Abstract
Chronic scrotal content pain remains one of the more challenging urological problems to manage. This is a frustrating disorder to diagnose and effectively treat for both the patient and clinician, as no universally accepted treatment guidelines exist. Many patients with this condition end up seeing physicians across many disciplines, further frustrating them. The pathogenesis is not clearly understood, and the treatment ultimately depends on the etiology of the problem. This article reviews the current understanding of chronic scrotal content pain, focusing on the diagnostic work-up and treatment options.
Keywords: Chronic pain, Epididymis, Epididymitis, Pelvic pain, Vasovasostomy
INTRODUCTION
Chronic scrotal content pain (CSCP) is defined by at least 3 months of chronic or intermittent scrotal content pain with severity that interferes with daily activities, prompting the patient to seek medical treatment [1]. CSCP may originate from the testicle, epididymis, paratesticular structures, and/or the spermatic cord. The etiology of the pain is idiopathic in up to 50% of patients [2]. Easily recognizable and reversible causes include varicocele, epididymitis, spermatocele, tumor, infection, and torsion.
This syndrome has been referred to by many names, including chronic orchialgia, testicular pain syndrome, testialgia, CSCP, post-vasectomy orchialgia, post-vasectomy pain syndrome (PVPS), congestive epididymitis, and chronic testicular pain. Presently, this problem is referred to as CSCP, as this term appears to best encompass the variety of structures that may be involved [3,4]. Approximately 2.5% of all urology visits are associated with scrotal content pain, resulting in a significant healthcare financial burden [1,5]. Many patients with this condition end up seeing physicians across many disciplines, further frustrating them [6,7].
In recent years, physicians are seeing more patients with CSCP due to the increased awareness of men's health [8]. It is estimated that around 100,000 men suffer from this problem, and the number will continue to increase [9]. It is estimated that chronic pain affects around 116 million Americans, leading to $635 billion being spent on medical expenses and lost productivity per year due to this problem [10].
Multiple algorithms have been proposed, but none have been validated [2,4]. It is recommended that pharmacotherapy options should be exhausted before considering surgical treatments, which include epididymectomy, microdenervation of the spermatic cord (MDSC), vasecptomy reversal, and finally orchiectomy. In this article, we aim to review the workup and treatment options available for CSCP.
METHODOLOGY
We conducted a computerized bibliographic search of the PubMed, Medline, Embase, and Cochrane databases for all reports pertaining to CSCP using the Medical Subject Headings keywords “Chronic scrotal content pain”, “Testicular pain”, “Orchialgia”, “Testicular Pain Syndrome”, “microdenervation of the spermatic cord”, “Post-vasectomy Pain Syndrome”, and “testialgia” through 15th September 2017. All studies pertaining to CSCP were included in the review. Studies were excluded if they were published in languages other than English.
ETIOLOGY/PATHOPHYSIOLOGY
The iliohypogastric, ilioinguinal, genitofemoral, and pudendal nerves originate from the L1∼L2 and S2∼S4 nerve roots and are responsible for innervation of the testes, epididymis, and scrotum [1,11]. These nerves innervate the scrotal content and travel through the spermatic cord. At least 50% of the nerves innervating those structures were identified near the vas deferens and 20% were identified in the cremasteric fascia [12]. The pathophysiology of CSPS is multifactorial and poorly understood. An injury to the testes or other scrotal content structures is typically the precursor of CSPS. This acute pain leads to nerve sensitization, resulting in modulation of the nerve pathways, causing hypersensitivity around the spermatic cord. This hypersensitivity has been proposed to follow neural injury, with resulting Wallerian degeneration (WD) in these peripheral nerves. WD is characterized as an auto-destructive change in both the proximal and distal nerve axons, which leads to an environment clear of inhibitory debris and supportive of axon regrowth and functional recovery [13]. Following nerve injury, calcium enters the cell, triggering the sealing of the proximal and distal axonal stump by the fusion of axolemmal vesicles around the injured axon endings. This also activates calcium-dependent protease pathways, such as phospholipases and M-calpain. M-calpain triggers the expression of further mediators of WD, such as interleukin 1α [14].
Any organ that shares the same nerve pathways with the scrotal content (L1∼L2 and S2∼S4) may refer pain to this area. Lower back pain due to irritation of the nerve root of T10∼L1 may radiate to the testicle, as they share the same innervation. Pain arising in the ureter due to obstruction, hip pain and intervertebral disc prolapse, or pudendal neuropathies can all result in CSCP [15]. The pain could also be part of chronic prostatitis/chronic pelvic pain syndrome, for which up to 50% of men have reported to also have pain in the testes [2]. Ultimately, CSCP can arise from any traumatic, infectious, or irritative stimulus to the nerves in the scrotum [16].
CLINICAL PRESENTATION AND EVALUATION
The signs and symptoms of CSCP include orchialgia, tender epididymis, tender vas, dyspareunia, pain with ejaculation, premature ejaculation, pain with straining, and pain after prolonged sitting. The key to a successful evaluation is to establish a good rapport with the patient. The evaluation of a patient presenting with scrotal content pain should include a thorough history and physical examination with a focused examination of the scrotal content. The history portion of the clinical visit should include the duration and nature of the pain (sharp, aching, burning, pressure, etc.), consistency (intermittent or constant), severity (on a 0∼10 visual analogue scale), variation in intensity, location, radiation, aggravating factors, associated symptoms, and previous therapeutic maneuvers. The patient should be asked whether the pain is associated with voiding, bowel movements, sexual or physical activities, or prolonged sitting. A surgical history pertaining to the spine, inguinal, scrotal, pelvic, and retroperitoneal space should also be documented. A thorough psychosocial exam to rule out depression, a history of sexual abuse, Munchausen syndrome, or other somatoform disorders should also be included in the evaluation [17,18].
A thorough physical examination focusing on the genitalia is essential for a patient presenting with scrotal content pain. The patient should be examined while standing and supine, beginning on the normal/less painful side. A focused examination of the testicles, epididymides, and vas deferens is recommended, as well as a 360° digital rectal exam to evaluate abnormalities of the prostate and hypertonicity or tenderness of the pelvic floor structures. A neurological examination of the lower limbs should also be performed to rule out radicular pain syndromes and neurosensory deficits. Laboratory investigation studies should include a urinalysis and urine and semen culture to rule out infection when indicated. Should microscopic hematuria be identified on the urinalysis, a computed tomography scan of the abdomen and pelvis is indicated, as obstructing stones in the ureter (particularly the intramural portion of the ureter) can result in scrotal content pain.
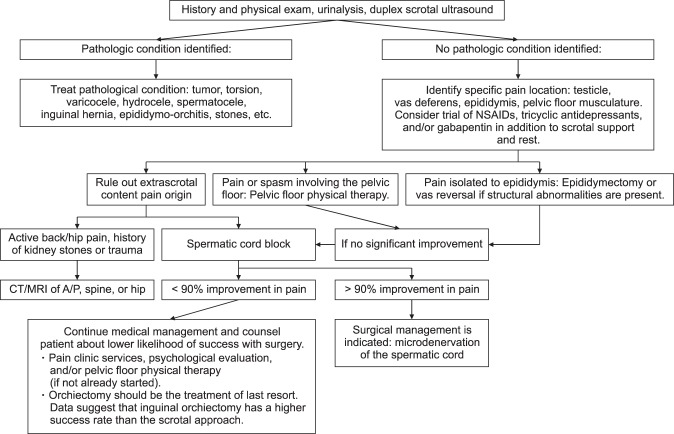
The use of scrotal ultrasound in men with chronic orchialgia has been suggested to have a low yield with respect to identifying pathology, but we recommend that men presenting with CSCP should undergo a high-resolution scrotal ultrasound with color-flow Doppler to rule out other pathological processes such as a testicular tumor, varicocele, or infection [19,20]. A magnetic resonance imaging scan of the spine and/or hips is also recommended when there is a history of back or hip pain to rule out nerve impingement. A spermatic cord block (SCB) should also be performed to determine if the pain is being generated from within the scrotum. We recommend that this block be performed by injecting 20 mL of 0.25% bupivacaine/ropivacaine without epinephrine into the spermatic cord at the level of the pubic tubercle [17]. If the pain signal is conducted via the spermatic cord nerves, the pain should be temporarily relived after performing the cord block. The differential diagnoses for CSPS include constipation; hydrocele; varicocele; epididymitis; tumor; intermittent testicular torsion; inguinal hernia; aortic or common iliac aneurysm; trauma; pelvic floor myalgia; groin, hip, spine, or pelvic floor referred pain; and psychogenic causes. CSCP is a diagnosis of exclusion, and the diagnosis should only be made after all these investigative studies have been performed. Fig. 1 summarizes our recommended algorithm for the evaluation and treatment of patients with scrotal content pain.
Fig. 1. Diagnosis and treatment algorithm for chronic scrotal content pain. NSAIDs: non-steroidal anti-inflammatory drugs, CT: computed tomography, MRI: magnetic resonance imaging, A/P: abdomen or pelvis.
TREATMENT
The treatment of idiopathic CSCP remains a therapeutic dilemma, as there are no published data providing good evidence regarding reliable non-surgical interventions. Diagnostic and treatment recommendations are currently based on expert opinion derived from small cohort studies. However, pharmacotherapy should be considered as the first-line treatment, since it is the least invasive option. Pelvic floor physical therapy (PFPT), acupuncture, and a psychological evaluation may also be beneficial.
Antibiotics should be initiated if the patient shows any signs of orchitis or epididymitis. Trimethoprim/sulfamethoxazole or a quinolone antibiotic for 2∼4 weeks is preferred due to their lipophilic nature, which allows them to penetrate the testis and epididymis well. Antibiotics are not recommended to empirically treat men for an infection in the absence of any signs or symptoms.
Initial pharmacological therapy includes non-steroidal anti-inflammatory drugs (NSAIDs) over a period of 4 weeks [10]. We generally start with a daily oral dose of 200 mg of celecoxib or 600 mg of ibuprofen administered orally 3 times daily. Long-term treatment with narcotic agents is not recommended, as this does not address the underlying etiology and carries the risk of addiction. We occasionally offer a short course of narcotics for the temporary relief of CSCP. If NSAIDs do not work, we recommend using a tricyclic antidepressant (TCA). We recommend 10∼20 mg of amitriptyline nightly. Sinclair et al [21] found that 66.6% of patients with idiopathic testicular pain showed improved pain after 3 months of nortriptyline therapy in a trial of 6 patients.
TCAs work by inhibiting the reuptake of norepinephrine and serotonin in the brain. They also inhibit sodium channel blockers and L-type calcium channels, and this is thought to be responsible for their analgesic effect by modulating first-order neuron synapses with second-order neuron synapses in the dorsal horn of the spinal cord. Tertiary amines (amitriptyline and clomipramine) have been reported to be more effective for neuropathic pain than secondary amines (desipramine and nortriptyline) [22,23]. However, tertiary amines are also associated with more sedation and postural hypotension [24]. A TCA may take 2∼3 weeks from initiation of therapy to be effective.
After a month of TCA therapy without success, we recommend adding an anticonvulsant. Anticonvulsants have also been shown to work for neuropathic pain. The 2 mainstays of anticonvulsants used for neuropathic pain are gabapentin and pregabalin, due to the paucity of side effects in the older-generation anticonvulsants. We recommend adding 75 mg of pregabalin orally 3 times a day. Sinclair et al [21] found that 61.5% of patients with idiopathic testicular pain showed improved pain after 3 months of gabapentin therapy in a trial of 13 patients. The limiting factors of that study include a small sample size and its retrospective nature. Unfortunately, due to the rarity of this disorder, this treatment is unlikely to be validated in a prospective randomized controlled study. However, gabapentin has been validated in multiple large, randomized, placebo-controlled trials to relieve pain in patients with diabetic polyneuropathy, post-herpetic neuralgia, and other types of neuralgia [25,26,27]. The proposed mechanism of gabapentin as an analgesic is that it modulates the α-2-d subunit of N-type calcium channels, which affects the afferent pain fibers. Pharmacological therapy is considered to have failed if pain persists after pregabalin has been administered for 4 weeks. It is crucial to taper patients off TCAs.
PFPT is also beneficial for patients with pelvic floor dysfunction, especially those who have muscle dysfunction or myofascial trigger points. In our practice, we routinely recommend specialized PFPT to patients with CSCP if they have pain on a 360° digital rectal exam, as chronic testicular pain can lead to chronic pelvic pain or vice versa. Farrell et al [28] showed that after a mean of 12 PFPT sessions, 50% of patients showed improved pain, and 13.3% of patients had complete resolution of the pain. Following PFPT, fewer subjects required pain medication than prior to PFPT (44.0% vs. 73.3%, p=0.03).
Other nonsurgical techniques include pulsed radiofrequency of the spermatic cord and the genital branch of the genitofemoral nerve for PVPS if the patient receives temporary relief from a SCB [29,30]. This technique has only been reported in small non-randomized trials.
We also offer our patients a series of SCBs with local anesthetic agents with or without steroids to disrupt the afferent pain pathway in order to relieve CSCP. SCB can be both diagnostic and therapeutic. However, studies have demonstrated that this technique rarely provides long-term relief, and often only lasts for the duration of the local anesthetic [17]. The block is performed by isolating the spermatic cord at the inguinal-scrotal junction. A 27-gauge needle is then introduced into the spermatic cord at the level of the pubic tubercle. We typically use 20 mL of 0.25% bupivacaine hydrochloride for the initial cord block. The patient is instructed to call the office in 24 hours to report the duration and level of relief, if any, from the block. Should he experience >90% temporary pain relief, we offer a series of cord blocks every 2 weeks for 4∼5 blocks using 9 mL of 0.75% bupivacaine hydrochloride combined with 1 mL (10 mg) of triamcinolone acetonide. If there is no alleviation of pain with a well-placed injection, we do not recommend repeating this treatment. In our experience, this technique is rarely successful for long-term pain relief, especially when the duration of chronic pain exceeds 6 months.
Patients in whom the above approach does not work should be considered for surgical intervention. Surgical interventions include epididymectomy, the excision of sperm granuloma, vasectomy reversal if the patient previously had a vasectomy, MDSC, and orchiectomy. The success rates of these procedures remain unclear because of the availability of only small case series of men undergoing surgical treatment for PVPS. Table 1 [1,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] presents the success rates of surgical treatments for men with CSCP. No clear predictors of success for any procedure have been reported, except as listed below.
Table 1. Surgical treatments of chronic scrotal content pain described in the literature.
| Reference | No. of case | Follow-up (mo) | No. of case of success (%) | ||
|---|---|---|---|---|---|
| Complete | Partial | No relief | |||
| Microsurgical denervation: | |||||
| Devine and Schellhammer [31] | 2 | N/A | 2 (100) | 0 | 0 |
| Choa and Swami [32] | 4 | 18.5 | 4 (100) | 0 | 0 |
| Levine et al [33] | 8 | 16.6 | 7 (88) | 1 (12) | 0 |
| Ahmed et al [34] | 17 | N/A | 13 (76) | 4 (24) | 0 |
| Levine et al [33] | 33 | 20 | 25 (76) | 3 (9) | 5 (15) |
| Heidenreich et al [35] | 35 | 31.5 | 34 (97) | 1 (3) | 0 |
| Strom and Levine [36] | 95 | 20.3 | 67 (71) | 17 (17) | 11 (12) |
| Oliveira et al [37] | 10 | 24 | 7 (70) | 2 (20) | 1 (10) |
| Marconi et al [38] | 50 | 6 | 40 (80) | 6 (12) | 4 (8) |
| Laparoscopic denervation: | |||||
| Cadeddu et al [39] | 9 | 25.1 | N/A | 7 (78) | 2 (22) |
| Vasectomy reversal: | |||||
| Shapiro and Silber [40] | 6 | N/A | 6 (100) | 0 | 0 |
| Myers et al [41] | 32 | 29 | N/A | 24 (75) | 8 (25) |
| Nangia et al [42] | 13 | 18 | 9 (69) | 4 (31) | 0 |
| Horovitz et al [43] | 14 | 7 (50) | 6 (43) | 1 (7) | |
| Epididymectomy: | |||||
| Davis et al [1] | 10 | N/A | 1 (10) | 9 (90) | N/A |
| West et al [44] | 16 | 66 | N/A | 14 (88) | N/A |
| Calleary et al [45] | 15 | 15.6 | 3 (20) | 5 (33) | 7 (47) |
| Padmore et al [46] | 21 | 27 | 5 (24) | 9 (43) | 7 (33) |
| Sweeney et al [47] | 10 | N/A | 0 (0) | 7 (70) | 3 (30) |
| Chen and Ball [48] | 7 | N/A | 6 (86) | 0 (0) | 1 (14) |
| Lee et al [49] | 21 | 88.8 | 6 (29) | 6 (28.57) | 12 (57) |
| Resection of the genitofemoral nerve: | |||||
| Ducic and Dellon [50] | 4 | 6 | 4 (100) | 0 | 0 |
| Orchiectomy: | |||||
| Davis et al [1] | |||||
| Inguinal orchiectomy | 15 | N/A | 11 (73) | 4 (27) | 0 |
| Scrotal orchiectomy | 9 | N/A | 5 (55) | 3 (33) | 1 (22) |
| Yamamoto et al (inguinal) [51] | 4 | N/A | 3 (75) | 1 (25) | 0 |
| Costabile et al [52] | 10 | N/A | 0 (0) | 2 (20) | 8 (80) |
N/A: not available.
1. Microdenervation of the spermatic cord
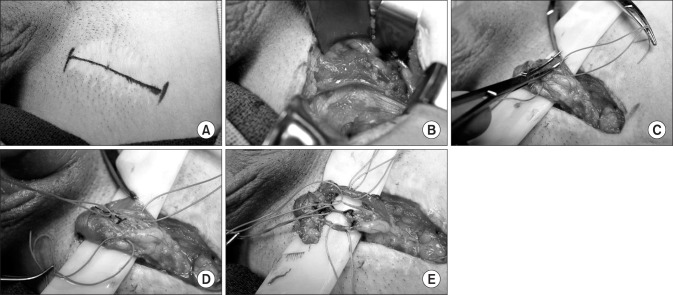
MDSC is a rather recent surgical option that has become more popular over the last 2 decades. A randomized controlled animal study showed a decrease in the median number of nerve fibers remaining around the vas deferens after MDSC compared to a sham treatment (MDSC, 3.5 nerves; sham, 15.5 nerves), showing that the majority of nerves were severed by MDSC [53]. The goal of the procedure involves transecting all the nerves in the spermatic cord while preserving all the arteries (testicular, cremasteric, and deferential) along with several lymphatic channels to reduce the likelihood of developing a hydrocele (Fig. 2) [54].
Fig. 2. Microdenervation of the spermatic cord. (A) Marking of inguinal site. (B) Dissection to expose the spermatic cord. (C) Spermatic cord supported by a Penrose drain with the cord fascia opened. (D) Arteries secured by a vessel loop. (E) After completion of the dissection, only the cremasteric artery, testicular artery, deferential artery, and lymphatics remain (top to bottom).
The patient should be made aware that the pain may persist, and occasionally worsen, following this procedure [36]. This is likely due to accessory fibers from the pudendal nerve, incomplete cord denervation, central nervous system sensitization, or malingering. Other complications include the development of a hydrocele (<1%) if the lymphatics of the testicles are injured and testicular atrophy (1%) if the arteries to the testicles are injured. Patients with bilateral scrotal content pain should undergo surgery on the more painful side first to avoid substantial prolonged scrotal edema and potential injury to both testicles, and also because the contralateral CSCP may resolve following MDSC.
Multiple studies have shown good success with MDSC for chronic testicular pain due to a variety of etiologies including idiopathic pain. A total of 152 out of 191 men (79.6%), based on all cases of MDSC in the English-language literature, experienced complete resolution of scrotal content pain. The most successful series was reported by Heidenreich et al [35], in which 34 of 35 patients (97%) experienced the complete resolution of pain and 1 patient experienced the partial resolution of pain following MDSC. In 2008, Strom and Levine [36] analyzed 95 patients who underwent MDSC, reporting durable relief in 71%. A further 17% of the patients reported partial relief, and 12% reported no change in pain, but no patients reported worsening pain. Marconi et al [38] published a recent series on MDSC for CSCP in which 50 patients were treated with MDSC. Forty patients (80.0%) were completely pain-free following the procedure and 4 patients (8.0%) did not experience any relief from surgery. The largest series to date was published by Calixte et al [13], who employed a modified robotic MDSC technique, and they found that 84% of patients experienced a reduction in pain (complete resolution in 63% and >50% resolution in 22%) in their sample of 772 men with a 1-year follow-up.
Larsen et al [55] also reported our institution's results of MDSC, showing that patients who had not undergone a prior attempt at surgical correction for scrotal content pain had a mean post-MDSC visual analog pain scale (VAPS) score of 2 (range, 0∼10) with an average pain decrease of 79%. In contrast, patients in whom prior surgical procedures failed, including epididymectomy, varicocelectomy, and vasectomy reversal, who then underwent MDSC reported a mean postoperative VAPS score of 3 (range, 0∼10) with an average decrease in pain of 67%. There was a complete response in 64% of patients in the surgery-naïve group compared to 50% in patients whom prior surgical correction for pain had failed. In a separate study, we also found that a positive response to a SCB, defined as at least a 50% temporary reduction of pain, was an independent predictor of successful MDSC [56].
2. Epididymectomy
Epididymectomy continues to remain a more popular approach than MDSC, especially in Europe. A survey among Swiss urologists in 2005 concluded that 74% of urologists would perform an epididymectomy, 7% would perform an inguinal orchiectomy, and 6% would perform MDSC for PVPS [5]. The reported success rates of epididymectomy range from 50% to 92%, and better results for relieving pain have been reported if a structural abnormality (cyst, granuloma, or mass) was noted in the epididymis on examination or ultrasonography [45,57,58,59]. When diffuse pain in the cord, epididymis, and/or testicle is noted during physical examination, this should lead to MDSC being performed rather than epididymectomy.
Multiple small series have been published on epididymectomy for CSCP. Chung et al [60] published a multicenter, randomized, controlled, single-blind study in 2013, in which 21 patients underwent epididymectomy alone and 22 patients underwent epididymectomy with the concurrent administration of hyaluronic acid and carboxymethyl cellulose to inhibit adhesion and fibrosis in cases of PVPS. At postoperative week 24, 15.8% of patients in the epididymectomy-only group were pain-free, whereas 57.1% of the patients who underwent an epididymectomy with the concurrent administration of hyaluronic acid and carboxymethyl cellulose were pain-free. A total of 31.6% of the patients from the epididymectomy-only group exhibited partial pain relief, in contrast to 9.5% of the patients who underwent epididymectomy with the concurrent administration of hyaluronic acid and carboxymethyl cellulose.
Epididymectomy is rarely performed in our practice, as most patients present with more diffuse pain, rather than pain limited to the epididymis. The ideal patient for an epididymectomy for CSCP is one in whom the pain is isolated to only the epididymis, especially when there is a cyst or granuloma identified on physical examination or ultrasonography.
3. Vasectomy reversal
Vasectomies are the most effective male contraceptive method available. It is estimated that 500,000 vasectomies are performed in the United States per annum, representing 10.2 of 1,000 men 25 to 49 years old [61]. Vasovasostomy may be beneficial in patients who have CSCP due to PVPS. Vasectomy reversal appears to be an intuitive solution to PVPS. The goal of the procedure is to relieve the pressure from the obstruction, thereby decreasing pain levels. Only data from small single-center studies are available. However, these studies show that up to 100% of patients experience some improvement in pain scores, and the complete resolution of pain ranges from 50% to 69% [40,41,42]. The benefits of this approach are the potential resolution of pain and preservation of all intrascrotal structures. However, this contradicts the purpose of the vasectomy, and the procedure may be costly and may not be covered by health insurance. Reasons that this approach may not succeed include non-obstructive etiologies of scrotal pain, such as nerve entrapment.
Polackwich et al [62] studied 26 patients who underwent a vasovasostomy and 7 patients who underwent an epididymovasostomy for PVPS. A total of 34% of patients had complete resolution of pain, and 59% of patients reported improvements in pain scores. Lee et al [63] identified 32 patients who underwent a vasectomy reversal for PVPS and noted that the improvement in the mean preoperative and postoperative VAPS was 6.00±1.25 (range, 4∼8) in the patency group (sperm in the ejaculate) and 4.43±0.98 (range, 3∼6) in the non-patency group (p=0.011). The authors concluded that there was a significant difference in pain reduction in patients who were patent following vasectomy reversal compared to those who remained obstructed. However, patients who remained obstructed had a decrease in VAPS, suggesting that the mechanism of action of PVPS may not be due to the obstructed vas deferens alone.
Horovitz et al [43] also published a series describing 14 patients who underwent vasectomy reversal for PVPS. Fifty percent of patients were rendered pain-free, and 93% showed improvements in pain. Myers et al [41] reviewed the records of 32 patients undergoing vasectomy reversal for PVPS and found that 24 patients experienced symptom relief after the initial procedure. Of the 8 men with recurrent or persistent pain, 6 underwent a second reversal, and 50% of those men subsequently experienced symptom relief.
4. Orchiectomy
Orchiectomy is considered to be the last resort in patients who do not respond to other means of therapy. There is little support for this procedure in the literature. Davis et al [1] reviewed 24 patients with chronic unilateral or bilateral orchialgia—not necessarily due to PVPS—who underwent inguinal orchiectomy. A total of 15 patients underwent inguinal orchiectomy, of whom 11 patients (73.3%) reported complete relief of pain, while 4 experienced partial relief. Of the 9 patients who underwent scrotal orchiectomy, 5 (55.6%) reported complete relief of pain, 3 (33.3%) had partial relief, and 1 (11.1%) reported no improvement [1]. Other studies have not shown the same success post-orchiectomy. Costabile et al [52] found that 80% of patients continued to have pain following orchiectomy for idiopathic chronic orchialgia. Based on these results, the authors recommended inguinal orchiectomy as the procedure of choice for the management of chronic testicular pain when other management is unsuccessful.
CONCLUSION
CSCP remains a challenge for clinicians, due to its poorly understood pathophysiology and variable response to current therapeutic options. Large, multicenter, well-constructed trials are essential in hopes of establishing level 1 evidence to facilitate a standardized algorithm to approach this problem more effectively. Increasing evidence indicates that psychological factors play an important role in genital pain when there is no identifiable organic cause, with the most consistent features being somatization disorder, major depression, anxiety, and sexual dysfunction. A multidisciplinary approach including pain clinics, psychologists/psychiatrists, and pelvic floor physical therapists, along with the urologist, is warranted before considering surgery. When nonsurgical treatments fail, MDSC remains a valuable approach, with high success rates and low complication rates, and should be considered for cases of CSCP that are refractory to medical therapy. MDSC appears to have the most success for patients who experience temporary relief from an SCB, and can significantly improve patients' quality of life and ability to return to daily activities.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contribution: Drafted the manuscript: Tan WP. Reviewed and edited the manuscript: Levine LA. Read and approved the final manuscript: all authors.
References
- 1.Davis BE, Noble MJ, Weigel JW, Foret JD, Mebust WK. Analysis and management of chronic testicular pain. J Urol. 1990;143:936–939. doi: 10.1016/s0022-5347(17)40143-1. [DOI] [PubMed] [Google Scholar]
- 2.Levine LA, Hoeh MP. Evaluation and management of chronic scrotal content pain. Curr Urol Rep. 2015;16:36. doi: 10.1007/s11934-015-0510-1. [DOI] [PubMed] [Google Scholar]
- 3.Christiansen CG, Sandlow JI. Testicular pain following vasectomy: a review of postvasectomy pain syndrome. J Androl. 2003;24:293–298. doi: 10.1002/j.1939-4640.2003.tb02675.x. [DOI] [PubMed] [Google Scholar]
- 4.Tatem A, Kovac JR. Chronic scrotal pain and microsurgical spermatic cord denervation: tricks of the trade. Transl Androl Urol. 2017;6:S30–S36. doi: 10.21037/tau.2017.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strebel RT, Leippold T, Luginbuehl T, Muentener M, Praz V, Hauri D. Chronic scrotal pain syndrome: management among urologists in Switzerland. Eur Urol. 2005;47:812–816. doi: 10.1016/j.eururo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Tan WP, Levine LA. An overview of the management of post-vasectomy pain syndrome. Asian J Androl. 2016;18:332–337. doi: 10.4103/1008-682X.175090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan WP, Levine LA. Micro-denervation of the spermatic cord for post-vasectomy pain management. Sex Med Rev. 2017 doi: 10.1016/j.sxmr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Ng CJ, Teo CH, Ho CC, Tan WP, Tan HM. The status of men's health in Asia. Prev Med. 2014;67:295–302. doi: 10.1016/j.ypmed.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Parekattil SJ, Cohen MS. Robotic surgery in male infertility and chronic orchialgia. Curr Opin Urol. 2010;20:75–79. doi: 10.1097/MOU.0b013e3283337aa0. [DOI] [PubMed] [Google Scholar]
- 10.Quallich SA, Arslanian-Engoren C. Chronic testicular pain in adult men: an integrative literature review. Am J Mens Health. 2013;7:402–413. doi: 10.1177/1557988313476732. [DOI] [PubMed] [Google Scholar]
- 11.Patel AP. Anatomy and physiology of chronic scrotal pain. Transl Androl Urol. 2017;6:S51–S56. doi: 10.21037/tau.2017.05.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oka S, Shiraishi K, Matsuyama H. Microsurgical anatomy of the spermatic cord and spermatic fascia: distribution of lymphatics, and sensory and autonomic nerves. J Urol. 2016;195:1841–1847. doi: 10.1016/j.juro.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 13.Calixte N, Brahmbhatt J, Parekattil S. Chronic testicular and groin pain: pathway to relief. Curr Urol Rep. 2017;18:83. doi: 10.1007/s11934-017-0722-7. [DOI] [PubMed] [Google Scholar]
- 14.Uçeyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Morgan RJ, Parry JR. Scrotal pain. Postgrad Med J. 1987;63:521–523. doi: 10.1136/pgmj.63.741.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belanger GV, VerLee GT. Diagnosis and surgical management of male pelvic, inguinal, and testicular pain. Surg Clin North Am. 2016;96:593–613. doi: 10.1016/j.suc.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Levine L. Chronic orchialgia: evaluation and discussion of treatment options. Ther Adv Urol. 2010;2:209–214. doi: 10.1177/1756287210390409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar P, Mehta V, Nargund VH. Clinical management of chronic testicular pain. Urol Int. 2010;84:125–131. doi: 10.1159/000277587. [DOI] [PubMed] [Google Scholar]
- 19.Kashanian JA, Mazur DJ, Hehemann MC, Morrison CD, Oberlin DT, Raup VT, et al. Scrotal ultrasound for pain: low frequency of absolute surgical indications. Urology. 2017;108:17–21. doi: 10.1016/j.urology.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 20.van Haarst EP, van Andel G, Rijcken TH, Schlatmann TJ, Taconis WK. Value of diagnostic ultrasound in patients with chronic scrotal pain and normal findings on clinical examination. Urology. 1999;54:1068–1072. doi: 10.1016/s0090-4295(99)00352-0. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair AM, Miller B, Lee LK. Chronic orchialgia: consider gabapentin or nortriptyline before considering surgery. Int J Urol. 2007;14:622–625. doi: 10.1111/j.1442-2042.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher RM. Management of neuropathic pain: translating mechanistic advances and evidence-based research into clinical practice. Clin J Pain. 2006;22:S2–S8. doi: 10.1097/01.ajp.0000193827.07453.d6. [DOI] [PubMed] [Google Scholar]
- 23.Jackson KC, 2nd, St Onge EL. Antidepressant pharmacotherapy: considerations for the pain clinician. Pain Pract. 2003;3:135–143. doi: 10.1046/j.1533-2500.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- 24.Sansone RA, Sansone LA. Pain, pain, go away: antidepressants and pain management. Psychiatry (Edgmont) 2008;5:16–19. [PMC free article] [PubMed] [Google Scholar]
- 25.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 26.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 27.Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–224. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 28.Farrell MR, Dugan SA, Levine LA. Physical therapy for chronic scrotal content pain with associated pelvic floor pain on digital rectal exam. Can J Urol. 2016;23:8546–8550. [PubMed] [Google Scholar]
- 29.Terkawi AS, Romdhane K. Ultrasound-guided pulsed radiofrequency ablation of the genital branch of the genitofemoral nerve for treatment of intractable orchalgia. Saudi J Anaesth. 2014;8:294–298. doi: 10.4103/1658-354X.130755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misra S, Ward S, Coker C. Pulsed radiofrequency for chronic testicular pain-a preliminary report. Pain Med. 2009;10:673–678. doi: 10.1111/j.1526-4637.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 31.Devine CJ, Jr, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchialgia. Trans Am Assoc Genitourin Surg. 1978;70:149–151. [PubMed] [Google Scholar]
- 32.Choa RG, Swami KS. Testicular denervation. A new surgical procedure for intractable testicular pain. Br J Urol. 1992;70:417–419. doi: 10.1111/j.1464-410x.1992.tb15800.x. [DOI] [PubMed] [Google Scholar]
- 33.Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol. 1996;155:1005–1007. doi: 10.1016/s0022-5347(01)66369-9. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed I, Rasheed S, White C, Shaikh NA. The incidence of post-vasectomy chronic testicular pain and the role of nerve stripping (denervation) of the spermatic cord in its management. Br J Urol. 1997;79:269–270. doi: 10.1046/j.1464-410x.1997.32221.x. [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich A, Olbert P, Engelmann UH. Management of chronic testalgia by microsurgical testicular denervation. Eur Urol. 2002;41:392–397. doi: 10.1016/s0302-2838(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 36.Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol. 2008;180:949–953. doi: 10.1016/j.juro.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira RG, Camara C, Alves Jde M, Coelho RF, Lucon AM, Srougi M. Microsurgical testicular denervation for the treatment of chronic testicular pain initial results. Clinics (Sao Paulo) 2009;64:393–396. doi: 10.1590/S1807-59322009000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marconi M, Palma C, Troncoso P, Dell Oro A, Diemer T, Weidner W. Microsurgical spermatic cord denervation as a treatment for chronic scrotal content pain: a multicenter open label trial. J Urol. 2015;194:1323–1327. doi: 10.1016/j.juro.2015.05.081. [DOI] [PubMed] [Google Scholar]
- 39.Cadeddu JA, Bishoff JT, Chan DY, Moore RG, Kavoussi LR, Jarrett TW. Laparoscopic testicular denervation for chronic orchalgia. J Urol. 1999;162:733–735. doi: 10.1097/00005392-199909010-00028. discussion 735-6. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro EI, Silber SJ. Open-ended vasectomy, sperm granuloma, and postvasectomy orchialgia. Fertil Steril. 1979;32:546–550. doi: 10.1016/s0015-0282(16)44357-8. [DOI] [PubMed] [Google Scholar]
- 41.Myers SA, Mershon CE, Fuchs EF. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;157:518–520. [PubMed] [Google Scholar]
- 42.Nangia AK, Myles JL, Thomas AJ., JR Vasectomy reversal for the post-vasectomy pain syndrome: a clinical and histological evaluation. J Urol. 2000;164:1939–1942. [PubMed] [Google Scholar]
- 43.Horovitz D, Tjong V, Domes T, Lo K, Grober ED, Jarvi K. Vasectomy reversal provides long-term pain relief for men with the post-vasectomy pain syndrome. J Urol. 2012;187:613–617. doi: 10.1016/j.juro.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 44.West AF, Leung HY, Powell PH. Epididymectomy is an effective treatment for scrotal pain after vasectomy. BJU Int. 2000;85:1097–1099. doi: 10.1046/j.1464-410x.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 45.Calleary JG, Masood J, Hill JT. Chronic epididymitis: is epididymectomy a valid surgical treatment? Int J Androl. 2009;32:468–472. doi: 10.1111/j.1365-2605.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 46.Padmore DE, Norman RW, Millard OH. Analyses of indications for and outcomes of epididymectomy. J Urol. 1996;156:95–96. [PubMed] [Google Scholar]
- 47.Sweeney P, Tan J, Butler MR, McDermott TE, Grainger R, Thornhill JA. Epididymectomy in the management of intrascrotal disease: a critical reappraisal. Br J Urol. 1998;81:753–755. doi: 10.1046/j.1464-410x.1998.00636.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen TF, Ball RY. Epididymectomy for post-vasectomy pain: histological review. Br J Urol. 1991;68:407–413. doi: 10.1111/j.1464-410x.1991.tb15362.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee JY, Lee TY, Park HY, Choi HY, Yoo TK, Moon HS, et al. Efficacy of epididymectomy in treatment of chronic epididymal pain: a comparison of patients with and without a history of vasectomy. Urology. 2011;77:177–182. doi: 10.1016/j.urology.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Ducic I, Dellon AL. Testicular pain after inguinal hernia repair: an approach to resection of the genital branch of genitofemoral nerve. J Am Coll Surg. 2004;198:181–184. doi: 10.1016/j.jamcollsurg.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto M, Hibi H, Katsuno S, Miyake K. Management of chronic orchialgia of unknown etiology. Int J Urol. 1995;2:47–49. doi: 10.1111/j.1442-2042.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 52.Costabile RA, Hahn M, McLeod DG. Chronic orchialgia in the pain prone patient: the clinical perspective. J Urol. 1991;146:1571–1574. doi: 10.1016/s0022-5347(17)38169-7. [DOI] [PubMed] [Google Scholar]
- 53.Laudano MA, Osterberg EC, Sheth S, Ramasamy R, Sterling J, Mukherjee S, et al. Microsurgical denervation of rat spermatic cord: safety and efficacy data. BJU Int. 2014;113:795–800. doi: 10.1111/bju.12421. [DOI] [PubMed] [Google Scholar]
- 54.Levine LA. Microsurgical denervation of the spermatic cord. J Sex Med. 2008;5:526–529. doi: 10.1111/j.1743-6109.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 55.Larsen SM, Benson JS, Levine LA. Microdenervation of the spermatic cord for chronic scrotal content pain: single institution review analyzing success rate after prior attempts at surgical correction. J Urol. 2013;189:554–558. doi: 10.1016/j.juro.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 56.Benson JS, Abern MR, Larsen S, Levine LA. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med. 2013;10:876–882. doi: 10.1111/j.1743-6109.2012.02937.x. [DOI] [PubMed] [Google Scholar]
- 57.Sweeney CA, Oades GM, Fraser M, Palmer M. Does surgery have a role in management of chronic intrascrotal pain? Urology. 2008;71:1099–1102. doi: 10.1016/j.urology.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 58.Granitsiotis P, Kirk D. Chronic testicular pain: an overview. Eur Urol. 2004;45:430–436. doi: 10.1016/j.eururo.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Kavoussi PK, Costabile RA. Orchialgia and the chronic pelvic pain syndrome. World J Urol. 2013;31:773–778. doi: 10.1007/s00345-013-1092-5. [DOI] [PubMed] [Google Scholar]
- 60.Chung JH, Moon HS, Choi HY, Jeong TY, Ha US, Han JH, et al. Inhibition of adhesion and fibrosis improves the outcome of epididymectomy as a treatment for chronic epididymitis: a multicenter, randomized controlled, single-blind study. J Urol. 2013;189:1730–1734. doi: 10.1016/j.juro.2012.11.168. [DOI] [PubMed] [Google Scholar]
- 61.Barone MA, Hutchinson PL, Johnson CH, Hsia J, Wheeler J. Vasectomy in the United States, 2002. J Urol. 2006;176:232–236. doi: 10.1016/S0022-5347(06)00507-6. discussion 236. [DOI] [PubMed] [Google Scholar]
- 62.Polackwich AS, Tadros NN, Ostrowski KA, Kent J, Conlin MJ, Hedges JC, et al. Vasectomy reversal for postvasectomy pain syndrome: a study and literature review. Urology. 2015;86:269–272. doi: 10.1016/j.urology.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 63.Lee JY, Chang JS, Lee SH, Ham WS, Cho HJ, Yoo TK, et al. Efficacy of vasectomy reversal according to patency for the surgical treatment of postvasectomy pain syndrome. Int J Impot Res. 2012;24:202–205. doi: 10.1038/ijir.2012.17. [DOI] [PubMed] [Google Scholar]