Abstract
Purpose
Gene therapy, stem cell therapy, and low-energy extracorporeal shockwave therapy (ESWT) have been investigated as treatments for refractory erectile dysfunction (ED), but inconclusive evidence has been obtained. We investigated the effect of a next-generation electromagnetic cylinder ESWT device on an animal model of ED.
Materials and Methods
Diabetes mellitus (DM)-induced rats were divided into 3 groups: group 1, control; group 2, DM; and group 3, DM+ESWT. Rats were treated with ESWT 3 times a week for 2 weeks. After the treatment course, intracavernous pressure was measured and the corpus cavernosum and cavernous nerve were evaluated.
Results
In the DM group, all parameters predicted to be significantly lower in the ED model had statistically significantly decreased (p<0.01). As a measurement of erectile function, intracavernous pressure was evaluated. The DM+ESWT group exhibited significantly restored erectile function compared to the DM group (p<0.05). Moreover, ESWT treatment restored smooth muscle content, as assessed by Masson's trichrome staining (p<0.05). Finally, corporal tissue and the dorsal nerve were evaluated by immunohistochemistry, Western blotting, and ELISA. After ESWT treatment, vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), platelet endothelial cell adhesion molecule-1, cyclic guanosine monophosphate, and neuronal nitric oxide synthase (nNOS) expression levels were restored to levels in the DM group (p<0.05).
Conclusions
Electromagnetic cylinder ESWT device resulted in increased VEGF, nNOS, and eNOS expression; reduced smooth muscle atrophy; and increased endothelial cell regeneration in a DM-associated ED model. Our data suggest that safe and effective application could be possible in future clinical studies.
Keywords: Animals, Diabetes mellitus, Erectile dysfunction, Vascular endothelial growth factor
INTRODUCTION
With the development of modern medicine, the human life span has been increasing and, with it, concerns related to sexual health and aging [1]. Among these concerns, erectile dysfunction (ED) is a relatively common health problem that impacts not only the quality of life of the affected man but also his spouse [2]. Neurologic injury secondary to prostatectomy and vascular lesions secondary to diabetes, among other causes of ED, are unlikely to respond well to phosphodiesterase type 5 inhibitors (PDE-5I) and are prone to developing into refractory disease [3]. PDE-5I is the most common treatment for ED. While some methods, such as the penile prosthesis, are available for patients who do not respond to PDE-5I, these methods are invasive and nonphysiologic. Therefore, various alternative therapies are being studied, such as gene therapy, stem cell therapy, and extracorporeal shockwave therapy (ESWT) [4,5].
Renal lithotripsy was introduced in the 1980s as a shockwave therapy and has since been studied worldwide for potential applications to other diseases [6,7,8]. Shockwaves are an acoustic wave type that transmit energy and have both destructive and regenerative effects on human tissues. In 1990, Young and Dyson [9] found that shockwaves stimulated angiogenesis, which aids in the expression of vascular endothelial growth factor (VEGF) [10,11]. Since this study, many clinicians have started using shockwaves to treat coronary artery disease, bone fracture, calcifying tendinitis, and diabetic foot ulcer. Angiogenesis is also a key factor in ED. Several clinical studies have been conducted and have shown good results for shockwave therapy [12,13]. The mechanism is believed to involve induction of mesenchymal stem cell recruitment, expression of growth factors, and expression of endothelial nitric oxide synthase (eNOS), which promotes angiogenesis; however, this mechanism has not yet been firmly established [14,15,16].
ESWT can be produced by several types of generators, including electromagnetic, electrohydraulic, and piezoelectric. The next-generation ESWT MT2000H (Urontech Korea, Hwaseong, Korea) is an electromagnetic cylinder ESWT device that has more advantages than the electrohydraulic ESWT device, which is the only device previously used in ED treatment.
To overcome the limitations of existing ESWT generators, we investigated here the effect of ESWT in an animal model of diabetes mellitus (DM)-induced ED using a next-generation electromagnetic cylinder ESWT device.
MATERIALS AND METHODS
1. Ethics statement
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of The Catholic University of Korea (approval number: CUMC-2016-0310-01).
2. Experimental animal and study design
Eight-week-old male Sprague-Dawley rats weighing 270~300 g (Orient Bio Co., Seongnam, Korea) were used in this study. DM was induced by an intraperitoneal injection of streptozotocin (STZ) (50 mg/kg in sodium citrate buffer, pH 4.5). After 72 hours, induction of DM was confirmed by testing blood samples obtained from tail veins for blood glucose levels using a commercially available kit. Rats with blood glucose above 300 mg/dL were considered diabetic. The rats were randomly divided into 3 groups (n=10 per group): normal, DM, and DM+ESWT. All rats underwent intracavernosal pressure (ICP) measurement and were sacrificed under anesthesia after the treatments.
3. Description of extracorporeal shockwave therapy device
An MT2000H ESWT electromagnetic generator was used. Generally, 3 types of ESWT devices are used in clinics: electrohydraulic, electromagnetic, and piezoelectric. Each device generates a shockwave through a different technique. In the electromagnetic technique, electric current is passed through a coil to produce a strong magnetic field. The amplitude of the focused waves increases nonlinearly when the acoustic wave propagates toward the focal point [17]. The coaxial configuration of an imaging device and a shockwave device has the advantage of more accurate focusing than the 2 devices used separately. Electromagnetic cylinder devices have many advantages, including power, reproducibility, dynamic range, lifetime, and precise identification of the targeted structures within the surrounding anatomy [18].
4. Extracorporeal shockwave therapy treatment
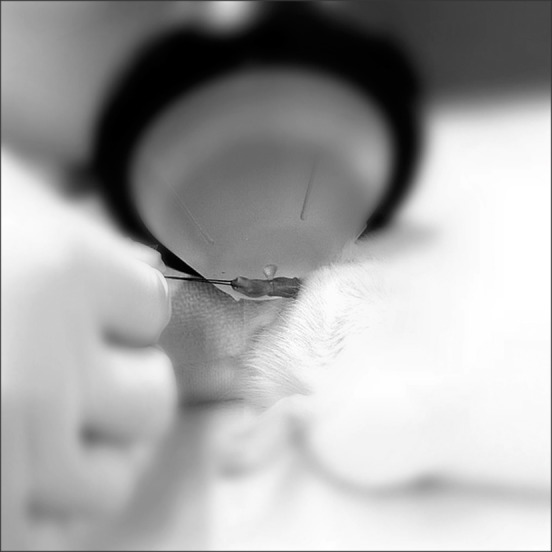
After 4 weeks of DM induction, rats in the DM+ESWT group underwent shockwave treatment. Under anesthesia, each rat was placed in the supine position, shaved, and the prepuce was degloved. Ultrasound gel was applied to the penis and a MT2000H shockwave applicator was placed at the penis. A total of 300 shocks were delivered at an energy level of 0.1 mL/mm2 and a frequency of 120 shocks/minute. This procedure was performed 3 times a week for 2 weeks, which is similar to the treatment course used for patients with ED in clinical practice (Fig. 1).
Fig. 1. Extracorporeal shockwave therapy (MT2000H) application to the rat penis. Under anesthesia, the prepuce was degloved and sutured to fix it.
5. Intracavernosal pressure measurement
Erectile function was assessed 1 week after the ESWT treatment course for all 3 groups (n=10 per group). After placing the rat in the supine position, the corpus cavernosum and crus of the penis were exposed and the pelvic ganglion was exposed through abdominal incision.
The carotid artery and cavernous nerve were exposed to detect the mean arterial pressure (MAP) and ICP. PE-50 tubing was inserted into the carotid artery to measure the MAP. At the same time, a 23-gauge butterfly needle filled with heparin was inserted in the corpus cavernosum, and then connected to a pressure transducer (Grass model S48K, Grass Instrument Division; Astro-Med, Inc., West Warwick, RI, USA) to measure the ICP. A bipolar electrical stimulator was used to stimulate the pelvic ganglion and the cavernosal nerve for 50 seconds at 10 V and 0.5 ms at 2.4 mA, respectively. ICP and MAP were recorded by a pressure transducer (Grass Stimulator Recorder, model S48K, Grass Instrument Division, Astro-Med Inc.). The maximal ICP was calculated and recorded on a computer with a PowerLab commercial data acquisition system (AD Instruments, Dunedin, New Zealand). BD Intramedic PE-50 tubing (BD, Franklin Lakes, NJ, USA) was used to measure MAP. The ICP/MAP ratio and ratio of the area under the curve (AUC) to MAP was measured to assess erectile function. After the stimulation test, the penis was harvested from each rat. Samples were fixed in 4% paraformaldehyde for 24 hours at 4℃ before paraffin blocks were created.
6. Masson's trichrome staining
Masson's trichrome staining was performed on corporal tissue sections. The middle portions of the penile samples were fixed overnight in 4% formalin, washed, and stored in 70% alcohol at 4℃ until processing for paraffin-embedded tissue sectioning (4 µm). After staining, the color distribution in the tissue was measured using Image Pro Plus version 5.0 (Media Cybernetics, Silver Spring, MD, USA). After the entire color distribution of the image was calculated, the muscle tissue was expressed using the color blue. The mean ratio of collagen and muscle fiber was calculated.
7. Immunohistochemical analysis of vascular endothelial growth factor and neuronal nitric oxide synthase expression
The corporal tissue and dorsal nerve of the penis tissues were immunostained with the following primary antibodies: anti-neuron-specific β-III tubulin (diluted 1:200; Abcam, Cambridge, MA, USA) and anti-neuronal nitric oxide synthase (nNOS, diluted 1:200; Santa Cruz Biotechnologies, Santa Cruz, CA, USA) for the dorsal penile nerve and anti-VEGF (diluted 1:200; Santa Cruz Biotechnologies) for the corporal tissue. Sections were mounted with 4′,6-diamidino-2-phenylindol to stain nuclei. Digital images were obtained using a Zeiss LSM 510 Meta confocal microscope (Zeiss, Oberkochen, Germany). The mean staining intensity was calculated using a ZEN 2009 instrument (Zeiss).
8. Western blot analysis of endothelial nitric oxide synthase and platelet endothelial cell adhesion molecule-1 expression
Corpus cavernosum tissue was homogenized using RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) containing an ethylenediam inetetraacetic acid-free protease inhibitor cocktail and a phosphatase inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). The resultant lysates were clarified by centrifugation at 12,000×g for 10 minutes at 4℃ and their supernatants extracted. Proteins were electrophoresed on NuPAGE 4% to 12% Bis-Tris gels (Invitrogen, Carlsbad, CA, USA) and transferred onto nitrocellulose membranes. The membranes were incubated with primary antibodies against platelet endothelial cell adhesion molecule-1 (PECAM-1) (diluted 1:500; Abcam), endothelial nitric oxide synthase (diluted 1:1,000; ab5589; Abcam), or phosphorylated eNOS (Ser1177, diluted 1:500; Cell Signaling Technology). Next, the membranes were incubated with secondary antibodies conjugated to horseradish peroxidase for 1 hour at room temperature. Enhanced chemiluminescence reagents (Amersham, Arlington Heights, IL, USA) were used for densitometric analysis of band intensity.
9. Cyclic guanosine monophosphate assay
Corporal tissues were treated with 350 mL of 0.1 M HCL, after which silica beads were added (BioSec Enviro, Inc., Guelph, ON, Canada). The resulting samples were processed through a homogenizer (Precellys 24; Bertin Technologies, Montigny-le-Bretonneux, France) andspun by centrifugation at 12,000×g for 10 minutes at 4℃, after which their supernatants were extracted. A cyclic guanosine monophosphate (cGMP) direct immunoassay kit (K372-100; BioVision, Mountain View, CA, USA) was used to measure corporal cGMP levels.
10. Statistical analysis
All data are presented as mean±standard deviation (SD). Data were analyzed using IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA). Data are expressed as mean±standard error. Results from different groups were compared using the Mann-Whitney U-test. A p-value less than 0.05 was considered tobe statistically significant.
RESULTS
1. In vivo assessment of erectile function
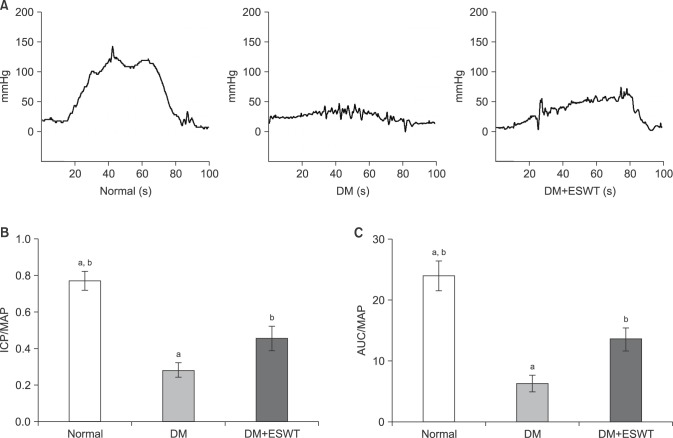
The DM-induced rats showed significantly decreased ICP values, and ICP/MAP and AUC/MAP ratios compared to the control rats; however, these values and ratios were significantly restored by ESWT, albeit only partially (Fig. 2). The mean peak ICP values for the control, DM, and DM+ESWT groups were 105.7±25.03 mmHg, 40.74±15.39 mmHg, and 60.84±24.09 mmHg, respectively. The mean ICP/MAP ratios±SDs for the control, DM, and DM+ESWT groups were 0.76±0.12, 0.27±0.09, and 0.45±0.16, respectively. The mean ICP/MAP ratio was significantly lower in the DM group compared to the control group (p<0.01). The ESWT treatment significantly improved the ratio in the DM rats relative to the untreated DM group (p<0.05), but the ESWT group did not recover to the control group level (p<0.01).The mean ratio of AUC/MAP±SD in the control, DM, and DM+ESWT groups were 23.85±5.88, 6.15±3.27, and 13.42±4.54. After DM induction, the ratio decreased significantly (p<0.05). ESWT significantly increased the ratio from the DM level but did not reach the control group level (p<0.05, p<0.05, respectively) (Fig. 2).
Fig. 2. Intracavernosal pressure (ICP), ICP/mean arterial pressure (MAP), and area under the curve (AUC)/MAP measurements of each group. (A) ICP recordings after cavernous nerve stimulation. (B) Comparison of ICP/MAP ratios of the different groups. (C) Comparison of AUC/MAP ratios. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.
2. Masson's trichrome staining
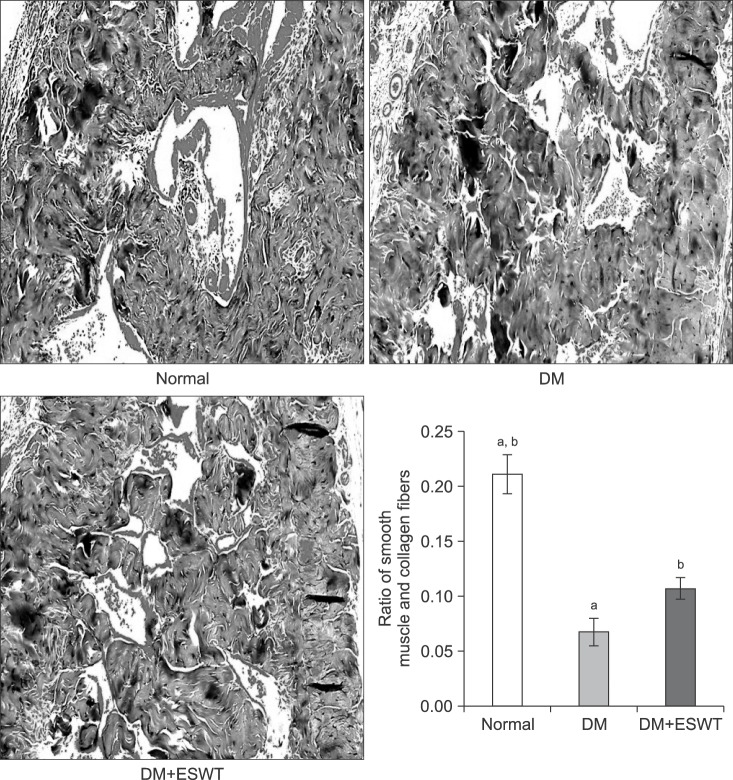
The DM group showed less corporal smooth muscle tissue and higher collagen deposition than the control group (Fig. 3). The mean muscle/collagen ratios±SDs for the control, DM, and DM+ESWT groups were 0.21±0.04, 0.06±0.02, and 0.10±0.02, respectively. The muscle/collagen ratio was significantly lower in the DM group compared to the control group (p<0.01), whereas ESWT treatment significantly improved this ratio in DM rats (p<0.05). But the ESWT treatment did not return the DM rats to the control group level (p<0.01).
Fig. 3. Smooth muscle content evaluation of each group by Masson's trichrome staining (×100). The graph shows comparison of the smooth muscle and collagen fiber ratios of the different groups. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.
3. Immunohistochemical analysis of vascular endothelial growth factor expression in corporal tissue
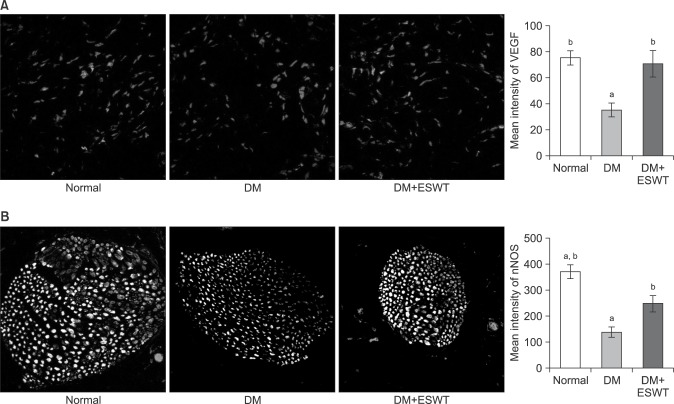
We next performed immunohistochemical staining to assess the expression of VEGF in corporal tissue (Fig. 4). The mean intensities±SDs of VEGF staining for the control, DM, and DM+ESWT groups were 75.30±13.21, 35.21±13.26, and 70.73±24.94, respectively. VEGF staining was significantly lower in the DM group compared to the control group (p<0.01), while ESWT treatment significantly improved VEGF staining in DM rats (p<0.05) to levels similar to those in normal control rats, that is, with no statistically significant difference.
Fig. 4. (A) Immunohistochemical staining (×200) of vascular endothelial growth factor (VEGF) expression in the corpus cavernosum of each group. The graph shows comparison of VEGF expression in the different groups. (B) Immunohistochemical staining (×400) of neuronal nitric oxide synthase (nNOS) expression in the dorsal penile nerve of each group. The graph shows a comparison of nNOS expression levels of the different groups. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.
4. Immunohistochemical analysis of neuronal nitric oxide synthase expression in the dorsal penile nerve
Next, nNOS expression in the penile dorsal nerve was analyzed by immunohistochemical staining (Fig. 4). STZ treatment significantly decreased the amount of nNOS in the nerve. The mean intensities±SDs of nNOS-positive areas for the control, DM, and DM+ESWT groups were 370.68±98.61, 136.91±40.38, and 247.44±70.65, respectively (Fig. 4). nNOS expression was significantly decreased in the DM group compared with the control group (p<0.01), whereas nNOS expression was significantly greater in the DM+ESWT group than in the DM group (p<0.05). ESWT treatment was not able to bring the DM rats back up to the control group nNOS level (p<0.05).
5. Western blot analysis of endothelial nitric oxide synthase and platelet endothelial cell adhesion molecule-1 levels in corporal tissue
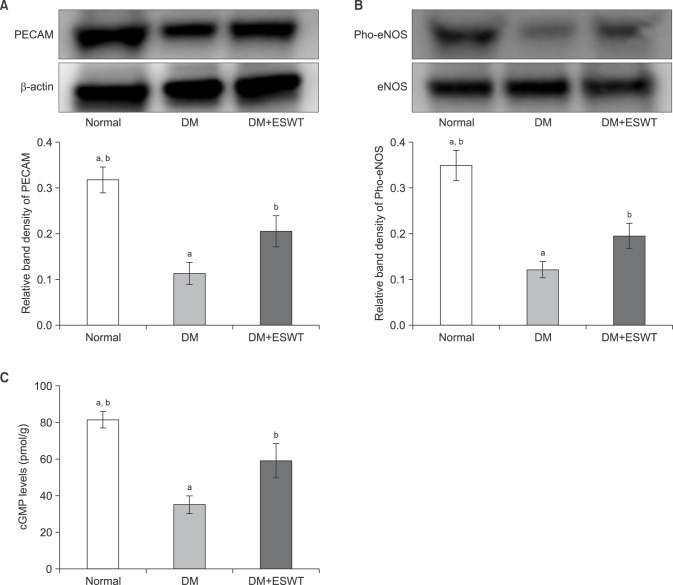
Western blotting was next used to measure eNOS and PECAM-1 expression levels in corporal tissue. eNOS expression was not statistically significantly different among any of the 3 groups. However, pho-eNOS expression was significantly lower in the DM group compared to the control group (p<0.01); of note, pho-eNOS levels were significantly higher with ESWT (p<0.05) but did not reach the level of the control group (p<0.05). The mean densities± SDs of pho-eNOS/eNOS for the control, DM, and DM+ESWT groups were 0.34±0.08, 0.11±0.04, and 0.19±0.06, respectively (Fig. 5).The mean densities±SDs of the PECAM/β-actin ratio for the control, DM, and DM+ESWT groups were 0.31±0.06, 0.11±0.05, and 0.20±0.08, respectively (Fig. 5). PECAM expression was significantly decreased in the DM group compared to the control group (p<0.01), whereas ESWT treatment significantly improved PECAM expression in DM rats (p<0.05) but the treatment did not return PECAM to the level of the control group (p<0.05).
Fig. 5. (A) Western blot images of platelet endothelial cell adhesion molecule-1 (PECAM-1).The graph shows a comparison of the relative PECAM-1/β-actin band densities of the different groups. (B) Pho-endothelial nitric oxide synthase (eNOS) expression in the corporal tissue of each group assessed by Western blotting. The graph shows a comparison of the relative band densities of Pho-eNOS/eNOS. (C) Cyclic guanosine monophosphate (cGMP) levels in the corporal tissue of each group as assessed by ELISA. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.
6. Enzyme-linked immunosorbent assay cyclic guanosine monophosphate assay
STZ treatment significantly decreased the cGMP level in corporal tissue (p<0.01); however, ESWT restored cGMP to a level closer to that in normal control rats (p<0.05) (Fig. 5) but not as high as the level in the control group (p<0.05). The mean densities±SDs of cGMP for the control, DM, and DM+ESWT groups were 81.16±10.97, 34.73±12.03, and 58.77±23.06, respectively (Fig. 5).
DISCUSSION
While the management of ED has undergone a great deal of development over the past decade, treatment of ED originating from diabetes is still difficult. Stem cell therapy and ESWT have been proposed as therapeutic strategies for ED in such cases [16]. ED can be induced in diabetic rats using STZ. ESWT has been shown to induce regeneration of nerves, endothelium, and smooth muscle, all of which are damaged by diabetes, thereby restoring erectile function [12]. Moreover, ESWT was shown to restore various parameters that were decreased in an animal model of DM-induced ED. However, electrohydraulic ESWT, the technique currently used, has a number of disadvantages, such as irregular shockwaves, constant focal zones, and the need for frequent replacement of consumables. By performing preclinical experiments, we investigated here the effects of an electromagnetic cylinder ESWT device that was developed to overcome these disadvantages.
The therapeutic mechanism of ESWT has been shown to be associated with increased expression of VEGF [10,11], which is thought to protect endothelial cells from apoptosis [19]. VEGF expression and ICP/MAP ratios were better for the DM+ESWT group compared to the DM group in our study, suggesting that ESWT improves penile blood flow. Elevated VEGF has been shown to induce phosphorylation of eNOS and to elevate cGMP levels [20]. Nitric oxide (NO) is the most important neurotransmitter for smooth muscle relaxation in the corpus cavernosum and acts as an endothelium-derived relaxation factor [21,22]. NO controls penile erection at the level of the paraventricular nucleus [23]. In addition, diabetic rats show statistically significantly reduced nNOS in penile tissue; a similar finding emerged from clinical studies [24,25,26]. We observed a statistically significant increase in nNOS in penile dorsal nerves and elevated eNOS and cGMP levels in corporal tissue in the shockwave-treated group compared to the diabetic group.
Several studies have observed endothelial injury in the corporal tissue in diabetic rats; similar results were obtained in human trials and were associated with ED [25]. Moreover, the diabetic rat model has been shown to have significantly reduced endothelial content [27]. We performed immunohistochemical staining to measure PECAM-1 expression and thereby assess recovery of erectile function in relation to endothelial cell regeneration [28].
The purpose of this study was to investigate the effect of an electromagnetic cylinder ESWT device on ED in diabetic rats. While we observed parameters related to the mechanism by which ESWT treats ED and confirmed that ESWT treatment improves ED, we could not find any new mechanisms. Second, the purpose of our study was to evaluate the effect of a new type of ESWT compared with conventional electrohydraulic and piezoelectric methods, but we were not able to include more groups that were treated with conventional methods as a comparison. The third limitation is that the optimal wave length and frequency of ESWT treatment have not yet been established, and various treatment parameters have not been tried and compared systematically. Fourth, there were no gross specific findings that differed between groups, and we note that separate safety tests were not carried out. Fifth, longer follow-up results are not available.
While several studies have compared the efficacies of electrohydraulic and electromagnetic ESWT for treating ureter stones, the results obtained with the 2 types of generators were not significantly different [29,30]. However, we anticipate that electromagnetic ESWT will be found to have theoretical advantages that could be applicable in clinical practice in the future.
CONCLUSIONS
We confirmed the efficacy of an electromagnetic cylinder ESWT device using a DM-associated ED model. This device restored erectile components (nerves, endothelium, and smooth muscle), albeit partially, in the ED animal model using DM rats. Safe and effective application of this technique will be tested in future clinical studies.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant HI17C1944).
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contribution: Research conception & design: Jeong HC, Kim KS, Kim SW. Performing the experiments: Jeon SH, Zhou GQ. Data acquisition: Bashraheel F, Choi SW. Data analysis and interpretation: Jeong HC, Kim SJ. Statistical analysis: Bae WJ, Cho HJ. Drafting of the manuscript: Jeong HC. Critical revision of the manuscript: Ha US, Hong SH, Lee JY, Moon DG. Receiving grant: Kim SW. Approval of final manuscript: all authors.
References
- 1.Montorsi F, Adaikan G, Becher E, Giuliano F, Khoury S, Lue TF, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010;7:3572–3588. doi: 10.1111/j.1743-6109.2010.02062.x. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159–166. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorsey P, Keel C, Klavens M, Hellstrom WJ. Phosphodiesterase type 5 (PDE5) inhibitors for the treatment of erectile dysfunction. Expert Opin Pharmacother. 2010;11:1109–1122. doi: 10.1517/14656561003698131. [DOI] [PubMed] [Google Scholar]
- 4.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum Gene Ther. 2006;17:1165–1176. doi: 10.1089/hum.2006.17.1165. [DOI] [PubMed] [Google Scholar]
- 5.Deng W, Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ. Gene and stem cell therapy for erectile dysfunction. Int J Impot Res. 2005;17(Suppl 1):S57–S63. doi: 10.1038/sj.ijir.3901430. [DOI] [PubMed] [Google Scholar]
- 6.Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;2:1265–1268. doi: 10.1016/s0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- 7.Chaussy C, Schmiedt E. Shock wave treatment for stones in the upper urinary tract. Urol Clin North Am. 1983;10:743–750. [PubMed] [Google Scholar]
- 8.Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann B, Walther V. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol. 1982;127:417–420. doi: 10.1016/s0022-5347(17)53841-0. [DOI] [PubMed] [Google Scholar]
- 9.Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol. 1990;16:261–269. doi: 10.1016/0301-5629(90)90005-w. [DOI] [PubMed] [Google Scholar]
- 10.Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J. 2003;26:220–232. [PubMed] [Google Scholar]
- 11.Nurzynska D, Di Meglio F, Castaldo C, Arcucci A, Marlinghaus E, Russo S, et al. Shock waves activate in vitro cultured progenitors and precursors of cardiac cell lineages from the human heart. Ultrasound Med Biol. 2008;34:334–342. doi: 10.1016/j.ultrasmedbio.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, Lue TF, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardi Y, Appel B, Kilchevsky A, Gruenwald I. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–1775. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 14.Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 15.Lee MC, El-Sakka AI, Graziottin TM, Ho HC, Lin CS, Lue TF. The effect of vascular endothelial growth factor on a rat model of traumatic arteriogenic erectile dysfunction. J Urol. 2002;167:761–767. doi: 10.1016/S0022-5347(01)69141-9. [DOI] [PubMed] [Google Scholar]
- 16.Lin CS, Xin ZC, Wang Z, Deng C, Huang YC, Lin G, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21:343–351. doi: 10.1089/scd.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogden JA, Tóth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. 2001;(387):8–17. doi: 10.1097/00003086-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Onose G, Daia Chendreanu C, Haras M, Spinu A, Andone I. Extracorporeal shock wave therapy: a new “wave” (also) in physiatry? Practica Medicala. 2011;6:35–42. [Google Scholar]
- 19.Park K, Ahn KY, Kim MK, Lee SE, Kang TW, Ryu SB. Intracavernosal injection of vascular endothelial growth factor improves erectile function in aged rats. Eur Urol. 2004;46:403–407. doi: 10.1016/j.eururo.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka M, Shirai M, Shiina H, Tanaka Y, Enokida H, Tsujimura A, et al. Vascular endothelial growth factor restores erectile function through inhibition of apoptosis in diabetic rat penile crura. J Urol. 2005;173:318–323. doi: 10.1097/01.ju.0000141586.46822.44. [DOI] [PubMed] [Google Scholar]
- 21.Lee NH, Seo CS, Lee HY, Jung DY, Lee JK, Lee JA, et al. Hepatoprotective and antioxidative activities of cornus officinalis against acetaminophen-induced hepatotoxicity in mice. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/804924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Xu J, Li L, Wang P, Ji X, Ai H, et al. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res Bull. 2010;83:196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Melis MR, Succu S, Mauri A, Argiolas A. Nitric oxide production is increased in the paraventricular nucleus of the hypothalamus of male rats during non-contact penile erections and copulation. Eur J Neurosci. 1998;10:1968–1974. doi: 10.1046/j.1460-9568.1998.00207.x. [DOI] [PubMed] [Google Scholar]
- 24.Dashwood MR, Crump A, Shi-Wen X, Loesch A. Identification of neuronal nitric oxide synthase (nNOS) in human penis: a potential role of reduced neuronally-derived nitric oxide in erectile dysfunction. Curr Pharm Biotechnol. 2011;12:1316–1321. doi: 10.2174/138920111798280965. [DOI] [PubMed] [Google Scholar]
- 25.Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25:129–136. doi: 10.1016/j.jdiacomp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhou F, Xin H, Liu T, Li GY, Gao ZZ, Liu J, et al. Effects of icariside II on improving erectile function in rats with streptozotocin-induced diabetes. J Androl. 2012;33:832–844. doi: 10.2164/jandrol.111.015172. [DOI] [PubMed] [Google Scholar]
- 27.Albersen M, Lin G, Fandel TM, Zhang H, Qiu X, Lin CS, et al. Functional, metabolic, and morphologic characteristics of a novel rat model of type 2 diabetes-associated erectile dysfunction. Urology. 2011;78:476, 476.e1–476.e8. doi: 10.1016/j.urology.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon MH, Ryu JK, Kim WJ, Jin HR, Song KM, Kwon KD, et al. Effect of intracavernous administration of angiopoietin-4 on erectile function in the streptozotocin-induced diabetic mouse. J Sex Med. 2013;10:2912–2927. doi: 10.1111/jsm.12278. [DOI] [PubMed] [Google Scholar]
- 29.Bhojani N, Mandeville JA, Hameed TA, Soergel TM, McAteer JA, Williams JC, Jr, et al. Lithotripter outcomes in a community practice setting: comparison of an electromagnetic and an electrohydraulic lithotripter. J Urol. 2015;193:875–879. doi: 10.1016/j.juro.2014.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mustafa M, Aburas H, Helo FM, Qarawi L. Electromagnetic and electrohydraulic shock wave lithotripsy-induced urothelial damage: is there a difference? J Endourol. 2017;31:180–184. doi: 10.1089/end.2016.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]