppressing 5-HT neuron generation (45). Tgf-beta signaling is then activated in Nkx2-2+ progenitors and serves as a switching signal to shut down Phox2b expression, restricting Nkx2-2+ progenitor potency and permitting the sequential generation of 5-HT neurons and OLPs (102).
Nkx2-2+ progenitors also transiently express Ascl1 and Foxa2 constituting a 5-HT progenitor stage gene regulatory network (GRN). This primary GRN activates a secondary GRN in postmitotic precursors that performs three critical functions: first, it directly selects terminal effector genes for coexpression in 5-HT neurons such as the 5-HT pathway genes. Second, 5-HT GRNs set the levels of transcription of these genes and therefore the level of 5-HT signaling. Third, they provide the regulatory apparatus to enable external stimuli to make adjustments to default transcriptomes according to the physiological demands placed on the animal (103).
While it is now evident (see below) that postmitotic 5-HT GRNs are heterogeneous, there is a set of core TFs, GATA2, GATA3, INSM1, LMX1B, PET1, that control co-expression of 5-HT synthesis, reuptake and vesicular transport effector genes (25, 104–108). Together, these TFs constitute a postmitotic stage master GRN that governs acquisition of 5-HT neuron identity in postmitotic 5-HT neurons from E9.5–E12.5 (Figure 5). Like the common 5-HT pathway genes, none of the TFs in 5-HT GRNs is expressed exclusively in 5-HT neuronal lineage and so it is their co-expression and presumed combinatorial function in the small number of cells fated to be 5-HT neurons that enables co-expression of 5-HT neuron identity genes.
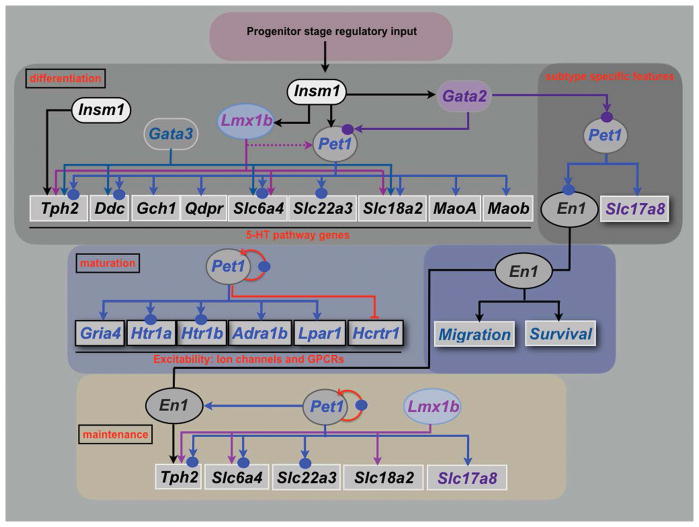
Figure 5. 5-HT neuron gene regulatory networks.
Depicted regulatory interactions (arrows) at the indicated stages of life are based on germ line or conditional loss of function data for each indicated TF. Terminal effector genes are depicted in rectangles and transcription factors in ovals. 5-HT pathway genes encoding 5-HT synthesis (Tph2, Ddc, Gch1, Qdpr), reuptake, (Slc6a4, Slc22a3), vesicular transport (Slc18a2) and metabolism (Maoa, Maob) are shown in rectangles with black letters. Terminal effector genes depicted in blue letters (Gria4, Htr1a, Htr1b, Adra1b, Lpar1, Hcrtr1) encode AMPA receptor subunit GLUR4 and GPCRs required for responses to diverse synaptic inputs. Arrows indicate that deficiency of a specific TF results in complete or partial loss of gene expression of a particular target gene in some or nearly all 5-HT neurons in which the TF expressed. Red blunted line from Pet1 to Hcrtr1 indicates that loss of Pet1 leads to upregulation of Hcrtr1. Slc17a8 (VGLUT3) is a subtype-specific terminal effector of 5-HT neurons. Homeobox gene, Engrailed1, (En1) encodes a 5-HT subtype specific TF as it is expressed only in r1-derived 5-HT and non-5-HT neurons but not at more posterior hindbrain levels. Dashed line indicates that Lmx1b controls maintenance of Pet1 at fetal stage. Solid circles indicate that evidence in support of direct regulation of a TF or terminal effector gene by a particular TF was obtained by chromatin immunoprecipitation (ChIP)–PCR or ChIP sequencing. In the case of Htr1a, evidence in support of direct regulation by Pet1 was obtained with cell line reporter assays and in vitro mobility shift DNA binding assays (187). Further ChIP studies may reveal PET1 occupancy at the other PET1 regulated effector genes shown in the scheme. Chromatin immunoprecipitation has not been reported for LMX1B in 5-HT neurons. Red curved arrow indicates positive autoregulation by Pet1.
Gata2 and Insm1 sit atop the regulatory hierarchy in postmitotic precursors to control induction of Gata3, Pet1 and Lmx1b (104, 106, 109) (Figure 5). Insm1 expression in 5-HT precursors is almost completely lost in Ascl−/− embryos (104) Chromatin immunoprecipitation (ChIP) analyses suggest ASCL1 directly induces Insm1 in 5-HT neuron precursors in cooperation with a presently unknown BRN-type POU TF (104, 110). A candidate POU factor is POU3F2 as presumed hypomorphic POU3F2 alleles led to deficits in the number of DRN TPH2+ cells (111). RNA-seq revealed POU3F2 expression in fetal 5-HT neurons but it is not yet known if it is coexpressed with ASCL1 at the progenitor/precursor stage. Insm-1 seems to be transiently expressed and is required in a subset of 5-HT neurons. GATA2 appears to directly control induction of Pet1 in 5-HT precursors as GATA2 binds near conserved GATA binding sites in the Pet1 upstream enhancer region and mouse transgenic studies indicate these sites are required for 5-HT neuron specific activity of the enhancer (112). Subsequently, Gata2 expression is dramatically downregulated as 5-HT neurons enter the maturation stage (28, 113).
Several groups have investigated the role of Gata3 in acquisition of 5-HT neuron-type identity (108, 109, 113, 114). In Gata3 null embryos there is an increasing requirement for Gata3 along the rostrocaudal hindbrain axis with a substantial depletion of 5-HT+ neurons in r8 and sparing of most 5-HT+ neurons in r1 (108, 114). Similarly, conditional targeting of Gata3 beginning at around E12.5 in newborn 5-HT neurons resulted in moderate deficiencies in 5-HT+ neurons in all raphe nuclei (113). However, a recent study of Gata3 in which earlier conditional targeting was achieved with an En1-cre transgene led to severe deficiencies of r1-derived TPH2+ and SERT+ neurons (109). The basis for the discrepancy between null vs. En1-cre targeting of Gata3 in r1-derived 5-HT neurons is presently unknown.
Lmx1b deficiency obtained through either germ line or 5-HT neuron-directed conditional targeting resulted in a nearly complete elimination of TPH2 and SERT expression and a near complete absence of 5-HT in the mouse brain and spinal cord (107, 115) (Figure 5). Although 5-HT cell bodies were reported missing in 5-HT conditionally targeted Lmx1b mice, the stage at which this occurs is not known (115). Expression of secretogranin II (SCGII) and calcitonin receptor (Ctr) was also eliminated in 5-HT conditionally targeted Lmx1b mice indicating that Lmx1b function is not limited to 5-HT pathway genes (115).
The ETS domain transcription factor gene, Pet1, (human ortholog, FEV) has a rostral to caudal temporal pattern of expression, similar to the rostral to caudal temporal sequence of 5-HT neurogenesis noted above. Pet1 expression precedes the appearance of 5-HT immunoreactivity in both cell groups by 6–12 hours (55, 56, 116). Nkx2-2+ progenitors in the ventral hindbrain generate Pet1+ postmitotic precursors through control of Pet1 by Gata2 (106, 109, 112). Similar to Lmx1b, Pet1 is required for acquisition of 5-HT transmitter identity in nearly all 5-HT precursors as SERT is strongly reduced in all raphe nuclei of Pet1 deficient mice (25, 113, 117). SERT radio-ligand binding is also reduced by 90% in Pet1−/− brain (118); the residual binding may reflect SERT expression on the remaining 5-HT neurons whose axons are concentrated in very specific brain areas (amygdala, paraventricular nucleus, midline thalamic nuclei). Moreover, expression of Slc22a3 (encoding the low affinity, high capacity 5-HT transporter, Oct3) and Htr1a is nearly eliminated in Pet1−/− 5-HT neurons further supporting the essential function of Pet1 in 5-HT neuron development (117). However, 5-HT synthesis is intact, albeit reduced, in about 25% of Pet1-deficient 5-HT neurons because of residual TPH2, VMAT2, and AADC expression (118). BAC transgenic rescue lines in which FEV is expressed at different levels in the Pet1−/− background led to corresponding graded levels of rescue of 5-HT identity (i.e., TPH2 and 5-HT) supporting the idea that the level of 5-HT GRN function determines the level of 5-HT signaling (23).
Pet1 and engrailed function in 5-HT neuron maturation
Pet1 expression continues through the maturation stage suggesting that in addition to controlling induction of the 5-HT pathway genes as 5-HT neurons are born it may directly control gene expression trajectories supporting 5-HT neuron maturation. In support of this idea, whole cell recordings in slices taken from P21 and adult animals indicated that passive and active membrane properties of Pet1−/− DRN 5-HT neurons were significantly different from those in wild type 5-HT neurons and in general resembled the immature properties of neonatal 5-HT neurons (71). Moreover, excitability was increased while EPSC amplitudes, noradrenergic input, 5-HT autoreceptor function, and G-protein signaling were significantly reduced in Pet1−/− 5-HT neurons; collectively these mutant properties were indicative of an immature state (86).
RNA-seq of E15.5 Pet1−/− 5-HT neurons revealed significantly altered expression of hundreds of other genes in addition to the common 5-HT pathway genes, hence revealing Pet1’s broad role in shaping the maturation of 5-HT neuron transcriptomes (71). For example, expression of several neurotransmitter-type GPCR and ligand-gated ion channel genes were decreased in Pet1−/− neurons including Adra1b, Htr1a, Lpar1, and Gria4 (Figure 5). Multielectrode array and whole cell recordings revealed severely reduced responses of mutant 5-HT neurons to agonists selective for each of the GPCRs (71). Therefore, Pet1 coordinates expression of different surface receptors required for the diverse neuromodulatory synaptic input to 5-HT neurons.
The engrailed homeodomain paralogs, En1 and En2, play important early roles in 5-HT neuron development although it is not clear whether this early role is in patterning of the mid/hindbrain territory or in 5-HT progenitor specification or both (119–121). En1 and En2 are also expressed in postmitotic DRN 5-HT neurons that are generated from progenitors in r1 but not in 5-HT neurons that develop at more posterior rhombomeres (54, 58, 122, 123). These expression patterns indicate that EN1 and EN2 are subtype-specific TFs of r1-derived 5-HT neurons (Figure 5). En1 persists into adulthood while En2 expression is extinguished at the late fetal stage.
Conditional targeting of En1/2 in postmitotic 5-HT neurons revealed a requirement for these TF genes in the positioning of 5-HT neurons in the DRN and thus maturation of DRN cytoarchitecture (122). These studies further indicated that En1/2 were required in the perinatal stage for maintenance of TPH2 expression in the DRN with En1 being the predominant functional paralog (Figure 5). Following this loss of identity, a large proportion of the En1/2-deficient DRN 5-HT neurons did not survive (122), which could result from a poor integration in functional neural circuits and lack of trophic interactions.
Regulation of 5-HT subtype identity
Insight into the development of VGLUT3+ neurons in the raphe has been gained from a number of recent studies. Genetic fate mapping has recently revealed previously unrecognized complexity in the cell lineages derived from ventral r1 Nkx2-2+ progenitors (109). While confirming that these progenitors give rise to Pet1+ precursors, which in turn differentiate into 5-HT neurons that populate the DRN it was reported that subsets of Pet1+ precursors also generate neurons with dual VGLUT3/5-HT identity and some with just VGLUT3 identity. However, quantitative estimates of the number of Pet1+ precursors that give to VGLUT3+/5-HT− neurons in the DRN were not reported and would require combined triple labeling studies of the three markers. VGLUT3+/TPH2− neurons clustered in the dmDRN may not derive from Nkx2-2/Pet1 lineage as expression of Pet1 in these cells is very low or absent (109).
Pet1 also controls Slc17a8 (VGLUT3) and peptide gene expression in the DRN (Figure 5). The control of Slc17a8 by Pet1 was initially shown with tamoxifen inducible targeting of Pet1 in adult 5-HT neurons, which resulted in a partial loss of Slc17a8 expression (113). More recently ISH and RNA-seq studies of flow-sorted fetal Pet1−/− 5-HT neurons revealed decreased Slc17a8 expression relative to wild type 5-HT neurons (4, 71). Similarly, several peptide genes were significantly decreased in flow-sorted fetal Pet1−/− 5-HT neurons suggesting Pet1 controls the complex patterns of peptidergic traits in 5-HT neurons (71). Pet1’s control of Slc17a8 and peptide genes indicates that it is not only required for expression of terminal identity features shared by all 5-HT neurons, but also for expression of subtype-specific features. Lmx1b has also been implicated in regulating the expression of several peptides in these neurons (124). Gata2 but not Gata3 is required for VGLUT3 expression in r1-derived DRN and MRN 5-HT neurons (109). Thus, a regulatory program comprising Nkx2-2, Gata2 and Pet1 controls Slc17a8/VGLUT3 expression in r1-derived 5-HT neurons.
How is Slc17a8 and peptide gene expression restricted to a subset of 5-HT neurons? One possibility is that Pet1 function is repressed in some 5-HT neurons by repressor TFs, which is a recently established mechanism through which neuronal subtype diversification is controlled in C. elegans (125). Alternatively, distinct Pet1 cofactor combinations may be involved that select subtype-specific traits in some 5-HT neurons, hence contributing to subtype-specific diversification. Although distinct Pet1 cofactor combinations have not yet been identified a large number of regulatory genes are differentially expressed in Pet1+ neurons across the various raphe nuclei including the MRN (54, 58).
In some Pet1+ cells of the DRN and MRN Tph2 expression could not be detected with ISH (126). The largest proportion of Pet1+/Tph2− relative to Pet1+/Tph2+ neurons was detected in the MRN B8 and B9 groups where it reached 26% whereas in the B6/B7 DRN the proportion was only about 1% (126). Thus, the vast majority of DRN Pet1+ neurons possess overt 5-HT neuron-type identity some of which express Slc17a8/VGLUT3 (Figure 3B). The presence of Pet1+/Tph2− neurons in the MRN was interpreted as evidence that some Pet1+ neurons are not 5-HT neurons (37, 126). However, recent single neuron RNAseq and two-color single molecule FISH demonstrated that virtually all Pet1+ neurons in the r2-derived MRN transcribe Tph2 although in some single Pet1+ neurons the level of Tph2 expression is much lower than in other Pet1+ neurons (58). These findings identify two 5-HT subtypes based on Tph2 and Slc17a8 expression patterns: high Tph2/low Slc17a8 expression and low Tph2/high Slc17a8 expression (Figure 3B). Gch1 and Slc6a4 expression also varied with Tph2 in r2-derived MRN raising a question about the 5-HT synthesis and reuptake capacity of some Pet1+ 5-HT MRN neurons. It is possible that this represents a reserve quiescent /dormant subset of 5-HT neurons, in which 5-HT identity features are normally repressed. An interesting idea yet to be investigated is that Pet1+ neurons are poised to switch between high Tph2/low Slc17a8 expressing and low Tph2/high Slc17a8 expressing identities in response to appropriate types of stimuli as has been described for sensory stimulus switching of dopamine (DA) vs. somatostatin identities of neurons in the hypothalamus (127).
Maintenance of 5-HT neurons
It is becoming increasingly evident that transcriptional programs actively maintain the identity and function of adult neurons (128). These programs often comprise continuously expressed TFs that in many instances play crucial early roles in controlling the acquisition of identity of the very neurons they work to maintain later in life. Further, there is now growing attention to the possibility that transcriptional destabilization of adult neuron-type identity may predispose neurons to degeneration. For example, the continuously expressed TF, NURR1, is necessary for the acquisition and maintenance of DA neuron identity. Conditional targeting of Nurr1 in adult DA neurons led to a phenotype with features of early stage Parkinson’s disease (PD) (129, 130). In addition, Nurr1 is also required to maintain DA neuroprotective pathways through its control of the glial-cell line derived neurotrophic factor (GDNF) receptor gene, Ret (131). Recent findings suggest that alpha-synuclein toxicity may lead to PD by interfering with Nurr1 regulatory programs that function to maintain GDNF/Ret trophic signaling in adult DA neurons (132).
Integrity of transcriptional maintenance programs may also be crucial for preserving the health of brain 5-HT neurons. Age-related progressive degeneration of 5-HT axonal morphology is evident as early as 12 months in rats and is followed over the ensuing several months by a progressive loss of forebrain 5-HT axonal density (133). Large deficits in the number of 5-HT neurons in the DRN and MRN are consistently observed in Alzheimer’s disease and some genetic mouse models of AD (134). 5-HT cell body deficits appear to follow the 5-HT axonal degeneration that is evident at earlier stages of AD (133, 134). These findings raise the potential importance of regulatory mechanisms that might act continuously to maintain 5-HT neuronal identity and the ability to appropriately respond to neurotoxic injury.
Tamoxifen inducible targeting approaches were used to investigate requirements for Lmx1b and Pet1 in adult 5-HT neurons (113, 135). The findings obtained made it clear that both TFs are needed in adulthood to maintain 5-HT neuron identity and 5-HT levels. Further, mice in which Pet1 was targeted in adult 5-HT neurons exhibited a robust anxiety-like behavioral phenotype suggesting that maintenance of 5-HT neuron identity in adulthood is required for normal emotional behaviors (113). While TPH2 and SERT expression was reduced after targeting of either Lmx1b or Pet1, VMAT2 was reduced only after Lmx1b targeting, suggesting distinct roles for the two TFs in maintenance of 5-HT neuron identity (Figure 5). Although these studies have revealed continued functions for Pet1 and Lmx1b in adult 5-HT neurons it remains far from clear to what extent they are required to regulate 5-HT gene expression in adulthood and preserve the health of 5-HT neurons.
Role of neural activity in specification of 5-HT neurons
Several environmental factors affecting the specification of 5-HT neurons have been suggested to converge on activity-dependent mechanisms. In Xenopus, expression of the TF LMX1B was controlled by neuronal activity during the period of specification of 5-HT neurons in the hindbrain (103). Increased activity inhibited Lmx1b and downstream Tph expression, while decreased activity increased Lmx1b expression, with no alterations of Nkx2-2+ progenitors. These activity-dependent mechanisms occur in a context of spontaneous neural activity observed in both chick and mouse embryos. Calcium imaging showed that in the embryonic mouse hindbrain spontaneous waves of activity are initiated in the midline, by E11–E13, and propagate rostrocaudally and mediolaterally (136, 137). The driver population of this activity appears to be the serotonergic neurons of the nascent raphe at the level of r2 (138). In addition, in the raphe region, more sustained calcium increases that can last minutes have been noted, that could act as important modulators of gene expression (139). Whether such activity-dependent mechanisms regulate the genesis of 5-HT neurons in mammals would be an important point to address in the future.
Terminal selector mechanisms in 5-HT gene regulatory networks
Pet1 possesses many of the defining properties of terminal selector-type transcription factors (140). Terminal selectors were originally described in C. elegans as TFs that are continuously expressed in postmitotic neurons and function to initiate and maintain coexpression of neuron-type identity genes through direct binding to common conserved motifs in cis-regulatory regions. Thus, terminal selector TFs “select” genes for coexpression to establish and sustain the differentiated state of postmitotic neurons (140–142). Some invertebrate terminal selectors have been shown to maintain their own expression via direct positive autoregulation and in some cases are sufficient for generating terminal neuron-type identities when ectopically expressed (143–147).
Pet1 is continuously expressed in postmitotic 5-HT neurons and continuously controls 5-HT neuron-type identity through common PET1/FEV ETS binding sites. Pet1 also appears to maintain its own expression through direct autoregulation. Lmx1b and Gata3 are also continuously expressed in 5-HT neurons and likely function as terminal selectors. Neither Pet1 nor Lmx1b appears to be sufficient for directing 5-HT neuron-type identity. However, together they are sufficient when coexpressed in ventral but not dorsal spinal cord (105). Recent findings described below have provided insight into how the Pet1 terminal selector controls 5-HT neuron-type identity.
Pet1 controls a postmitotic regulatory network
Studies in C. elegans have shown that terminal selectors are not regulatory endpoints in that they not only directly control terminal effector genes but also other TFs (143). These secondary TFs act in regulatory subroutines to control specific terminal identity features of a neuron (142). Similarly, Pet1 is not a regulatory terminus in postmitotic 5-HT neurons and part of the mechanism through which it regulates maturation and maintenance of 5-HT neurons is by controlling the expression of a downstream TF network (71). Although this postmitotic TF network is just beginning to be understood recent studies have established a regulatory link between Pet1 and the En genes.
RNA-seq and ISH showed that Pet1 is required to maintain expression of En1 in r1-derived 5-HT neurons (71). In support of direct control of En1 by PET1, ChIP-seq studies revealed PET1 occupancy near several high affinity PET1/FEV binding motifs upstream and downstream of En1. Together, these findings support a 5-HT neuron subtype-specific transcriptional subroutine in which Pet1 directly maintains En1 in r1-derived postmitotic 5-HT neurons to enable expression of an En1-dependent program needed for maturation of the DRN, maintenance of 5-HT identity in the DRN and survival of some DRN 5-HT neurons (Figure 5).
Autoregulation
ChIP experiments have indicated that PET1 binding is enriched in the immediate 2kb upstream region of Pet1 near conserved high affinity PET1 DNA binding motifs (71, 113). This upstream region, which functions as an enhancer, is sufficient to direct 5-HT neuron-specific transgene expression in the mouse brain (112, 148, 149). Tamoxifen-inducible targeting of Pet1 in adult 5-HT neurons led to a loss of enhancer activity suggesting that Pet1 was required to maintain its own expression through direct binding to its upstream enhancer region (113). With Pet1 expression locked in by autoregulation, the expression and function of other continuously expressed TFs can be maintained through direct control by Pet1, for example En1, thereby preserving 5-HT identity (Figure 5). However, Pet1 autoregulation appears to be critical only later in life, as Lmx1b is necessary to maintain Pet1 at fetal stages but not in adult 5-HT neurons (105, 115, 135). Maintenance of Lmx1b expression is also not dependent on Pet1 in adult 5-HT neurons and whether Lmx1b autoregulates to maintain expression has not been investigated. Neither Pet1 nor Lmx1b are required to control the induction of each other. Interestingly, the loss of expression of some 5-HT neuron identity genes seems to worsen as Pet1−/− mice age suggesting that deficiency of a single TF does not simply result in a static unitary mutant phenotype (71). These findings suggest a continuing decay of 5-HT neuron cell state with mutant cells taking on phenotypes progressively less like that of a 5-HT neuron. Perhaps, the progressive destabilization of terminal identity in Pet1−/− neurons is caused by a loss of Pet1 autoregulation and a continuing collapse in reinforcing terminal selector and other TF interactions governed by PET1. Later changes in Pet1−/− could also be due to indirect consequences of a lack of proper integration in a cellular network, with the resultant changes in evoked activity and trophic interactions.
Repression helps to shape 5-HT neuron identity
RNA-seq studies of flow sorted Pet1 deficient 5-HT neurons revealed more upregulated genes than downregulated ones (71). Upregulated were numerous peptide, ion channel, GPCR, and TF genes. ChIP-seq revealed PET1 occupancy near PET1/FEV high affinity binding sites located within 5kb upstream of transcriptional start sites or downstream of transcriptional termination sites of numerous upregulated genes in Pet1 deficient neurons. The potential for Pet1 to repress transcription is not unexpected as cell line reporter assays published many years ago indicated that PET1 and FEV can repress through high affinity ETS binding sites (150, 151). Further, the alanine-rich carboxyl-terminal region of FEV is a strong transcriptional repressor domain that acts independently of the ETS DNA binding domain (151).
One such gene whose expression was significantly increased in Pet1 deficient 5-HT neurons was the hypocretin receptor 1 (Hcrtr1) gene (71) (Figure 5). Hcrtr1 is a GPCR gene that is normally expressed in many but not all DRN 5-HT neurons and is required on 5-HT neuron plasma membranes for modulation of their activity by direct orexigenic synaptic input (152, 153). Loss of Pet1 appeared to derepress Hcrtr1 in the DRN so that more 5-HT neurons expressed the GPCR or the levels of Hcrtr1 were increased in 5-HT neurons that already expressed it (71). Similar effects on Hcrtr1 expression were observed after conditional deletion of Pet1 in the early postnatal period (71). These findings suggest ongoing Pet1-mediated repression may determine the number of 5-HT neurons that express subtype-specific characteristics and in the case of Hcrtr1 set the level of orexinergic input to the 5-HT system. It is not yet known how Pet1 terminal selector function is modified to enable transactivation of some genes and repression of others in the same neuron-type.
Terminal selector target switching
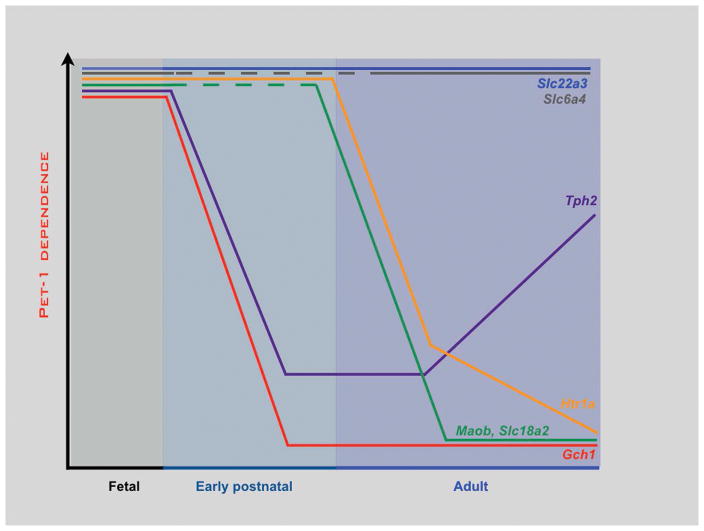
Another intriguing aspect of Pet1 terminal selector function revealed through temporal conditional targeting is that the dependency of transcriptional targets on Pet1 appear to shift as 5-HT neurons mature (71). In constitutive Pet1−/− mice, expression levels of 5-HT synthesis genes, Tph2, Gch1, Gchfr are severely reduced to about 10–15% normal levels. However, when Pet1 is targeted in the early postnatal period with AAV-Cre (Pet-1floxed/−;AAV−Cre), expression of these genes became nearly insensitive to Pet1 deficiency. Instead, a different group of target genes (GPCRs: Adra1b, Htr1a) that function to control 5-HT neuron excitability and whose expression is upregulated around birth were highly sensitive to postnatal loss of Pet1. These findings suggest an intriguing regulatory strategy for a continuously expressed neuronal terminal selector: as postmitotic development proceeds there is a switch in Pet1 targets from those required in newborn 5-HT neurons for initiation of 5-HT synthesis to those required postnatally for maturation of extrinsically controlled 5-HT neuron excitability.
Some genes such as Slc22a3, which encodes a low affinity, high capacity 5-HT transporter, remains completely dependent on Pet1 at all stages of life. In contrast, Htr1a requires Pet1 for its induction and also its maintenance but only up to the early postnatal stages of life as it loses sensitivity to loss of Pet1 and so becomes independent of Pet1 at later stages (Figure 6). Thus, there is a postmitotic sensitive period for Pet1 regulation of Htr1a (71). Transcriptional dependencies of some target genes to particular terminal selector TFs may actually be dynamic throughout life. For example, Tph2 appears to regain sensitivity to loss of Pet1 in adulthood (113). Moreover, although Slc18a2 loses dependence on Pet1 it remains highly sensitive to Lmx1b loss in adult 5-HT neurons (113, 135). These findings suggest that terminal effector genes possess distinct and dynamic temporal dependencies on specific terminal selectors at different stages of postmitotic life (Figure 6). A similar type of target switching has also been uncovered for the bHLH TF, HAND2, during peripheral sympathetic neuron maturation suggesting switching may be a common regulatory strategy of continuously expressed TFs in postmitotic neurons (154). Understanding the importance of terminal selector target switching and the mechanism though which switching is controlled should provide further insight into stage specific control of postmitotic neuronal gene expression by terminal selectors.
Figure 6. Temporally dynamic Pet1 target dependencies in postmitotic 5-HT neurons.
Stage-specific conditional targeting with AAV-Cre and tamoxifen-inducible approaches reveal changing sensitivities of target genes to loss of Pet1 in postmitotic 5-HT neurons. Target sensitivities to Pet1 loss (y-axis) are presented in arbitrary scale based on RT-qPCR and in situ hybridization assays of target gene expression. Dashed line segments indicate that no data is available for early postnatal targeting of Pet1. Slc6a4, high affinity, low capacity 5-HT plasma membrane transporter; Slc22a3, low affinity, high capacity 5-HT plasma membrane transporter; Slc18a2, vesicular monoamine transporter 2; Tph2, tryptophan hydroxylase 2; Gch1, GTP cyclohydrolase 1; Htr1a, 5-HT1a receptor; Maob, monoamine oxidase b.
Genetic and environmental perturbations of 5-HT neuron migration
Although the precise mechanisms controlling raphe neuron migration are still not elucidated, several genetic and environmental factors have been found to play a role. As discussed above, En1 and En2 are required in a cell-autonomous manner for midline fusion of raphe 5-HT neurons (122). Selective deletion of En1/En2 in maturing 5-HT neurons prevented their normal migration resulting in many mis-positioned neurons laterally on either side of the midline (122).
Another gene involved in the maturation and migration of 5-HT raphe neurons is Necdin (Ndn), whose action is most conspicuous for the maturation of the posterior raphe cluster. Ndn is one of the genes involved in Prader-Willi syndrome that associates an autistic syndrome and breathing deficits. Ndn is expressed in the early born medullary 5-HT neurons and controls their growth and positioning (155). Ndn knock out mice show alterations in the maturation of medullary raphe neurons, with perturbed serotoninergic transmission that in turn causes breathing defects (155).
Mouse models of Zellweger syndrome were also reported to show an altered maturation of 5-HT raphe neurons. These mice are deficient in brain peroxisome biogenesis through Nestin-cre targeting of Pex13, which encodes a protein component (PEX13) of the peroxisome protein import machinery (156). Pex13 deficient mice exhibit numerous dystrophic serotonergic axons, reduced numbers and abnormal distribution of 5-HT neurons (157). However these alterations likely reflect secondary neurodegeneration rather than primary developmental defects.
In addition to genetic factors, environmental exposure to toxics such as ethanol, valproate, and thalidomide at the time of early serotonergic maturation modifies the migration of 5-HT neurons and reduces their numbers. After an ethanol diet during gestation (E8–E10), 5-HT raphe neurons of the exposed embryos were clustered along the midline as though unable to migrate out of the neurogenic zone (158). Similar observations were made after exposure to valproate or thalidomide at mid-gestation (159, 160). Further studies found that these chemicals reduce sonic-hedgehog expression in the hindbrain, which could explain the developmental alterations. The consequences on the final number of 5-HT neurons were estimated differently according to species. In zebrafish, VPA exposure resulted in an important failure of 5-HT neurons specification whereas no difference in the final number of 5-HT neurons generated was observed in VPA-treated rodents (161), suggesting compensatory mechanisms such as increases of Gata3 expression (162).
Overall, it is likely that diverse genetic and environmental factors acting during early stages of raphe neuron development -mid embryonic life in rodents, first trimester of gestation in humans - could influence the morphogenesis of 5-HT neurons. This could then impact 5-HT neurotransmission and hence have a role in the pathophysiology of brain disorders, such as autism or sudden infant death syndrome (51).
Molecular control of 5-HT axon growth
Surprisingly, given the extensive influence of 5-HT on CNS circuitry, and the remarkable capacity of 5-HT axons to regrow in response to injury, very few studies have analyzed the molecular control of raphe axon growth and guidance. This is likely due to a prevailing view of 5-HT neurons as a diffuse, highly collateralized system with limited specificity. This view has changed in recent years in particular with increasing availability of mouse genetic models and pan-genomic analyses showing that developing 5-HT neurons express a wide array of receptors for guidance molecules. These analyses further indicated that expression of guidance molecules varied between the different raphe neurons (54, 58) and could therefore be key to construct their efferent and afferent connectivity.
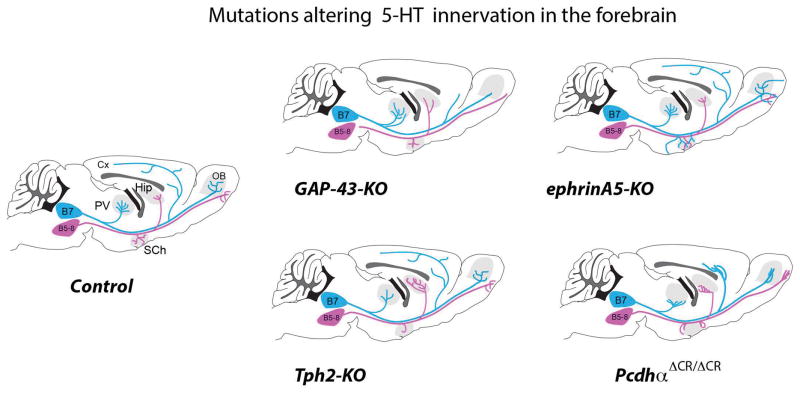
Several broadly expressed growth factors such as BDNF, S100β and GAP43 were found to promote the growth and branching of 5-HT axons (163–168). These effects were particularly marked for GAP43, a phosphoprotein that is associated with the membrane skeleton and is particularly enriched in growing and regenerating axons (169). Suppressing GAP43 expression has widespread adverse effects on axon outgrowth. In particular, the 5-HT raphe axons of Gap43−/− mice failed to invade the cerebral cortex, with axon stalling in subcortical brain areas and increased 5-HT innervation of the brainstem and thalamus (164) (Figure 7). GAP43, together with integrin beta, are molecules with continued high expression in adult 5-HT neurons that could be involved in their remarkable regenerative properties (97, 98).
Figure 7.
Schematic representation of the ascending 5-HT raphe axons from the DRN (B7) and the MRN (B5–B8). Different patterns of altered 5-HT terminal innervation are noted in Gap43−/− (164), Tph2−/− (90), ephrin A5−/− (179), and Pcdhα ΔCR/ΔCR (176), reflecting the requirement of trophic (5-HT) or guidance molecules for the establishment of the 5-HT raphe circuits.
An autocrine trophic effect of 5-HT has recently been clearly demonstrated. As mentioned earlier, 5-HT is concentrated in growth cones and likely released by the growing axons. While lack of 5-HT does not impact early raphe development it plays a role during the later period of target invasion in postnatal life. In Tph2−/− mice and Slc18a2−/− mice that are unable to synthesize or store 5-HT, the raphe neurons develop normally and the main axon tracts are unaffected (170, 171). However alterations in the distribution of 5-HT terminal innervation was found at later developmental stages. This was made possible with Tph2−/− mice in which a green fluorescent (GFP) reporter is knocked into the 5-HT locus, allowing unambiguous labeling of the 5-HT raphe neurons despite their lack of 5-HT. In these mutants, terminal raphe innervation was decreased in selected areas, such as the suprachiasmatic nucleus and the thalamic paraventricular nucleus, and increased in the hippocampus (90) (Figure 7). Similar effects were observed after adult conditional depletion of Tph2 in this same model (172), indicating that 5-HT terminal innervation is highly plastic.
Besides these general trophic factors, a handful of more specific axon guidance molecules have been shown to act in the selective orientation and targeting of the 5-HT raphe axons. WNT signaling has a major role in the initial polarity and orientation along the anterior to posterior axis (173). WNT is a secreted morphogen that controls neuron specification and axon growth. Different WNT sources are present in the brainstem close to the site of 5-HT neuron precursors. WNT5 is highly expressed in the isthmus (at the mid-hindbrain boundary) and caudally in the spinal cord. Gain and loss of function experiments indicated that WNTs act as chemoattractants for both the ascending and the descending 5-HT raphe neurons. Embryonic (E11, E12) raphe neurons express FRIZZLED3, CELSR3 and VANGL2, which are WNT receptors of the planar cell polarity complex. Mice deficient for one or another of these receptors showed abnormal orientations of raphe 5-HT axons. The rostrally-oriented axons were misrouted laterally and caudally. Conversely 5-HT axons arising from the posterior cluster showed aberrant rostral orientation of their axons. As a result, abnormal ingrowth of 5-HT was observed in r4, which is normally devoid of 5-HT axons during early embryonic development (173).
5-HT axon tract organization is in part controlled by SLIT/ROBO signaling (174). The repellent molecules, SLITs, are highly expressed along the midline and ROBO1/2 receptors are expressed in the embryonic raphe 5-HT neurons. In Slit1−/−/Slit2−/− mice, a large fraction of the raphe axons are directed away from the mfb toward the hypothalamus, whereas such midline crossing is more limited in WT mice.
Invasion of 5-HT axons in their targets is controlled by protocadherins that play an important role in the tiling of 5-HT axons in brain targets (175, 176). Protocadherins are a large family of genes whose combinatorial expression is thought to play a role in conferring specific neuronal identities. Katori et al. (2009) showed that the protocaherin alpha genes are strongly expressed in the developing rostral 5-HT raphe groups. Mouse mutants that lack the common cytoplasmic region of protocaherin alpha, have normal early axon growth until P7, but thereafter abnormal clustering of axons is visible in the periphery of target structures such as the globus pallidus, substantia nigra or hippocampus, resulting in an abnormal distribution of 5-HT terminal innervation (176) (Figure 7). This abnormal innervation might explain the anxiety and learning deficits observed in these mutants (177). Recently, these observations were extended by the use of 5-HT-specific deletion of the 3 main protocadherin families, which demonstrated a cell-autonomous role of protocadherin alpha for 5-HT axon tiling in target structures, while protocadherin beta and gamma are dispensable (175). Repulsive interactions between the different 5-HT ingrowing axons are thought to mediate these effects (175).
Other guidance molecules such as ephrins appear to be involved in the selective targeting of brain areas by different 5-HT raphe nuclei. Ephrin signaling plays a major role in the establishment of a variety of topographic sensory maps (178). Recent evidence shows that it is involved in the selective targeting of dorsal (DRN) versus median raphe (MRN) nuclei. Expression of the EphA5 receptor gene is unequally distributed across the raphe nuclei with a rostral to caudal gradient: DRN 5-HT neurons have high EphA5 expression and growth cones from these axons are repelled by ephrin A in vitro and in vivo (179). This correlates with the fact that DRN axons avoid innervating brain areas expressing high levels of ephrinA5. Conversely, MRN 5-HT neurons with low EphA5 expression innervate areas with high ephrinA expression. This pattern was particularly clear in the olfactory bulb, and in several hypothalamic nuclei. In ephrinA5 knock out mice (efna5−/−), DRN 5-HT axons are mistargeted to these structures (Figure 7). These results indicated that ephrinA5 signaling contributes to the selective targeting of 5-HT axons by repelling the ingrowth of 5-HT axons originating from the DRN, while being permissive to 5-HT axons originating from the MRN (179). These findings could explain increased hypothalamic 5-HT innervation and altered aggressive behavior observed in EphA5−/− mice (180).
Concluding remarks and future directions
This review has highlighted the impressive progress that has been made in understanding the specification, differentiation, and maturation of the small number of brain neurons that make and use 5-HT as a neurotransmitter. The continuing application of classical histological approaches together with more recently established molecular genetic approaches has further refined our understanding of the neuroanatomical development of 5-HT neurons and the topographic organization of their axonal projections. These approaches have also uncovered an astonishing diversity of 5-HT neuron molecular subtypes, which suggest that no two 5-HT neurons in the brain are likely to share an identical transcriptome. Key TFs that compose 5-HT GRNs have been identified and their respective functions in controlling acquisition of 5-HT neuron transmitter identity are understood in some depth. Focus is beginning to shift to the study of how other common and subtype-specific characteristics of these neurons are controlled, and how they contribute to specific circuits and functions.
New knowledge of developmental 5-HT GRNs and the transcriptomes they create have revealed genetic loci whose allelic variants could in theory produce a spectrum of 5-HT signaling capacities. Two open and important questions are which variants cause alterations in 5-HT signaling and what is the extent to which they increase vulnerability for behavioral disorders. Knowledge of 5-HT GRNs and 5-HT transcriptomes has been applied to create strategies for efficient production of neurons with 5-HT identities from human pluripotent stem cells and human fibroblasts (181–183). These strategies could be used for examining the effects of allelic variants of human 5-HT neuron expressed genes on 5-HT signaling capacity and to screen for potential new therapeutic agents that target the 5-HT system.
Still many questions remain. First, an important challenge is to better understand the molecular and functional diversity of 5-HT neurons. Second, it is not well understood whether activity and environmental factors such as acute and chronic stress alter 5-HT GRNs and produce functionally consequential changes in 5-HT neuron identities. Third, a reasonable estimate is that for each 5-HT neuron there are roughly 200,000 non-5-HT neurons in the human brain (184–186). Yet, despite their miniscule numbers 5-HT neurons are able to reach and influence nearly all CNS circuitry through an impressive axonal architecture. Although GAP43, Ephs/ephrins, and protocadherins have been implicated in the patterning of 5-HT axons, the intrinsic regulatory mechanisms that enable the remarkable growth and connectivity of 5-HT axonal architecture throughout the brain and spinal cord are poorly understood. Finally 5-HT neurons have become a good model with which to investigate how continuously expressed vertebrate terminal selector-type transcription factors function. Further study of Pet1 and Lmx1b in 5-HT neurons is likely to reveal how neurons control patterns of gene expression at different stages of life.
Acknowledgments
The authors’ research is funded by the NIH RO1MH062723 and P50MH096972 (E.S.D.) and by Agence Nationale de la Recherche (ANR-11-0004-02, ANR-15-0179, ANR-16-0162), the Inserm, and Université Pierre et Marie Curie (PG). We thank Clay Spencer, Lauren Donovan, and Mariano Soiza-Reilly for comments.
REFERNCES
- 1.Twarog BM, Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953 Oct;175(1):157–61. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Dahlstrom A, Fuxe K. Evidence for the existence of monamine-containing neurons in the central nervous system. I. Demonstration of monamines in the cell bodies of brainstem neurons. Acta Physiol Scand. 1964;62(Suppl 232):1–55. [PubMed] [Google Scholar]
- 3.Flames N, Hobert O. Transcriptional control of the terminal fate of monoaminergic neurons. Annual review of neuroscience. 2011;34:153–84. doi: 10.1146/annurev-neuro-061010-113824. Epub 2011/06/23. eng. [DOI] [PubMed] [Google Scholar]
- 4.Gaspar P, Lillesaar C. Probing the diversity of serotonin neurons. Philos Trans R Soc Lond B Biol Sci. 2012 Sep 5;367(1601):2382–94. doi: 10.1098/rstb.2011.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008 Mar 25; doi: 10.1016/j.bbr.2008.03.020. Eng. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003 Dec;4(12):1002–12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 8.Trakhtenberg EF, Goldberg JL. The role of serotonin in axon and dendrite growth. Int Rev Neurobiol. 2012;106:105–26. doi: 10.1016/B978-0-12-407178-0.00005-3. [DOI] [PubMed] [Google Scholar]
- 9.Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007 Oct 15;159(1):85–101. doi: 10.1016/j.resp.2007.06.002. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Prog Brain Res. 2014;209:207–33. doi: 10.1016/B978-0-444-63274-6.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayan P, Huys QJ. Serotonin in affective control. Annu Rev Neurosci. 2009;32:95–126. doi: 10.1146/annurev.neuro.051508.135607. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez SP, Gaspar P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology. 2012 Sep 21;62:144–54. doi: 10.1016/j.neuropharm.2011.08.049. Epub 2011/10/04. Eng. [DOI] [PubMed] [Google Scholar]
- 13.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998 Aug 1;44(3):151–62. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 14.Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004 Oct;9(10):908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 15.Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003 Nov 15;54(10):960–71. doi: 10.1016/s0006-3223(03)00696-6. eng. [DOI] [PubMed] [Google Scholar]
- 16.Frick A, Ahs F, Engman J, Jonasson M, Alaie I, Bjorkstrand J, et al. Serotonin Synthesis and Reuptake in Social Anxiety Disorder: A Positron Emission Tomography Study. JAMA psychiatry. 2015 Aug;72(8):794–802. doi: 10.1001/jamapsychiatry.2015.0125. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez SP, Muzerelle A, Scotto-Lomassese S, Barik J, Gruart A, Delgado-Garcia JM, et al. Constitutive and Acquired Serotonin Deficiency Alters Memory and Hippocampal Synaptic Plasticity. Neuropsychopharmacology. 2017 Jan;42(2):512–23. doi: 10.1038/npp.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nature communications. 2016 Jan 28;7:10503. doi: 10.1038/ncomms10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012 Apr 03;109(14):5469–74. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waider J, Araragi N, Gutknecht L, Lesch KP. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: a perspective. Psychoneuroendocrinology. 2011 Apr;36(3):393–405. doi: 10.1016/j.psyneuen.2010.12.012. Epub 2011/01/25. eng. [DOI] [PubMed] [Google Scholar]
- 21.Angoa-Perez M, Kane MJ, Sykes CE, Perrine SA, Church MW, Kuhn DM. Brain serotonin determines maternal behavior and offspring survival. Genes, brain, and behavior. 2014 Sep;13(7):579–91. doi: 10.1111/gbb.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009 Jun 11;106:10332–7. doi: 10.1073/pnas.0810793106. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008 Aug 17;11:1001–3. doi: 10.1038/nn.2176. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pourin S, Muller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–6. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003 Jan 23;37(2):233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 26.Niederkofler V, Asher TE, Okaty BW, Rood BD, Narayan A, Hwa LS, et al. Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior. Cell reports. 2016 Nov 15;17(8):1934–49. doi: 10.1016/j.celrep.2016.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–8. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 28.Alonso A, Merchan P, Sandoval JE, Sanchez-Arrones L, Garcia-Cazorla A, Artuch R, et al. Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain structure & function. 2013 Sep;218(5):1229–77. doi: 10.1007/s00429-012-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordes SP. Molecular genetics of the early development of hindbrain serotonergic neurons. Clinical genetics. 2005 Dec;68(6):487–94. doi: 10.1111/j.1399-0004.2005.00534.x. eng. [DOI] [PubMed] [Google Scholar]
- 30.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 31.Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964 Jul 15;20(7):398–9. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- 32.Olson L, Seiger A. Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Zeitschrift fur Anatomie und Entwicklungsgeschichte. 1972;137(3):301–16. doi: 10.1007/BF00519099. [DOI] [PubMed] [Google Scholar]
- 33.Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004 Jul;5(7):545–52. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- 34.Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron. 2012 Oct 4;76(1):175–91. doi: 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG, et al. Serotonin transporter gene polymorphism and antidepressant response. Neuroreport. 2000 Jan 17;11(1):215–9. doi: 10.1097/00001756-200001170-00042. [DOI] [PubMed] [Google Scholar]
- 36.Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982 May 20;207(3):239–54. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- 37.Sos KE, Mayer MI, Cserep C, Takacs FS, Szonyi A, Freund TF, et al. Cellular architecture and transmitter phenotypes of neurons of the mouse median raphe region. Brain structure & function. 2017 Jan;222(1):287–99. doi: 10.1007/s00429-016-1217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vertes RP, Crane AM. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol. 1997 Feb 17;378(3):411–24. doi: 10.1002/(sici)1096-9861(19970217)378:3<411::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Bang SJ, Commons KG. Forebrain GABAergic projections from the dorsal raphe nucleus identified by using GAD67-GFP knock-in mice. J Comp Neurol. 2012 Dec 15;520(18):4157–67. doi: 10.1002/cne.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain structure & function. 2016 Jan;221(1):535–61. doi: 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aitken AR, Tork I. Early development of serotonin-containing neurons and pathways as seen in wholemount preparations of the fetal rat brain. J Comp Neurol. 1988 Aug 1;274(1):32–47. doi: 10.1002/cne.902740105. [DOI] [PubMed] [Google Scholar]
- 42.Lidov HGW, Molliver ME. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- 43.Wallace JA, Lauder JM. Development of the serotonergic system in the rat embryo: an immunocytochemical study. Brain Res Bull. 1983 Apr;10(4):459–79. doi: 10.1016/0361-9230(83)90144-2. [DOI] [PubMed] [Google Scholar]
- 44.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008 Apr;11(4):417–9. doi: 10.1038/nn2050. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, et al. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003 Mar 15;17(6):729–37. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978 Jun 01;179(3):641–67. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 47.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999 May 17;407(4):555–82. Epub 1999/05/11. eng. [PubMed] [Google Scholar]
- 48.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998 Jan;82(2):443–68. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 49.Dorocic IP, Furth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, et al. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014 Aug 6;83(3):663–78. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron. 2014 Aug 6;83(3):645–62. doi: 10.1016/j.neuron.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinney HC, Broadbelt KG, Haynes RL, Rognum IJ, Paterson DS. The serotonergic anatomy of the developing human medulla oblongata: implications for pediatric disorders of homeostasis. J Chem Neuroanat. 2011 Jul;41(4):182–99. doi: 10.1016/j.jchemneu.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olson L, Boreus LO, Seiger A. Histochemical demonstration and mapping of 5-hydroxytryptamine- and catecholamine-containing neuron systems in the human fetal brain. Zeitschrift fur Anatomie und Entwicklungsgeschichte. 1973 Apr 16;139(3):259–82. doi: 10.1007/BF00519968. [DOI] [PubMed] [Google Scholar]
- 53.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011 Apr 21;472(7343):347–50. doi: 10.1038/nature09972. Epub 2011/04/23. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, et al. Distinct transcriptomes define rostral and caudal serotonin neurons. J Neurosci. 2010 Jan 13;30(2):670–84. doi: 10.1523/JNEUROSCI.4656-09.2010. Epub 2010/01/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lillesaar C, Tannhauser B, Stigloher C, Kremmer E, Bally-Cuif L. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev Dyn. 2007 Apr;236(4):1072–84. doi: 10.1002/dvdy.21095. eng. [DOI] [PubMed] [Google Scholar]
- 56.Jacob J, Ferri AL, Milton C, Prin F, Pla P, Lin W, et al. Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat Neurosci. 2007 Nov;10(11):1433–9. doi: 10.1038/nn1985. eng. [DOI] [PubMed] [Google Scholar]
- 57.Altman J, Bayer SA. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. J Comp Neurol. 1981;198:677–716. doi: 10.1002/cne.901980409. [DOI] [PubMed] [Google Scholar]
- 58.Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, deBairos D, et al. Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron. 2015 Nov 18;88(4):774–91. doi: 10.1016/j.neuron.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez SP, Cauli B, Cabezas C, Muzerelle A, Poncer JC, Gaspar P. Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain structure & function. 2016 Nov 25;221(8):4007–25. doi: 10.1007/s00429-015-1142-4. [DOI] [PubMed] [Google Scholar]
- 60.Spaethling JM, Piel D, Dueck H, Buckley PT, Morris JF, Fisher SA, et al. Serotonergic neuron regulation informed by in vivo single-cell transcriptomics. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 Feb;28(2):771–80. doi: 10.1096/fj.13-240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan-Palay V, Jonsson G, Palay SL. Serotonin and substance P coexist i, neurons of the rat’s central nervous system. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1582–6. doi: 10.1073/pnas.75.3.1582. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell reports. 2014 Dec 24;9(6):2152–65. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hennessy ML, Corcoran AE, Brust RD, Chang Y, Nattie EE, Dymecki SM. Activity of Tachykinin1-Expressing Pet1 Raphe Neurons Modulates the Respiratory Chemoreflex. J Neurosci. 2017 Feb 15;37(7):1807–19. doi: 10.1523/JNEUROSCI.2316-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kast RJ, Wu HH, Williams P, Gaspar P, Levitt P. Specific Connectivity and Unique Molecular Identity of MET Receptor Tyrosine Kinase Expressing Serotonergic Neurons in the Caudal Dorsal Raphe Nuclei. ACS chemical neuroscience. 2017 May 17;8(5):1053–64. doi: 10.1021/acschemneuro.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lv J, Wang L, Gao Y, Ding YQ, Liu F. 5-hydroxytryptamine synthesized in the aorta-gonad-mesonephros regulates hematopoietic stem and progenitor cell survival. J Exp Med. 2017 Feb;214(2):529–45. doi: 10.1084/jem.20150906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohta Y, Kosaka Y, Kishimoto N, Wang J, Smith SB, Honig G, et al. Convergence of the insulin and serotonin programs in the pancreatic beta-cell. Diabetes. 2011 Dec;60(12):3208–16. doi: 10.2337/db10-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiyasova V, Bonnavion P, Scotto-Lomassese S, Fabre V, Sahly I, Tronche F, et al. A subpopulation of serotonergic neurons that do not express the 5-HT1A autoreceptor. ACS chemical neuroscience. 2013 Jan 16;4(1):89–95. doi: 10.1021/cn300157s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hokfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, Pieribone V, et al. Multiple messengers in descending serotonin neurons: localization and functional implications. J Chem Neuroanat. 2000 Feb;18(1–2):75–86. doi: 10.1016/s0891-0618(99)00037-x. eng. [DOI] [PubMed] [Google Scholar]
- 69.Hokfelt T, Ljungdahl A, Steinbusch H, Verhofstad A, Nilsson G, Brodin E, et al. Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience. 1978;3(6):517–38. doi: 10.1016/0306-4522(78)90017-9. [DOI] [PubMed] [Google Scholar]
- 70.Fu W, Le Maitre E, Fabre V, Bernard JF, David Xu ZQ, Hokfelt T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010 Sep 1;518(17):3464–94. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- 71.Wyler SC, Spencer WC, Green NH, Rood BD, Crawford L, Craige C, et al. Pet-1 Switches Transcriptional Targets Postnatally to Regulate Maturation of Serotonin Neuron Excitability. J Neurosci. 2016 Feb 3;36(5):1758–74. doi: 10.1523/JNEUROSCI.3798-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14488–93. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002 Jul 1;22(13):5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, et al. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010 Mar 1;518(5):668–86. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- 75.Voisin AN, Mnie-Filali O, Giguere N, Fortin GM, Vigneault E, El Mestikawy S, et al. Axonal Segregation and Role of the Vesicular Glutamate Transporter VGLUT3 in Serotonin Neurons. Frontiers in neuroanatomy. 2016;10:39. doi: 10.3389/fnana.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gagnon D, Parent M. Distribution of VGLUT3 in highly collateralized axons from the rat dorsal raphe nucleus as revealed by single-neuron reconstructions. PLoS One. 2014;9(2):e87709. doi: 10.1371/journal.pone.0087709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014 Mar 19;81(6):1360–74. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, et al. Fast synaptic subcortical control of hippocampal circuits. Science. 2009 Oct 16;326(5951):449–53. doi: 10.1126/science.1178307. eng. [DOI] [PubMed] [Google Scholar]
- 79.Sengupta A, Bocchio M, Bannerman DM, Sharp T, Capogna M. Control of Amygdala Circuits by 5-HT Neurons via 5-HT and Glutamate Cotransmission. J Neurosci. 2017 Feb 15;37(7):1785–96. doi: 10.1523/JNEUROSCI.2238-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron. 1994 Feb;12(2):433–42. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 81.Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010 Feb 10;30(6):2198–210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Domonkos A, Nikitidou Ledri L, Laszlovszky T, Cserep C, Borhegyi Z, Papp E, et al. Divergent in vivo activity of non-serotonergic and serotonergic VGluT3-neurones in the median raphe region. J Physiol. 2016 Jul 01;594(13):3775–90. doi: 10.1113/JP272036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spencer WC, Deneris ES. Regulatory Mechanisms Controlling Maturation of Serotonin Neuron Identity and Function. Frontiers in cellular neuroscience. 2017;11(215) doi: 10.3389/fncel.2017.00215. Epub 19 July 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lidov HG, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res Bull. 1982 Apr;8(4):389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- 85.Morton RA, Yanagawa Y, Valenzuela CF. Electrophysiological Assessment of Serotonin and GABA Neuron Function in the Dorsal Raphe during the Third Trimester Equivalent Developmental Period in Mice. eNeuro. 2015 Nov-Dec;2(6) doi: 10.1523/ENEURO.0079-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rood BD, Calizo LH, Piel D, Spangler ZP, Campbell K, Beck SG. Dorsal raphe serotonin neurons in mice: immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J Neurosci. 2014 Apr 2;34(14):4809–21. doi: 10.1523/JNEUROSCI.1498-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hawthorne AL, Wylie CJ, Landmesser LT, Deneris ES, Silver J. Serotonergic neurons migrate radially through the neuroepithelium by dynamin-mediated somal translocation. J Neurosci. 2010 Jan 13;30(2):420–30. doi: 10.1523/JNEUROSCI.2333-09.2010. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levitt P, Moore RY. Developmental organization of raphe serotonin neuron groups in the rat. Anat Embryol (Berl) 1978 Sep 27;154(3):241–51. doi: 10.1007/BF00345655. [DOI] [PubMed] [Google Scholar]
- 89.Maddaloni G, Bertero A, Pratelli M, Barsotti N, Boonstra A, Giorgi A, et al. Development of Serotonergic Fibers in the Post–Natal Mouse Brain. Frontiers in cellular neuroscience. 2017;11(202) doi: 10.3389/fncel.2017.00202. Epub 14 July 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013 Oct;18(10):1106–18. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- 91.Teissier A, Soiza-Reilly M, Gaspar P. Refining the Role of 5-HT in Postnatal Development of Brain Circuits. Frontiers in cellular neuroscience. 2017;11:139. doi: 10.3389/fncel.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaspar P, Febvret A, Colombo J. Serotonergic sprouting in primate MTP-induced hemiparkinsonism. Exp Brain Res. 1993;96(1):100–6. doi: 10.1007/BF00230443. [DOI] [PubMed] [Google Scholar]
- 93.Gagnon D, Gregoire L, Di Paolo T, Parent M. Serotonin hyperinnervation of the striatum with high synaptic incidence in parkinsonian monkeys. Brain structure & function. 2016 Sep;221(7):3675–91. doi: 10.1007/s00429-015-1125-5. [DOI] [PubMed] [Google Scholar]
- 94.Descarries L, Soghomonian JJ, Garcia S, Doucet G, Bruno JP. Ultrastructural analysis of the serotonin hyperinnervation in adult rat neostriatum following neonatal dopamine denervation with 6-hydroxydopamine. Brain Res. 1992 Jan 08;569(1):1–13. doi: 10.1016/0006-8993(92)90363-e. [DOI] [PubMed] [Google Scholar]
- 95.Mullner A, Gonzenbach RR, Weinmann O, Schnell L, Liebscher T, Schwab ME. Lamina-specific restoration of serotonergic projections after Nogo-A antibody treatment of spinal cord injury in rats. Eur J Neurosci. 2008 Jan;27(2):326–33. doi: 10.1111/j.1460-9568.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- 96.von Euler M, Janson AM, Larsen JO, Seiger A, Forno L, Bunge MB, et al. Spontaneous axonal regeneration in rodent spinal cord after ischemic injury. J Neuropathol Exp Neurol. 2002 Jan;61(1):64–75. doi: 10.1093/jnen/61.1.64. [DOI] [PubMed] [Google Scholar]
- 97.Hawthorne AL, Hu H, Kundu B, Steinmetz MP, Wylie CJ, Deneris ES, et al. The unusual response of serotonergic neurons after CNS Injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. J Neurosci. 2011 Apr 13;31(15):5605–16. doi: 10.1523/JNEUROSCI.6663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin Y, Dougherty SE, Wood K, Sun L, Cudmore RH, Abdalla A, et al. Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury. Neuron. 2016 Aug 17;91(4):748–62. doi: 10.1016/j.neuron.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deneris ES, Wyler SC. Serotonergic transcriptional networks and potential importance to mental health. Nature neuroscience. 2012 Feb 26; doi: 10.1038/nn.3039. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kiyasova V, Gaspar P. Development of raphe serotonin neurons from specification to guidance. Eur J Neurosci. 2011 Nov;34(10):1553–62. doi: 10.1111/j.1460-9568.2011.07910.x. [DOI] [PubMed] [Google Scholar]
- 101.Scott MM, Deneris ES. Making and breaking serotonin neurons and autism. Int J Dev Neurosci. 2005 Apr-May;23(2–3):277–85. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 102.Dias JM, Alekseenko Z, Applequist JM, Ericson J. Tgfbeta signaling regulates temporal neurogenesis and potency of neural stem cells in the CNS. Neuron. 2014 Dec 03;84(5):927–39. doi: 10.1016/j.neuron.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 103.Demarque M, Spitzer NC. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron. 2010 Jul 29;67(2):321–34. doi: 10.1016/j.neuron.2010.06.006. Epub 2010/07/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacob J, Storm R, Castro DS, Milton C, Pla P, Guillemot F, et al. Insm1 (IA-1) is an essential component of the regulatory network that specifies monoaminergic neuronal phenotypes in the vertebrate hindbrain. Development. 2009 Jul;136(14):2477–85. doi: 10.1242/dev.034546. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, et al. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003 Nov 5;23(31):9961–7. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Craven SE, Lim KC, Ye W, Engel JD, De Sauvage F, Rosenthal A. Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development. 2004 Mar;131(5):1165–73. doi: 10.1242/dev.01024. [DOI] [PubMed] [Google Scholar]
- 107.Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, et al. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003 Sep;6(9):933–8. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 108.van Doorninck JH, van Der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, et al. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci. 1999 Jun 15;19(12):RC12. doi: 10.1523/JNEUROSCI.19-12-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haugas M, Tikker L, Achim K, Salminen M, Partanen J. Gata2 and Gata3 regulate the differentiation of serotonergic and glutamatergic neuron subtypes of the dorsal raphe. Development. 2016 Dec 01;143(23):4495–508. doi: 10.1242/dev.136614. [DOI] [PubMed] [Google Scholar]
- 110.Castro DS, Skowronska-Krawczyk D, Armant O, Donaldson IJ, Parras C, Hunt C, et al. Proneural bHLH and Brn proteins coregulate a neurogenic program through cooperative binding to a conserved DNA motif. Dev Cell. 2006 Dec;11(6):831–44. doi: 10.1016/j.devcel.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Nasu M, Yada S, Igarashi A, Sutoo D, Akiyama K, Ito M, et al. Mammalian-specific sequences in pou3f2 contribute to maternal behavior. Genome biology and evolution. 2014 Apr 07;6(5):1145–56. doi: 10.1093/gbe/evu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krueger KC, Deneris ES. Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors. J Neurosci. 2008 Nov 26;28(48):12748–58. doi: 10.1523/JNEUROSCI.4349-08.2008. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010 Oct;13(10):1190–8. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pattyn A, Simplicio N, Van Doorninck JH, Goridis C, Guillemot F, Brunet JF. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat Neurosci. 2004 May 9;7:589–95. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- 115.Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006 Dec 6;26(49):12781–8. doi: 10.1523/JNEUROSCI.4143-06.2006. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hendricks T, Francis N, Fyodorov D, Deneris E. The ETS Domain Factor Pet-1 is an Early and Precise Marker of Central 5-HT Neurons and Interacts with a Conserved Element in Serotonergic Genes. J Neurosci. 1999;19:10348–56. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wyler SC, Donovan LJ, Yeager M, Deneris E. Pet-1 Controls Tetrahydrobiopterin Pathway and Slc22a3 Transporter Genes in Serotonin Neurons. ACS chemical neuroscience. 2015 Feb 18; doi: 10.1021/cn500331z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, et al. A Genetically Defined Morphologically and Functionally Unique Subset of 5-HT Neurons in the Mouse Raphe Nuclei. J Neurosci. 2011 Feb 23;31(8):2756–68. doi: 10.1523/JNEUROSCI.4080-10.2011. Epub 2011/03/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simon HH, Scholz C, O’Leary DD. Engrailed genes control developmental fate of serotonergic and noradrenergic neurons in mid- and hindbrain in a gene dose-dependent manner. Mol Cell Neurosci. 2005 Jan;28(1):96–105. doi: 10.1016/j.mcn.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 120.Genestine M, Lin L, Durens M, Yan Y, Jiang Y, Prem S, et al. Engrailed-2 (En2) deletion produces multiple neurodevelopmental defects in monoamine systems, forebrain structures and neurogenesis and behavior. Hum Mol Genet. 2015 Oct 15;24(20):5805–27. doi: 10.1093/hmg/ddv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Viaggi C, Gerace C, Pardini C, Corsini GU, Vaglini F. Serotonin abnormalities in Engrailed-2 knockout mice: New insight relevant for a model of Autism Spectrum Disorder. Neurochemistry international. 2015 Aug;87:34–42. doi: 10.1016/j.neuint.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 122.Fox SR, Deneris ES. Engrailed is required in maturing serotonin neurons to regulate the cytoarchitecture and survival of the dorsal raphe nucleus. J Neurosci. 2012 Jun 6;32(23):7832–42. doi: 10.1523/JNEUROSCI.5829-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zervas M, Millet S, Ahn S, Joyner AL. Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron. 2004 Aug 5;43(3):345–57. doi: 10.1016/j.neuron.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 124.Yan R, Huang T, Xie Z, Xia G, Qian H, Zhao X, et al. Lmx1b controls peptide phenotypes in serotonergic and dopaminergic neurons. Acta biochimica et biophysica Sinica. 2013 May;45(5):345–52. doi: 10.1093/abbs/gmt023. [DOI] [PubMed] [Google Scholar]
- 125.Kerk SY, Kratsios P, Hart M, Mourao R, Hobert O. Diversification of C. elegans Motor Neuron Identity via Selective Effector Gene Repression. Neuron. 2017 Jan 04;93(1):80–98. doi: 10.1016/j.neuron.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pelosi B, Migliarini S, Pacini G, Pratelli M, Pasqualetti M. Generation of Pet1210-Cre transgenic mouse line reveals non-serotonergic expression domains of Pet1 both in CNS and periphery. PLoS One. 2014;9(8):e104318. doi: 10.1371/journal.pone.0104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013 Apr 26;340(6131):449–53. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- 128.Deneris ES, Hobert O. Maintenance of postmitotic neuronal cell identity. Nat Neurosci. 2014 Jun 15; doi: 10.1038/nn.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kadkhodaei B, Alvarsson A, Schintu N, Ramskold D, Volakakis N, Joodmardi E, et al. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2360–5. doi: 10.1073/pnas.1221077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009 Dec 16;29(50):15923–32. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Decressac M, Volakakis N, Bjorklund A, Perlmann T. NURR1 in Parkinson disease--from pathogenesis to therapeutic potential. Nature reviews Neurology. 2013 Nov;9(11):629–36. doi: 10.1038/nrneurol.2013.209. [DOI] [PubMed] [Google Scholar]
- 132.Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A. alpha-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med. 2012 Dec 5;4(163):163ra56. doi: 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- 133.Rodriguez JJ, Noristani HN, Verkhratsky A. The serotonergic system in ageing and Alzheimer’s disease. Prog Neurobiol. 2012 Oct;99(1):15–41. doi: 10.1016/j.pneurobio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, et al. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2008 Dec 17;28(51):13805–14. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song NN, Xiu JB, Huang Y, Chen JY, Zhang L, Gutknecht L, et al. Adult raphe-specific deletion of lmx1b leads to central serotonin deficiency. PLoS One. 2011;6(1):e15998. doi: 10.1371/journal.pone.0015998. Epub 2011/01/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hughes SM, Easton CR, Bosma MM. Properties and mechanisms of spontaneous activity in the embryonic chick hindbrain. Developmental neurobiology. 2009 Jul;69(8):477–90. doi: 10.1002/dneu.20712. [DOI] [PubMed] [Google Scholar]
- 137.Hunt PN, McCabe AK, Gust J, Bosma MM. Spatial restriction of spontaneous activity towards the rostral primary initiating zone during development of the embryonic mouse hindbrain. J Neurobiol. 2006 Sep 15;66(11):1225–38. doi: 10.1002/neu.20260. [DOI] [PubMed] [Google Scholar]
- 138.Hunt PN, McCabe AK, Bosma MM. Midline serotonergic neurones contribute to widespread synchronized activity in embryonic mouse hindbrain. J Physiol. 2005 Aug 1;566(Pt 3):807–19. doi: 10.1113/jphysiol.2005.089581. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Watari H, Tose AJ, Bosma MM. Looping circuit: a novel mechanism for prolonged spontaneous [Ca2+]i increases in developing embryonic mouse brainstem. J Physiol. 2014 Feb 15;592(4):711–27. doi: 10.1113/jphysiol.2013.265892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A. 2008 Dec 23;105(51):20067–71. doi: 10.1073/pnas.0806070105. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Allan DW, Thor S. Transcriptional selectors, masters, and combinatorial codes: regulatory principles of neural subtype specification. Wiley interdisciplinary reviews Developmental biology. 2015 Apr 8; doi: 10.1002/wdev.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hobert O. A map of terminal regulators of neuronal identity in Caenorhabditis elegans. Wiley interdisciplinary reviews Developmental biology. 2016 Jul;5(4):474–98. doi: 10.1002/wdev.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Etchberger JF, Lorch A, Sleumer MC, Zapf R, Jones SJ, Marra MA, et al. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev. 2007 Jul 1;21(13):1653–74. doi: 10.1101/gad.1560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tursun B, Patel T, Kratsios P, Hobert O. Direct conversion of C. elegans germ cells into specific neuron types. Science. 2011 Jan 21;331(6015):304–8. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Way JC, Chalfie M. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 1989 Dec;3(12A):1823–33. doi: 10.1101/gad.3.12a.1823. [DOI] [PubMed] [Google Scholar]
- 146.Wenick AS, Hobert O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell. 2004 Jun;6(6):757–70. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 147.Xue D, Finney M, Ruvkun G, Chalfie M. Regulation of the mec-3 gene by the C.elegans homeoproteins UNC-86 and MEC-3. EMBO J. 1992 Dec;11(13):4969–79. doi: 10.1002/j.1460-2075.1992.tb05604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scott MM, Krueger KC, Deneris ES. A differentially autoregulated Pet-1 enhancer region is a critical target of the transcriptional cascade that governs serotonin neuron development. J Neurosci. 2005 Mar 9;25(10):2628–36. doi: 10.1523/JNEUROSCI.4979-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16472–7. doi: 10.1073/pnas.0504510102. Epub 2005/10/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fyodorov D, Nelson T, Deneris E. Pet-1, a novel ETS domain factor that can activate neuronal nAchR gene transcription. J Neurobiol. 1998;34:151–63. [PubMed] [Google Scholar]
- 151.Maurer P, T’Sas F, Coutte L, Callens N, Brenner C, Van Lint C, et al. FEV acts as a transcriptional repressor through its DNA-binding ETS domain and alanine-rich domain. Oncogene. 2003 Jun 22;22(21):3319–29. doi: 10.1038/sj.onc.1206572. [DOI] [PubMed] [Google Scholar]
- 152.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002 Nov 1;22(21):9453–64. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang QP, Koyama Y, Guan JL, Takahashi K, Kayama Y, Shioda S. The orexinergic synaptic innervation of serotonin- and orexin 1-receptor-containing neurons in the dorsal raphe nucleus. Regulatory peptides. 2005 Mar 15;126(1–2):35–42. doi: 10.1016/j.regpep.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 154.Stanzel S, Stubbusch J, Pataskar A, Howard MJ, Deller T, Ernsberger U, et al. Distinct roles of hand2 in developing and adult autonomic neurons. Developmental neurobiology. 2016 Oct;76(10):1111–24. doi: 10.1002/dneu.22378. [DOI] [PubMed] [Google Scholar]
- 155.Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, Gire C, et al. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J Neurosci. 2008 Feb 13;28(7):1745–55. doi: 10.1523/JNEUROSCI.4334-07.2008. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gould SJ, Kalish JE, Morrell JC, Bjorkman J, Urquhart AJ, Crane DI. Pex13p is an SH3 protein of the peroxisome membrane and a docking factor for the predominantly cytoplasmic PTs1 receptor. The Journal of cell biology. 1996 Oct;135(1):85–95. doi: 10.1083/jcb.135.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rahim RS, Meedeniya AC, Crane DI. Central serotonergic neuron deficiency in a mouse model of Zellweger syndrome. Neuroscience. 2014 Aug 22;274:229–41. doi: 10.1016/j.neuroscience.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 158.Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li T. Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Brain Res Dev Brain Res. 2001 Feb 28;126(2):147–55. doi: 10.1016/s0165-3806(00)00144-9. [DOI] [PubMed] [Google Scholar]
- 159.Miyazaki K, Narita N, Narita M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: implication for pathogenesis of autism. Int J Dev Neurosci. 2005 Apr-May;23(2–3):287–97. doi: 10.1016/j.ijdevneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 160.Oyabu A, Narita M, Tashiro Y. The effects of prenatal exposure to valproic acid on the initial development of serotonergic neurons. Int J Dev Neurosci. 2013 May;31(3):202–8. doi: 10.1016/j.ijdevneu.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 161.Kuwagata M, Ogawa T, Shioda S, Nagata T. Observation of fetal brain in a rat valproate-induced autism model: a developmental neurotoxicity study. Int J Dev Neurosci. 2009 Jun;27(4):399–405. doi: 10.1016/j.ijdevneu.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 162.Rout UK, Clausen P. Common increase of GATA-3 level in PC-12 cells by three teratogens causing autism spectrum disorders. Neurosci Res. 2009 Jun;64(2):162–9. doi: 10.1016/j.neures.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Azmitia EC, Dolan K, Whitaker-Azmitia PM. S-100B but not NGF, EGF, insulin or calmodulin is a CNS serotonergic growth factor. Brain Res. 1990 May 21;516(2):354–6. doi: 10.1016/0006-8993(90)90942-5. [DOI] [PubMed] [Google Scholar]
- 164.Donovan SL, Mamounas LA, Andrews AM, Blue ME, McCasland JS. GAP-43 is critical for normal development of the serotonergic innervation in forebrain. J Neurosci. 2002 May 1;22(9):3543–52. doi: 10.1523/JNEUROSCI.22-09-03543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fournet V, Jany M, Fabre V, Chali F, Orsal D, Schweitzer A, et al. The deletion of the microtubule-associated STOP protein affects the serotonergic mouse brain network. J Neurochem. 2010 Dec;115(6):1579–94. doi: 10.1111/j.1471-4159.2010.07064.x. [DOI] [PubMed] [Google Scholar]
- 166.Liu JP, Lauder JM. S-100 beta and insulin-like growth factor-II differentially regulate growth of developing serotonin and dopamine neurons in vitro. J Neurosci Res. 1992 Oct;33(2):248–56. doi: 10.1002/jnr.490330208. [DOI] [PubMed] [Google Scholar]
- 167.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999 Dec 21;96(26):15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000 Jan 15;20(2):771–82. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends in neurosciences. 1997 Feb;20(2):84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 170.Narboux-Neme N, Sagne C, Doly S, Diaz SL, Martin CB, Angenard G, et al. Severe serotonin depletion after conditional deletion of the vesicular monoamine transporter 2 gene in serotonin neurons: neural and behavioral consequences. Neuropsychopharmacology. 2011 Nov;36(12):2538–50. doi: 10.1038/npp.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gutknecht L, Waider J, Kraft S, Kriegebaum C, Holtmann B, Reif A, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008 Aug;115(8):1127–32. doi: 10.1007/s00702-008-0096-6. eng. [DOI] [PubMed] [Google Scholar]
- 172.Pratelli M, Migliarini S, Pelosi B, Napolitano F, Usiello A, Pasqualetti M. Perturbation of Serotonin Homeostasis during Adulthood Affects Serotonergic Neuronal Circuitry. eNeuro. 2017 Mar-Apr;4(2) doi: 10.1523/ENEURO.0376-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010 Nov 24;30(47):16053–64. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, et al. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002 Jan 17;33(2):233–48. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- 175.Chen WV, Nwakeze CL, Denny CA, O’Keeffe S, Rieger MA, Mountoufaris G, et al. Pcdhalphac2 is required for axonal tiling and assembly of serotonergic circuitries in mice. Science. 2017 Apr 28;356(6336):406–11. doi: 10.1126/science.aal3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, et al. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci. 2009 Jul 22;29(29):9137–47. doi: 10.1523/JNEUROSCI.5478-08.2009. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fukuda E, Hamada S, Hasegawa S, Katori S, Sanbo M, Miyakawa T, et al. Down-regulation of protocadherin-alpha A isoforms in mice changes contextual fear conditioning and spatial working memory. Eur J Neurosci. 2008 Oct;28(7):1362–76. doi: 10.1111/j.1460-9568.2008.06428.x. [DOI] [PubMed] [Google Scholar]
- 178.Klein R, Kania A. Ephrin signalling in the developing nervous system. Curr Opin Neurobiol. 2014 Aug;27:16–24. doi: 10.1016/j.conb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 179.Teng T, Gaillard A, Muzerelle A, Gaspar P. EphrinA5 Signaling Is Required for the Distinctive Targeting of Raphe Serotonin Neurons in the Forebrain. eNeuro. 2017 Jan-Feb;4(1) doi: 10.1523/ENEURO.0327-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mamiya PC, Hennesy Z, Zhou R, Wagner GC. Changes in attack behavior and activity in EphA5 knockout mice. Brain Res. 2008 Apr 18;1205:91–9. doi: 10.1016/j.brainres.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lu J, Zhong X, Liu H, Hao L, Huang CT, Sherafat MA, et al. Generation of serotonin neurons from human pluripotent stem cells. Nat Biotechnol. 2016 Jan;34(1):89–94. doi: 10.1038/nbt.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vadodaria KC, Mertens J, Paquola A, Bardy C, Li X, Jappelli R, et al. Generation of functional human serotonergic neurons from fibroblasts. Mol Psychiatry. 2016 Jan;21(1):49–61. doi: 10.1038/mp.2015.161. [DOI] [PubMed] [Google Scholar]
- 183.Xu Z, Jiang H, Zhong P, Yan Z, Chen S, Feng J. Direct conversion of human fibroblasts to induced serotonergic neurons. Mol Psychiatry. 2016 Jan;21(1):62–70. doi: 10.1038/mp.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baker KG, Halliday GM, Halasz P, Hornung JP, Geffen LB, Cotton RG, et al. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991 Apr;7(4):301–20. doi: 10.1002/syn.890070407. [DOI] [PubMed] [Google Scholar]
- 185.Baker KG, Halliday GM, Tork I. Cytoarchitecture of the human dorsal raphe nucleus. J Comp Neurol. 1990 Nov 8;301(2):147–61. doi: 10.1002/cne.903010202. [DOI] [PubMed] [Google Scholar]
- 186.Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003 Dec;26(4):331–43. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 187.Jacobsen KX, Czesak M, Deria M, Le Francois B, Albert PR. Region-specific regulation of 5-HT1A receptor expression by Pet-1-dependent mechanisms in vivo. J Neurochem. 2011 Dec 23; doi: 10.1111/j.1471-4159.2010.07161.x. Epub 2010/12/25. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]