Abstract
Objective
In resistance arteries, endothelial cell (EC) extensions can make contact with smooth muscle cells (SMC), forming myoendothelial junctions (MEJ) at holes in the internal elastic lamina (IEL). At these holes in the IEL, calcium signaling is tightly regulated. Because calreticulin (Calr) can buffer ~50% of endoplasmic reticulum calcium and is expressed throughout IEL holes in small arteries, the only place where MEJs form, we investigated the effect of EC-specific Calr deletion on calcium signaling and vascular function.
Approach and Results
We found Calr expressed in nearly every IEL hole in third-order mesenteric arteries, but not other ER markers. Because of this, we generated an EC specific, tamoxifen inducible, Calr knockout mouse (EC Calr Δ/Δ). Using this mouse, we tested third-order mesenteric arteries for changes in calcium events at holes in the IEL and vascular reactivity after application of carbachol (CCh) or phenylephrine (PE). We found arteries from EC Calr Δ/Δ mice stimulated with CCh had unchanged activity of calcium signals and vasodilation; however, the same arteries were unable to increase calcium events at holes in the IEL in response to PE. This resulted in significantly increased vasoconstriction to PE, presumably due to inhibited negative feedback. In line with these observations, the EC Calr Δ/Δ had increased blood pressure. Comparison of ER calcium in arteries and use of an ER-specific GCaMP indicator in vitro revealed no observable difference in ER calcium with Calr knockout. Using selective detergent permeabilization of the artery and inhibition of Calr translocation, we found that the observed Calr at holes in the IEL may not be within the ER.
Conclusions
Our data suggests Calr specifically at holes in the IEL may act in a non-ER dependent manner to regulate arteriolar heterocellular communication and blood pressure.
Keywords: calreticulin, myoendothelial junction, heterocellular signaling, endothelial cell, calcium
Subject codes: Cell signaling/signal transduction, endothelium, physiology, vascular biology, blood pressure
INTRODUCTION
Maintenance of normal blood pressure relies on a balance of small diameter artery constriction and dilation which contributes to peripheral vascular resistance.1 Receptor-mediated signaling that occurs within endothelial cells (ECs) and smooth muscle cells (SMCs) is particularly key to vascular tone regulation.2, 3 Any perturbation in these signaling processes in resistance arteries can shift blood pressure away from homeostasis and towards hypertension.4, 5
Calcium is a tightly regulated signaling molecule in the vascular wall with cytosolic increases having opposing effects on vascular tone.6 Unstimulated cells maintain cytosolic calcium at nanomolar levels, thus increases in EC cytosolic calcium result in vasodilation; increased SMC cytosolic calcium results in vasoconstriction. However, increases in SMC calcium and inositol 1,4,5 trisphosphate (IP3) via α1D-adrenergic receptor stimulation with phenylephrine have been shown to elicit subsequent increases in EC calcium7–9, indicating heterocellular communication occurs between the two cell types. In resistance arteries, direct cytoplasmic contact between EC and SMC can occur via cellular projections through holes in the internal elastic lamina (IEL), resulting in the formation of a myoendothelial junction (MEJ).10, 11
The MEJ predominantly originates from the EC, where focal increases in intracellular calcium occur, activating a well-established signaling cascade, resulting in vasodilation or initiation of negative feedback mechanisms after vasoconstriction.7, 12–18 In particular, endoplasmic reticulum (ER) -based calcium signals can occur via IP3 receptors on the ER that are present within or close proximity to the MEJs.13–15, 17, 19 Extracellular influx of calcium via transient receptor potential vanilloid 4 (TRPV4) channels has been shown to regulate calcium influx into the MEJ and vascular tone.12, 16 However, the role of ER calcium release in this signaling cascade is still unclear, due primarily to the inability to specifically manipulate ER-based proteins in vivo.
Calreticulin (Calr), well-known for sequestering ER calcium within the ER lumen20, 21, was shown by our lab to be highly expressed at the MEJ in vitro.22, 23 In experiments focused on Calr cell biology, the knockdown or overexpression of Calr has profound effects on ER calcium; deletion results in reduced ER calcium and overexpression increases ER calcium.24–26 Furthermore, Calr deficient cells have impairments in IP3 stimulated calcium mobilization.27–29 Until now, the role of Calr in the context of vascular tone has not been investigated. In this study, to avoid issues with Calr mediation of organogenesis during development 27, 30, 31, we used a novel mouse model in which we could reliably induce the deletion of Calr specifically in the endothelium . Given the effects of Calr on ER calcium and ER calcium release in vitro, we predicted we would see similar impairments in IP3 mediated signaling and hypothesized this would result in changes in calcium signaling, vascular tone and blood pressure.
Surprisingly, we found that Calr localized to MEJs is unique compared to the Calr in the EC monolayer of intact resistance arteries, and that EC deletion of Calr results in a differential response to IP3 stimulation depending on receptor activation. This alteration in signaling affects vascular tone and blood pressure. Importantly, we show this polarization of response is likely not due to differences in ER calcium content, and may instead be due to MEJ Calr localization outside the ER.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Calr is enriched at holes in the internal elastic lamina
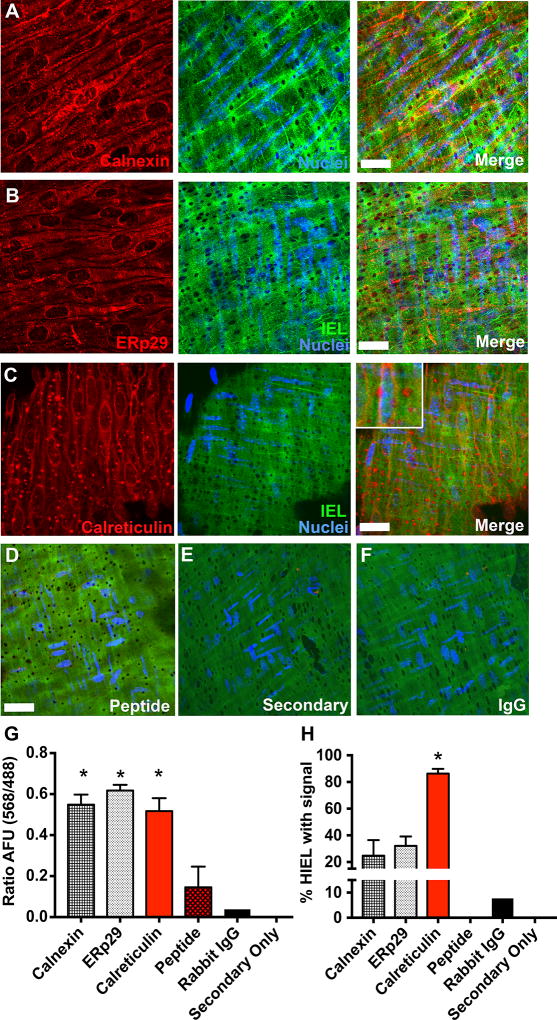
We investigated the expression of various ER localized proteins in the context of expression at holes in the internal elastic lamina (HIEL). Calnexin (Figure 1A) and ERp29 (Figure 1B) were throughout the EC monolayer (Figure 1G), but less so (~30%) within HIEL, the only place where MEJs form. However, Calr was present in EC monolayers and nearly all the HIEL (Figure 1C). Pre-incubation with Calr peptide before immunofluorescence with the Calr antibody, secondary antibody alone and IgG negative controls could not detect any positive staining (Figure 1D–F). Quantification of positive Calr staining was found in >80% of the HIEL (Figure 1H). We used immuno-electron microscopy and confirmed Calr expression in EC monolayer and MEJs (Supplemental Figure I) and validated the staining with multiple Calr antibodies (Supplemental Figure II).
Figure 1. Calreticulin is enriched in the majority of holes in the artery internal elastic lamina (IEL).
Confocal Z-stack of en face immunofluorescence for established endoplasmic reticulum (ER) markers, including A calnexin (red), B ERp29 (red), or C calreticulin (Calr; red) in a third order mesenteric artery with autofluorescence of the internal elastic lamina (IEL; green) and DAPI stained nuclei from endothelial cell (EC; blue). Merge indicates overlay of the three fluorescent channels. D, Arteries were incubated with Calr peptide prior to Calr primary antibody. E, Separate experiments show a representative artery incubated with secondary antibody only or F rabbit IgG instead of primary antibody. G, Quantification of EC monolayer fluorescence (568 nm) normalized to IEL autofluorescence (488 nm) in arbitrary fluorescent units (AFU). * p<0.05 versus peptide, rabbit IgG and secondary only. H, Percentage of HIEL with positive staining. * p<0.05 versus calnexin, ERp29, peptide, rabbit IgG and secondary only. Calreticulin/Secondary only/Rabbit IgG, n=6 fields of view. ERp29/calnexin/peptide, n=3 fields of view. Scale bar=10µm. Samples were compared using a one-way ANOVA with Sidak's multiple comparison test to compare to Calr expression.
Generation of tamoxifen inducible, endothelial specific, Calr knockout mouse
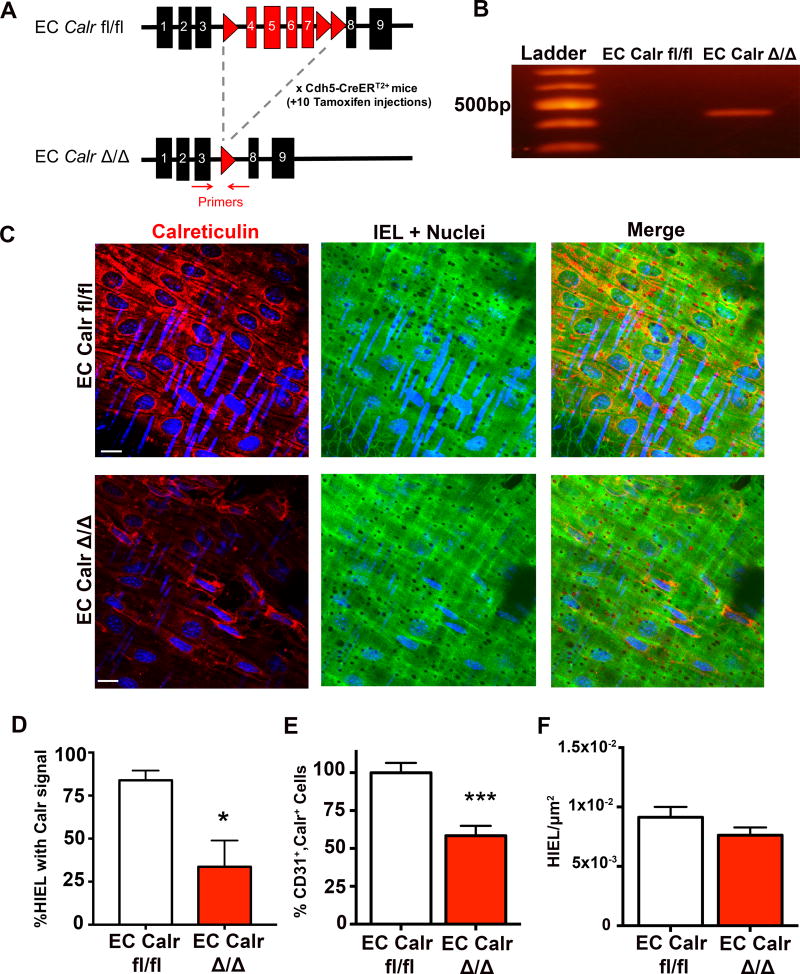
Because Calr is expressed in ECs and within MEJs, and ER calcium could be an important regulator of vascular function, we hypothesized that EC deletion of Calr could alter heterocellular calcium signaling. For these reasons, we made a tamoxifen inducible, EC-specific Calr knockout mouse (EC Calr Δ/Δ; Figure 2A). To check the efficiency of Calr knockout, we used primers designed to detect the truncated Calr gene, with a size of approximately 400bp only seen in EC Calr Δ/Δ mice30 (Figure 2B). Third order mesenteric arteries from these mice were stained for Calr (Figure 2C; Supplemental Figures III–IV) and a significant reduction in Calr at HIEL was identified in EC Calr Δ/Δ mice (Figure 2D). To more specifically look at EC Calr expression, we isolated microvascular diaphragm ECs and found EC Calr Δ/Δ mice had significantly fewer double positive cells (CD31+/Calr+ cells) (Figure 2E; Supplemental Figure V). There were no differences in the density of HIEL (Figure 2F) or blood cell counts (Supplemental Figure VI) between mice. We injected EC Calr fl/fl mice lacking the EC cre recombinase with tamoxifen and did not see any recombination or difference in vascular function or physiology versus EC Calr fl/fl (Supplemental Figure VII)
Figure 2. Generation of EC specific, tamoxifen inducible Calr knockout mice.
A, Gene map showing loxP sites (red triangles) around exons 4–7 in the Calr gene. The Calr floxed mice were bred with EC specific promoter mice (Cdh5-CreERT2+). All EC Calr fl/fl mice were injected for 10 days with vehicle control (peanut oil, EC Calr fl/fl) or tamoxifen (EC Calr Δ/Δ). Red arrows in A indicate location of primers for B, Representative end-point PCR gel indicating excision of Calr exons only in EC Calr Δ/Δ with a product of approximately 400 kilobase pairs (kb). C, Representative images of Calr (red) en face immunofluorescence in arteries (green=autofluorescence of IEL, blue= nuclei). Scale bar=10µm. D, Percentage of HIEL with Calr signal in EC Calr fl/fl and Δ/Δ arteries (EC Calr fl/fl n=5 fields of view; EC Calr Δ/Δ n=5 fields of view). E, Diaphragm microvasculature was digested, stained for CD31 (EC marker) and Calr and analyzed via flow cytometry. (EC Calr fl/fl n=6, EC Calr Δ/Δ n=5) F, HIEL were quantified to approximate the number of myoendothelial junctions (MEJ). Images were from en face arteries using autofluorescence of the IEL (EC Calr fl/fl n=22 fields of view, EC Calr Δ/Δ n=19 fields of view). * indicates p<0.05, *** p<0.001. Groups were compared using an unpaired student's t-test.
Calcium events in EC monolayer and HIEL of EC Calr Δ/Δ arteries
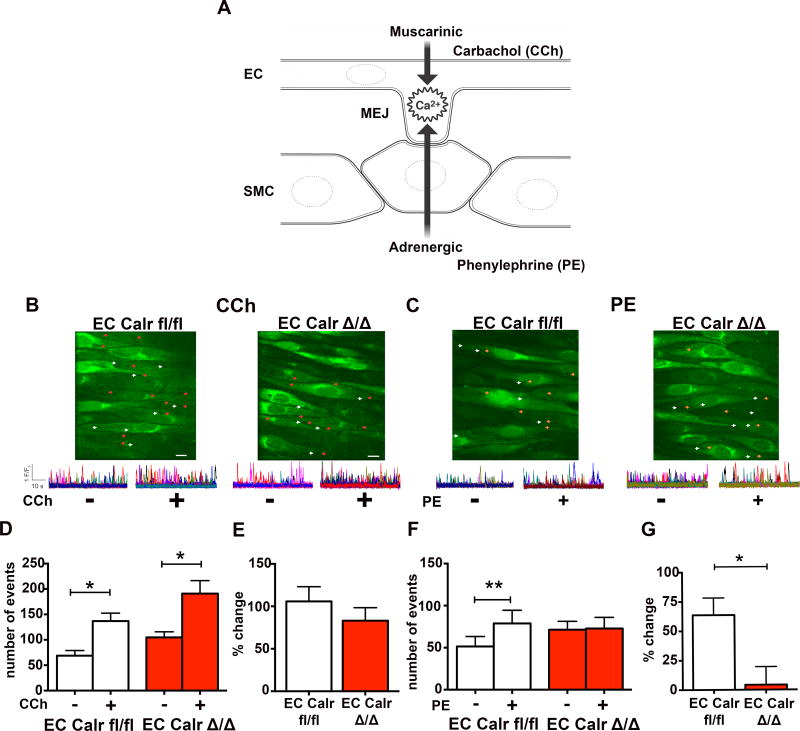
To investigate the physiological effect of EC Calr knockout, a muscarinic agonist (carbachol; CCh) and an adrenergic agonist (phenylephrine; PE) were used because of their ability to activate calcium events at HIEL (Figure 3A, Supplemental Figure VIII). When we focused on calcium events occurring in the endothelium specifically at HIEL (Figure 3B–C), CCh significantly increased calcium events at HIEL in both the EC Calr fl/fl and EC CalrΔ/Δ arteries (Figure 3D–E). Addition of PE to the arteries caused a significant increase in EC Calr fl/fl calcium events, however EC Calr Δ/Δ arteries did not exhibit this same increase (Figure 3F–G). There was no difference in the PE calcium response in SMC (Supplemental Figure IX)
Figure 3. EC Calr Δ/Δ arteries have differential, internal elastic lamina (IEL)-localized calcium responses to adrenergic versus muscarinic agonists.
A, Schematic showing both muscarinic stimulation (via carbachol; CCh) and adrenergic stimulation (via phenylephrine; PE) cause increases in calcium events at the MEJ. En face preparations of third order mesenteric arteries were loaded with fluo-4AM, incubated in physiological salt solution and maintained at 37°C. B, C, Representative images show locations of baseline (white arrows) and stimulated (red or orange arrows) events occurring within holes of the IEL (HIEL). Scale bar=10µm. Representative F/F0 traces show calcium release events occurring before and after CCh or PE. Each color trace represents distinct holes in the IEL and the peaks represent the calcium events that occur within the holes of the IEL. D, Quantification of the absolute number of calcium events occurring at HIEL before and after CCh stimulation. E, Percent increase in calcum events with CCh compared to baseline. F, Quantification of absolute number of calcium events before and after PE stimulation. G, Percent increase in calcium events with PE compared to baseline. (EC Calr fl/fl n=5 arteries from 5 mice, EC Calr Δ/Δ n=5 arteries from 5 mice). *p<0.05, **p<0.01. Baseline and drug values were compared using a paired student's t-test while EC Calr fl/fl and EC Calr Δ/Δ changes were compared using an unpaired student's t-test.
Calcium events also occur outside of the HIEL in the rest of the EC ("EC monolayer"). Carbachol stimulation caused a significant increase in calcium events in the EC monolayer in both EC Calr fl/fl and Δ/Δ mice (Supplemental Figure X). Phenylephrine application had no effect on calcium events in the EC monolayer (Supplemental Figure X), indicating that PE can only stimulate calcium events within the holes of the IEL. Thus, EC Calr Δ/Δ arteries are able to significantly increase calcium events in response to CCh, but not PE, and these differences occur at the HIEL.
Constriction and dilation of EC Calr Δ/Δ arteries
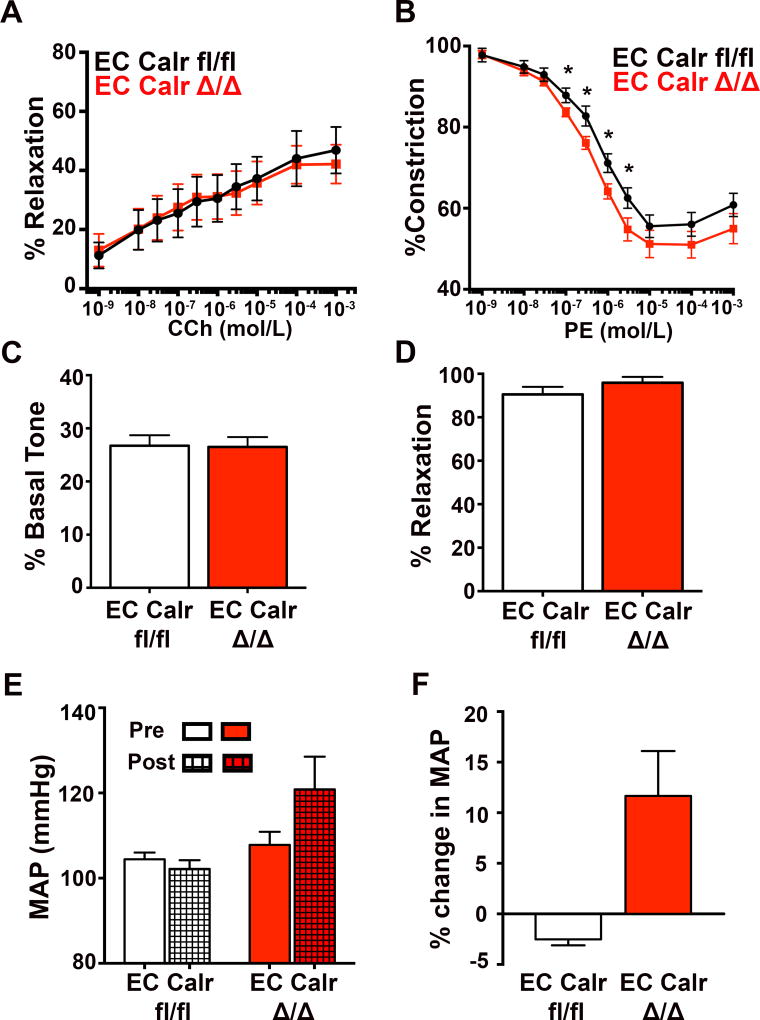
Because localized calcium events in the HIEL activate signaling cascades that affect vascular contractility12, 16, the effect of EC Calr Δ/Δ on dilation to CCh and constriction to PE was investigated. In line with our calcium data, dilation to CCh over a range of concentrations was not different between groups (Figure 4A). However, EC Calr Δ/Δ arteries constricted significantly more to PE than control (Figure 4B), functional evidence that correlates with their failure to increase calcium events in response to PE.
Figure 4. EC Calr Δ/Δ arteries have differential vasoreactive responses to adrenergic versus muscarinic agonists that results in a higher mean arterial pressure.
Third order mesenteric arteries were dissected clean of fat, cannulated and pressurized to 80mmHg. Arteries were maintained at 37°. A, Quantification of diameter response to CCh (EC Calr fl/fl n=3; EC Calr Δ/Δ n=3). B, Quantification of diameter response to PE (EC Calr fl/fl n=12 arteries from 8 mice; EC Calr Δ/Δ n=11 arteries from 8 mice; *p<0.06). Smooth muscle cell (SMC) function and viability was assessed via C, initial tone at 80 mmHg (EC Calr fl/fl n=12 arteries from 8 mice; EC Calr Δ/Δ n=11 arteries from 8 mice). D, NS309 (1µM) dilation indicates intact signaling at the myoendothelial junction (MEJ) along with endothelial viability (EC Calr fl/fl n=12 arteries from 8 mice; EC Calr Δ/Δ n=11 arteries from 8 mice.) E, Radiotelemetry catheters were implanted and daily averages for mean arterial pressure (MAP) were recorded over the course of 50 days. Each mouse was compared back to its initial baseline MAP. The average MAP 5 days prior to injections (left, solid bars) versus after injections (right, patterned bars). F, Percent change from baseline to the end of the study (EC Calr fl/fl n=3; EC Calr Δ/Δ n=3). *p<0.05. Comparisons were made using unpaired student's t-test at each drug dose, with the exception of F, which used Mann-Whitney test.
This is likely not due to alteration of intrinsic SMC signaling as myogenic tone at 80mmHg (Figure 4C) was not different between groups. Similarly, NS309-induced dilation was equivalent (Figure 4D) and sodium nitroprusside induced dilation and KCl induced constriction were not different (Supplemental Figure XI). To look at the contribution of endothelial derived hyperpolarization, nitric oxide and prostanoids in the CCh mesenteric response, we performed dose response curves in the presence of L-NAME and indomethacin (Supplemental Figure XII). To compare the small artery response to a conduit vessel, we performed PE and CCH dose response curves on carotid arteries and saw no difference in PE constriction or CCh dilation (Supplemental Figure XIII)
Given the calcium imaging and vascular reactivity results, we next investigated whole animal mean arterial pressure (MAP) via radiotelemetry catheters. Before tamoxifen injection, MAP was not different (Figure 4E, solid bars) but after induction of EC Calr deletion, EC Calr Δ/Δ mice increased their MAP relative to each mouse's own pre-injection baseline. (Figure 4F). The heart rate was not different before or after injection (Supplemental Figure XIV).
Calr does not affect the level of endoplasmic reticulum calcium or expression of ER localized channels
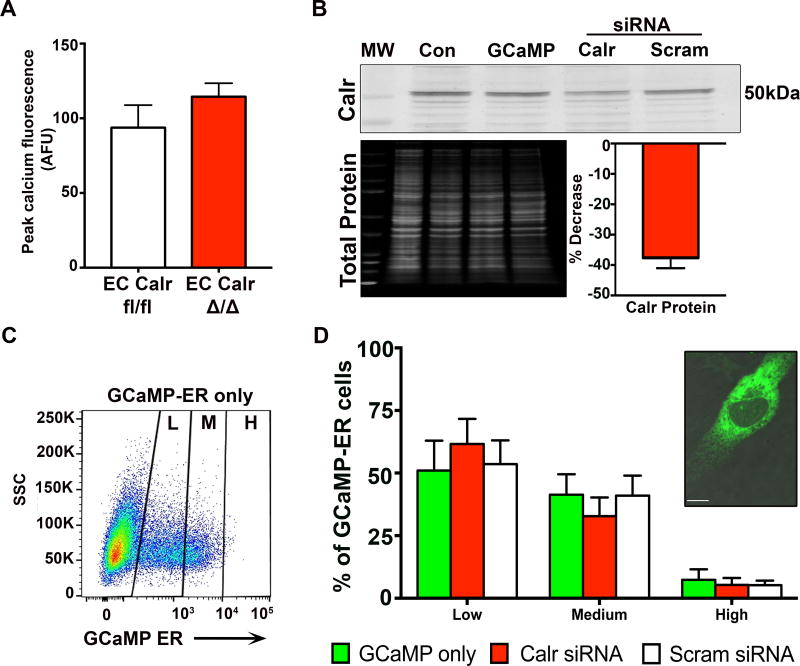
In order to determine if the EC Calr deletion was altering the ER calcium, we first incubated arteries with 0 mM extracellular calcium (to prevent extracellular calcium from entering the cell) followed by CPA (to block SERCA mediated refilling of the ER). Peak cytosolic calcium, specifically released from the ER, did not show any difference in ER calcium content in the arteries (Figure 5A). Next, primary human EC were transfected with GCaMP-ER to specifically visualize ER calcium bound to the low affinity indicator (Supplemental Figure XV–XVI). Co-transfection of human EC with Calr siRNA resulted in a ~40% decrease in Calr protein as measured by western blot (Figure 5B) which was concordant with our whole animal knockdown (Figure 2). GCaMP-ER fluorescence was measured via flow cytometry in live EC and divided into low, medium and high fluorescence intensity (Figure 5C). There was no difference in the amount of fluorescence between GCaMP alone, GCaMP co-transfected with Calr siRNA or GCaMP co-transfected with scrambled siRNA (Figure 5D). This data coincides with the intact arteries indicating the knockdown of Calr did not alter free ER Ca2+ levels. Furthermore, we investigated IP3R and SERCA2 expression and found no significant differences in mRNA levels between EC Calr fl/fl and Δ/Δ arteries (Supplemental Figure XVII).
Figure 5. Calreticulin knockdown in endothelial cells does not affect the level of endoplasmic reticulum calcium.
A, Third order mesenteric arteries from EC Calr fl/fl and EC Calr Δ/Δ mice were loaded with Fluo-4AM. Arteries were incubated in 0 mM extracellular calcium, along with cyclopiazonic acid (20µM) to inhibit SERCA refilling of ER calcium stores. This provides an indirect measurement of ER calcium stores (EC Calr fl/fl n=3; EC Calr Δ/Δ n=3, compared using unpaired student's t-test). B, Representative western blot of primary human aortic EC, which were transfected with GCaMP-ER plasmid to specifically detect ER calcium fluorescence. Then EC were transfected with either calreticulin siRNA or scrambled siRNA. Knockdown was assessed via western blot for calreticulin. Values were normalized to total protein for each lane.and compared to scrambled siRNA. Control EC were untransfected. MW=molecular weight ladder. C, Representative flow cytometry plot for EC transfected with GCaMP-ER to visualize ER calcium, divided into low (L), medium (M) and high (H) fluorescent signal. SSC=side scatter. D, Quantification of GCaMP-ER signal for EC co-transfected with Calr or scrambled siRNA. Inset: Representative confocal image of an EC with GCaMP-ER signal (green, visualized with GFP laser) indicating calcium within the ER. (GCaMP-ER only n=3, Calr siRNA n=3, Scram siRNA n=3, compared using one-way ANOVA. No comparisons were made between Low/Med/High expression.) Scale bar=10µm.
Subcellular localization of Calr in the MEJ
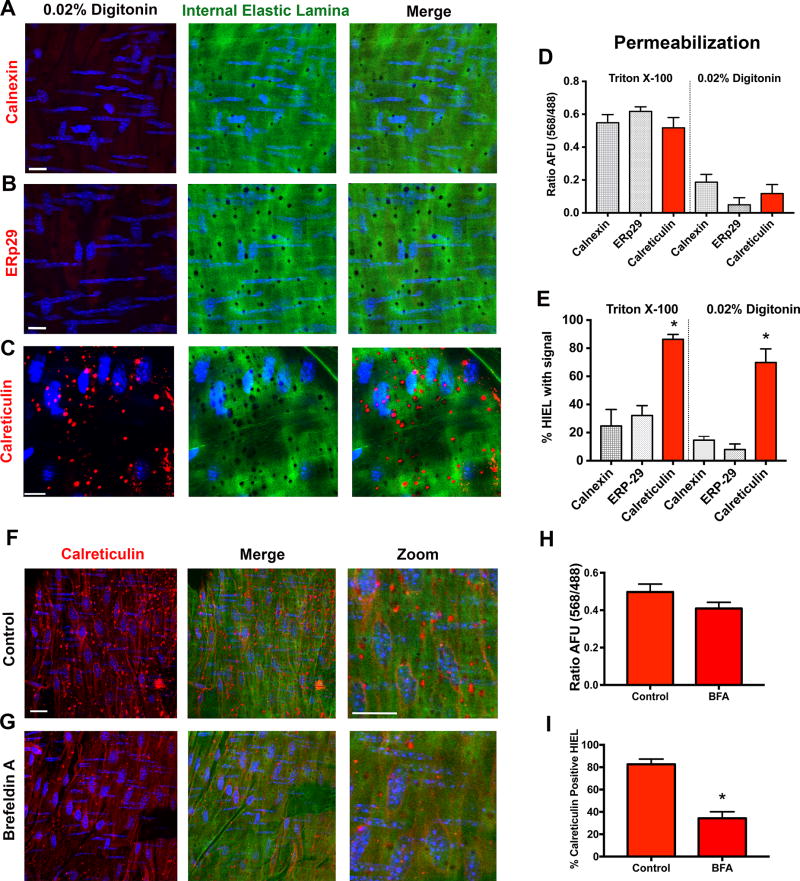
Due to the lack of changes in total ER calcium content, we further hypothesized that the Calr at the HIEL and MEJ may be regulating ER calcium signals independently of its calcium buffer function within the ER. In order to determine if Calr at HIEL was different from the Calr in the EC monolayer, we used proximity ligation assay; the protein ERp57 is a widely recognized binding partner of ER-localized Calr.32 We found association of the two proteins throughout the EC monolayer of arteries, but few in the HIEL (Supplemental Figure XVIII). Next, using digitonin to selectively permeabilize the plasma membrane (but not ER33, 34), we repeated the en face immunofluorescence for calnexin (Figure 6A), ERp29 (Figure 6B) and Calr (Figure 6C) in wild-type mouse mesenteric arteries. In both the EC monolayer and the HIEL, there was no signal for calnexin and ERp29 (Figure 6D–E). The Calr staining exhibited strong punctate signal in the HIEL (Figure 6E). In contrast to the rigorous permeabilization of TritonX-100 which permeabilizes all membranes, digitonin preferentially permeabilizes the plasma membrane but not the ER membrane. Thus, incubation with digitonin resulted in no observable staining in the EC monolayer (Figure 6D). This was seen with all of the ER-markers, including Calr. Next, we attempted to inhibit Calr expression at HIEL. Because Calr in the cytosol is derived from the ER35 and could be trafficked out of the ER36 we incubated arteries with Brefeldin A, which inhibits anterograde traffic out of the ER. This treatment caused a significant decrease in the Calr fluorescence in HIEL, but not the EC monolayer (Figure 6F–H). Further evidence of Calr polarization of function was found from immunoprecipitated calreticuin in vascular cell co-cultures, which demonstrated selective binding to binding immunoglobulin protein (glucose regulated protein 78/BiP) in EC monolayers (an ER chaperone protein), and binding to thrombospondin-1 in the MEJ fractions (an association found outside of the ER; Supplemental Figure XIX). This data suggests the Calr in endothelial monolayers of arteries is found in the ER, but the Calr at HIEL may not be ER-based.
Figure 6. Calreticulin at myoendothelial junctions may be localized outside the endoplasmic reticulum.
Representative en face immunofluorescence for A, calnexin B, ERp29 and C, Calr (red) with 0.02% digitonin permeabilization. This selectively permeabilizes plasma membrane and not the intracellular ER membrane. D, Quantification of EC monolayer fluorescence (568 nm) normalized to IEL autofluorescence (488 nm) in arbitrary fluorescent units (AFU). E, Percentage of holes in the IEL (HIEL) with positive staining. (Calreticulin/ERp29/calnexin n=3) Representative arteries were incubated F, without or G, with Brefeldin A (BFA; 1µg/mL), fixed with 4% paraformaldehyde, and stained for Calr (red). Scale bar=10µm. H, Quantification of EC monolayer fluorescence (568) normalized to IEL autofluorescence (488) in AFU. I, Percentage of HIEL with calreticulin signal. (Control/BFA n=4) *p<0.05. Digitonin experiments were compared using one way ANOVA with Sidak's multiple comparisons test. BFA experiments were compared using unpaired student's t-test.
DISCUSSION
In approximately 30% of holes in the IEL, we observed all three well-established markers for the ER, which indicate the presence of ER at the MEJ in some instances. This finding is consistent in the literature.17, 19, 37 However, the fact that Calr was found in nearly every hole in the IEL (~80%) was unexpected and markedly different than the other ER markers. The permeabilization of arteries with digitonin and the inhibition of Calr transfer to holes in the IEL with brefeldin A further suggest that the pool of Calr found in nearly every hole in the IEL of the arteries was not ER-based and could be indicative of a functionally unique pool of Calr. Our evidence in EC Calr Δ/Δ mice confirm this; in third-order mesenteric arteries, the ECs no longer respond with increases in calcium after SMC stimulation (but there is no impairment in the response to muscarinic stimulation) and pressurized arteries are more reactive to PE (but there is no impairment in the response to muscarinic dilation). In addition, the mice have an increase in mean arterial pressure. This phenotype occurred despite no evident change in global ER calcium in the ECs. The sum of the evidence appears to indicate that non-ER based Calr in the holes of the IEL of mesenteric arteries is an important, and unexpected, component to regulating negative feedback from smooth muscle to endothelium.
The finding that traditional ER markers are not expressed in the majority of IEL holes was also surprising. Although we use IEL holes as a surrogate for MEJ expression and they are the only place where MEJ form, not every IEL hole contains a corresponding functional MEJ.38 Previous research using en face immunofluorescence has shown calnexin in mouse mesenteric arteries is present in IEL holes15, but there was no quantification or data from multiple fields of view to know if the proportion of expression in IEL holes was similar to our results. While the ER is a continuous compartment, it could be possible that calnexin and ERp29 are differentially segregated to areas of the ER separate from Calr. The data presented here now suggest there may also be a population of IEL holes that do not exhibit the presence of ER, which lends support to the conclusion that Calr may be mediating heterocellular signaling from outside the ER.
Because global Calr deletion in mice is embryonically lethal at E1427 and there is sparse evidence on cell specific Calr knockout mice30, 39, we have no direct comparison to our knockout efficiency in vivo. Other researchers have attempted to knock out Calr in ECs and achieved only ~50% decreases in protein in vitro.40 Using the same Calr fl/fl mouse (but without the Cre recombinase), Zimmerman et al reported a knock out efficiency of 60% in SMC transfected with Cre-recombinase IRES-GFP.39 This may be because the half-life of Calr was calculated to be 4.5 days in an untreated HL-60 cell line. These same authors also noted "Calr proved to be an exceedingly stable protein".41 It is likely we have achieved maximal knockout in our models and further research into Calr may explain why it is so difficult to achieve >60% deletion in vivo and in vitro. Nevertheless, we see significant differences in physiology using a mouse model with significant but not complete deletion of EC Calr. This has allowed us to dissect out a differential role for Calr at the MEJ versus Calr in the rest of the EC.
Smaller, more localized changes in ER calcium or compensatory increases in calcium influx (independent of TRPV4) in the EC cannot be ruled out in EC Calr Δ/Δ arteries. However, our evidence does not show any significant differences in ER calcium within the mesenteric arteries or in human ECs transfected with GCaMP-ER. These findings correspond to data from other groups that have shown Calr deletion or knockdown does not affect IP3 mediated calcium stores.42 In their experiments, both resting and stimulated conditions did not change calcium levels in embryonic stem cells with or without Calr.42 The lack of difference between the groups in their calcium signaling and dilation to CCh supports the finding that ER calcium is not globally changed in our model. However, altering EC calcium mobilization has been shown to increase constriction to PE19, which is consistent with our results in isolated mesenteric arteries. Vascular responses such as basal myogenic tone at 80 mmHg and direct activation of endothelial and MEJ localized calcium-activated potassium channels with NS309 indicated intact MEJ signaling and endothelial integrity in both groups. This confirms the differential response to PE is not simply due to an experimental artifact.
One possible explanation for our results is the role of non-ER based Calr as a mediator of integrin-based cell adhesion. There is strong evidence that indicates there is a calcium-dependent interaction between Calr and the cytoplasmic tail of alpha integrin43, 44. Calr downregulation in cells decreases their adhesive capacity and migration31, 40, 42, 45 and some of these effects may be mediated by c-Src and calcium-calmodulin kinase II signaling.46, 47 Furthermore, overexpression studies have also shown alterations in adhesion.48 This would be important in terms of resistance arteries as the EC and MEJ must be coupled to the SMC in order to receive the PE signal.49 Knockout of Calr has been shown to inhibit attachment to the extracellular matrix44 and downregulate cell adhesion-related genes.50 One of the upregulated genes was Notch1, which has been shown to induce SMC adhesion to the EC basement membrane via integrin signaling.51 Our results show no changes in IEL hole number in our arteries, but elucidation of adhesion mechanisms could be more sophisticated than simply changing the hole formation. Cell adhesion at the MEJ, in addition to integrin expression and function has not been characterized. This is an intriguing future direction of exploration, particularly in the context of resistance artery function and control of blood pressure.
Beyond its role in cell adhesion and migration, Calr can be a major ER chaperone, key in maintaining proper protein folding, a calcium-dependent process.52 The knockdown of Calr protein has been shown by multiple groups to induce ER stress.53–55 There is evidence that ER stress via tunicamycin causes endothelial dysfunction by decreased eNOS phosophorylation.56 Furthermore, ER stress inhibition in angiotensin II-induced hypertension improved vascular reactivity in mesenteric arteries.57 We cannot exclude ER stress in our mouse model, especially in polarization of calcium responses and reactivity. However, we note several observations in the EC that lead us to conclude that uncontrolled ER stress may not be occurring in our model. These include the intact CCh calcium responses and vasodilation, as well as the undiminished responses to calcium-activated potassium channels with NS309. In both cases, significant ER stress would be expected to alter these fundamental vascular responses. Based on the evidence of Calr localization at the MEJ, it seems unlikely that Calr is acting as an ER chaperone at the MEJ. This may be more likely in the EC monolayer as we see a greater interaction with GRP78/BiP (in vitro) and ERp57 (en face arteries) versus at the MEJ/in the holes of the IEL. It is also possible that our induction of Calr deletion post development (at 6 weeks of age) and without a concomitant pathological situation does not induce an ER stress response.
Calr has been reported in a number of subcellular compartments beyond the ER depending on the cell type, pathological condition and context that is investigated.58 Our data demonstrate differences between Calr in the EC monolayer, which is perinuclear with typical ER-like staining, and the IEL holes, which have strong punctate staining. When the arteries are permeabilized with digitonin and not Triton, the typical EC monolayer staining is not visible while the MEJ Calr remains very similar (Figure 6). Because digitonin is a mild, non-ionic detergent that preferentially interacts with cholesterol, it does not permeabilize intracellular membranes such as the mitochondrial, lysosomal, nuclear or importantly, the ER membrane.33, 34 Some evidence suggests Calr is the only ER-based mammalian protein able to retrotranslocate out of the ER independently of a protein degradation signal.35 Exactly how Calr is mediating calcium signaling outside of the ER at the MEJ may become evident by looking at downstream signaling targets.
The movement of IP3 from SMCs to ECs through gap junctions has been established (e.g.,13, 15, 19, 59), however we cannot rule out the role of calcium also moving from smooth muscle to endothelium (e.g.,13, 60). Although the type of second messenger moving between cells was not the focus of the study, our use of nifedipine to close L-type calcium channels (used experimentally to limit movement of the artery on the microscope) focus the smooth muscle to endothelial calcium events more specifically on IP3. In this way, we can speculate there is a role for Calr affecting calcium release from IP3 receptors. There is evidence for this in the literature, where decreased Calr can up regulate CamKII activity47 and active CaMKII can inhibit IP3 receptor-mediated calcium release at HIEL.17 If Ca2+ can also move from smooth muscle to endothelium as part of the heterocellular signaling process, it would be interesting to speculate how calcium and IP3 may interact with, or be influenced by Calr, to discretely regulate the calcium events at the holes in the IEL.
In conclusion, we have identified and characterized a non-ER pool of Calr in resistance arteries, found almost exclusively in the holes of IEL. This non-ER pool of Calr can influence heterocellular calcium signaling, vasoreactivity, and physiological blood pressure control. The data suggests that modulating Calr expression or its protein interactions outside of the ER may prove to be a viable target for antihypertensive therapy.
Supplementary Material
HIGHLIGHTS.
-
-
Calreticulin (Calr) is highly expressed in the holes of the internal elastic lamina, which are the only places myoendothelial junctions (MEJs) form.
-
-
Inducible endothelial Calr knockout (EC Calr Δ/Δ) does not affect endothelial dependent calcium signals or vasodilation, but results in an impaired calcium mobilization to phenylephrine, thereby negating a key negative feedback pathway. Over time, these mice have a greater increase in blood pressure compared to baseline.
-
-
MEJ localized Calr may have a different subcellular localization and function versus Calr in the rest of the endothelial cell.
Acknowledgments
We thank Adam Straub for acquiring the representative Calr TEM image, as well as the University of Virginia School of Medicine Advanced Microscopy Facility and Histological Core Facilities. We also thank Nenja Krueger for technical support with qPCR and siRNA experiments and Pooneh Bagher and Marek Michalak for critical reading of this manuscript.
Sources of funding: This work was supported by the Plumeri Award for Faculty Excellence (RLW), American Heart Association predoctoral award 14PRE20420024 (LAB), NIH HL007284 (training grant support to LAB and MEG), NIH HL131399 (MEG), NIH HL121484 (SKS) and NIH HL088554 (BEI).
Non-standard abbreviations and acronyms
- Calr
calreticulin
- CCh
carbachol
- CPA
cyclopiazonic acid
- EC
endothelial cell
- ER
endoplasmic reticulum
- IEL
internal elastic lamina
- IP3
inositol (1,4,5) trisphosphate
- HIEL
holes in the internal elastic lamina
- MAP
mean arterial pressure
- MEJ
myoendothelial junction
- PE
phenylephrine
- SERCA
sarco-endoplasmic reticulum calcium ATP-ase
- SMC
smooth muscle cell
Footnotes
Disclosures: None of the authors declare any conflicts of interest with the work in this manuscript.
References
- 1.Beevers G, Lip GY, O'Brien E. ABC of hypertension: The pathophysiology of hypertension. BMJ. 2001;322:912–6. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev. 2014;66:513–69. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med. 2011;17:1402–9. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 4.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761–76. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 5.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–40. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 6.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 7.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94:6529–34. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yashiro Y, Duling BR. Integrated Ca(2+) signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–54. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- 9.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–46. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: connections that deliver the message. Physiology (Bethesda) 2014;29:242–9. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CH, Welsh DG, Plane F. Endothelial feedback and the myoendothelial projection. Microcirculation. 2012;19:416–22. doi: 10.1111/j.1549-8719.2012.00187.x. [DOI] [PubMed] [Google Scholar]
- 12.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci U S A. 2012;109:18174–9. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121:3664–73. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–54. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 15.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105:9627–32. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toussaint F, Charbel C, Blanchette A, Ledoux J. CaMKII regulates intracellular Ca(2)(+) dynamics in native endothelial cells. Cell Calcium. 2015;58:275–85. doi: 10.1016/j.ceca.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Boerman EM, Everhart JE, Segal SS. Advanced age decreases local calcium signaling in endothelium of mouse mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol. 2016;310:H1091–6. doi: 10.1152/ajpheart.00038.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012;302:C1226–42. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–6. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–66. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- 22.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491:473–7. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biwer LA, Taddeo EP, Kenwood BM, Hoehn KL, Straub AC, Isakson BE. Two functionally distinct pools of eNOS in endothelium are facilitated by myoendothelial junction lipid composition. Biochim Biophys Acta. 2016;1861:671–9. doi: 10.1016/j.bbalip.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biol Chem. 1996;271:9332–9. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- 25.Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995;130:847–55. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnaudeau S, Frieden M, Nakamura K, Castelbou C, Michalak M, Demaurex N. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem. 2002;277:46696–705. doi: 10.1074/jbc.M202395200. [DOI] [PubMed] [Google Scholar]
- 27.Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–68. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu N, Fine RE, Simons E, Johnson RJ. Decreasing calreticulin expression lowers the Ca2+ response to bradykinin and increases sensitivity to ionomycin in NG-108-15 cells. J Biol Chem. 1994;269:28635–9. [PubMed] [Google Scholar]
- 29.Nakamura K, Zuppini A, Arnaudeau S, Lynch J, Ahsan I, Krause R, Papp S, De Smedt H, Parys JB, Muller-Esterl W, Lew DP, Krause KH, Demaurex N, Opas M, Michalak M. Functional specialization of calreticulin domains. J Cell Biol. 2001;154:961–72. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokuhiro K, Satouh Y, Nozawa K, Isotani A, Fujihara Y, Hirashima Y, Matsumura H, Takumi K, Miyano T, Okabe M, Benham AM, Ikawa M. Calreticulin is required for development of the cumulus oocyte complex and female fertility. Sci Rep. 2015;5:14254. doi: 10.1038/srep14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauch F, Prud'homme J, Arabian A, Dedhar S, St-Arnaud R. Heart, brain, and body wall defects in mice lacking calreticulin. Exp Cell Res. 2000;256:105–11. doi: 10.1006/excr.2000.4818. [DOI] [PubMed] [Google Scholar]
- 32.Oliver JD, Roderick HL, Llewellyn DH, High S. ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol Biol Cell. 1999;10:2573–82. doi: 10.1091/mbc.10.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamur MC, Oliver C. Permeabilization of cell membranes. Methods Mol Biol. 2010;588:63–6. doi: 10.1007/978-1-59745-324-0_9. [DOI] [PubMed] [Google Scholar]
- 34.Schulz I. Permeabilizing cells: some methods and applications for the study of intracellular processes. Methods Enzymol. 1990;192:280–300. doi: 10.1016/0076-6879(90)92077-q. [DOI] [PubMed] [Google Scholar]
- 35.Afshar N, Black BE, Paschal BM. Retrotranslocation of the chaperone calreticulin from the endoplasmic reticulum lumen to the cytosol. Mol Cell Biol. 2005;25:8844–53. doi: 10.1128/MCB.25.20.8844-8853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiersma VR, Michalak M, Abdullah TM, Bremer E, Eggleton P. Mechanisms of Translocation of ER Chaperones to the Cell Surface and Immunomodulatory Roles in Cancer and Autoimmunity. Front Oncol. 2015;5:7. doi: 10.3389/fonc.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maarouf N, Sancho M, Furstenhaupt T, Tran CH, Welsh DG. Structural analysis of endothelial projections from mesenteric arteries. Microcirculation. 2017;24 doi: 10.1111/micc.12330. In Press. [DOI] [PubMed] [Google Scholar]
- 38.Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: relationship to function? J Anat. 2009;214:258–66. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmerman KA, Xing D, Pallero MA, Lu A, Ikawa M, Black L, Hoyt KL, Kabarowski JH, Michalak M, Murphy-Ullrich JE. Calreticulin Regulates Neointima Formation and Collagen Deposition following Carotid Artery Ligation. J Vasc Res. 2015;52:306–20. doi: 10.1159/000443884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu FF, Tao TQ, Wang XR, Li YZ, Song DD, Liu M, Liu XH. Cytosolic calreticulin inhibits microwave radiation-induced microvascular endothelial cell injury through the integrin-focal adhesion kinase pathway. Microcirculation. 2014;21:717–29. doi: 10.1111/micc.12153. [DOI] [PubMed] [Google Scholar]
- 41.Clark RA, Li SL, Pearson DW, Leidal KG, Clark JR, Denning GM, Reddick R, Krause KH, Valente AJ. Regulation of calreticulin expression during induction of differentiation in human myeloid cells. Evidence for remodeling of the endoplasmic reticulum. J Biol Chem. 2002;277:32369–78. doi: 10.1074/jbc.M205269200. [DOI] [PubMed] [Google Scholar]
- 42.Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–7. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- 43.Coppolino M, Leung-Hagesteijn C, Dedhar S, Wilkins J. Inducible interaction of integrin alpha 2 beta 1 with calreticulin. Dependence on the activation state of the integrin. J Biol Chem. 1995;270:23132–8. doi: 10.1074/jbc.270.39.23132. [DOI] [PubMed] [Google Scholar]
- 44.Leung-Hagesteijn CY, Milankov K, Michalak M, Wilkins J, Dedhar S. Cell attachment to extracellular matrix substrates is inhibited upon downregulation of expression of calreticulin, an intracellular integrin alpha-subunit-binding protein. J Cell Sci. 1994;107(Pt 3):589–600. [PubMed] [Google Scholar]
- 45.Rojiani MV, Finlay BB, Gray V, Dedhar S. In vitro interaction of a polypeptide homologous to human Ro/SS-A antigen (calreticulin) with a highly conserved amino acid sequence in the cytoplasmic domain of integrin alpha subunits. Biochemistry. 1991;30:9859–66. doi: 10.1021/bi00105a008. [DOI] [PubMed] [Google Scholar]
- 46.Papp S, Fadel MP, Kim H, McCulloch CA, Opas M. Calreticulin affects fibronectin-based cell-substratum adhesion via the regulation of c-Src activity. J Biol Chem. 2007;282:16585–98. doi: 10.1074/jbc.M701011200. [DOI] [PubMed] [Google Scholar]
- 47.Papp S, Szabo E, Kim H, McCulloch CA, Opas M. Kinase-dependent adhesion to fibronectin: regulation by calreticulin. Exp Cell Res. 2008;314:1313–26. doi: 10.1016/j.yexcr.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Ihara Y, Inai Y, Ikezaki M. Alteration of integrin-dependent adhesion and signaling in EMT-like MDCK cells established through overexpression of calreticulin. J Cell Biochem. 2011;112:2518–28. doi: 10.1002/jcb.23176. [DOI] [PubMed] [Google Scholar]
- 49.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol. 2008;155:514–24. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faustino RS, Chiriac A, Niederlander NJ, Nelson TJ, Behfar A, Mishra PK, Macura S, Michalak M, Terzic A, Perez-Terzic C. Decoded calreticulin-deficient embryonic stem cell transcriptome resolves latent cardiophenotype. Stem Cells. 2010;28:1281–91. doi: 10.1002/stem.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheppke L, Murphy EA, Zarpellon A, Hofmann JJ, Merkulova A, Shields DJ, Weis SM, Byzova TV, Ruggeri ZM, Iruela-Arispe ML, Cheresh DA. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood. 2012;119:2149–58. doi: 10.1182/blood-2011-04-348706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szestakowska DSE, Eggleton P, Opas M, Young P. 7th International Workshop on Calreticulin, Niagara Falls, Canada The Complexities of Calreticulin From Protein Folding to Disease Prevention and Therapeutic Application. Calcium Binding Proteins. 2006;1:135–139. [Google Scholar]
- 53.Dey S, Matsunami H. Calreticulin chaperones regulate functional expression of vomeronasal type 2 pheromone receptors. Proc Natl Acad Sci U S A. 2011;108:16651–6. doi: 10.1073/pnas.1018140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knee R, Ahsan I, Mesaeli N, Kaufman RJ, Michalak M. Compromised calnexin function in calreticulin-deficient cells. Biochem Biophys Res Commun. 2003;304:661–6. doi: 10.1016/s0006-291x(03)00643-0. [DOI] [PubMed] [Google Scholar]
- 55.Bernard-Marissal N, Moumen A, Sunyach C, Pellegrino C, Dudley K, Henderson CE, Raoul C, Pettmann B. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J Neurosci. 2012;32:4901–12. doi: 10.1523/JNEUROSCI.5431-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galan M, Kassan M, Kadowitz PJ, Trebak M, Belmadani S, Matrougui K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta. 2014;1843:1063–75. doi: 10.1016/j.bbamcr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32:1652–61. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–83. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca(2+) events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–46. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garland CJ, Bagher P, Powell C, Ye X, Lemmey HAL, Borysova L, Dora KA. Voltage-dependent Ca2+ entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci Signal. 2017;10 doi: 10.1126/scisignal.aal3806. In Press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.