Abstract
Naked mole rats (NMRs) are among the most hypoxia-tolerant mammals. Other species respond to hypoxia by either escaping the hypoxic environment or drastically decreasing behavioural activity and body temperature (Tb) to conserve energy. However, NMRs rarely leave their underground burrows, which are putatively hypoxic and thermally stable near the NMRs' preferred Tb. Therefore, we asked whether NMRs are able to employ behavioural and thermoregulatory strategies in response to hypoxia despite their need to remain active and the minimal thermal scope in their burrows. We exposed NMRs to progressively deeper levels of hypoxia (from 21 to 0% O2) while measuring their behaviour and Tb. Behavioural activity decreased 40–60% in hypoxia and Tb decreased slightly in moderate hypoxia (5–9%) and then further with deeper hypoxia (3% O2). However, even at 3% O2 NMRs remained somewhat active and warm, and continued to explore their environment. Remarkably, NMRs were active for greater than 90 s in acute anoxia and Tb and metabolic rate decreased rapidly. We conclude that NMRs are adapted to remain awake and functional even at the extremes of their hypoxia-tolerance. This adaptation likely reflects variable and challenging levels of environmental hypoxia in the natural habitat of this species.
Keywords: metabolic rate depression, hypoxia-tolerant, body temperature
1. Introduction
Naked mole rats (NMRs; Heterocephalus glaber) are among the most hypoxia-tolerant mammals and are able to survive hours at 3% O2, days at 8% O2 and 18 min in anoxia in a laboratory setting [1–3]. Mammals rely on continuous oxygen delivery for aerobic energy production but oxygen availability is often limited by environmental factors, such as life in densely populated burrows. Matching metabolic demand to energy supply is the key to tolerating prolonged hypoxia and vertebrates have evolved adaptive strategies that contribute to this balance. These strategies can be grouped into two categories: (i) increasing oxygen delivery to tissues and (ii) reducing energy demand via metabolic rate depression [4]. This second mechanism is successfully employed by the most anoxia-tolerant vertebrates, which typically reduce body temperature (Tb) and enter into a coma-like state during seasonal periods of severe hypoxia or anoxia. Conversely, it is speculated that in the wild—given their deep nests and the large number of animals within the colony—that NMRs likely encounter chronic hypoxia throughout their lives. Therefore, it is likely that NMRs not only cannot escape this environment but also must perform their daily activities with reduced O2 availability. Furthermore, NMR burrows have a stable ambient temperature (Ta) within a few degrees Celsius of their preferred Tb [5,6], which offers minimal scope for thermoregulatory energy savings in hypoxia.
Despite these apparent restrictions on the use of behavioural and thermoregulatory strategies in response to hypoxia within their burrows, we hypothesized that NMRs would nonetheless use these strategies to the maximum degree permitted by their ecophysiology. Previous reports of behavioural and thermal responses to extreme hypoxia in NMRs are mostly observational and these parameters have not been evaluated empirically. To address this knowledge gap, we held awake and freely behaving NMRs at their typical burrow Ta (approx. 30.5°C), and exposed them to progressively deeper levels of hypoxia while tracking behavioural activity and Tb. In addition, we exposed NMRs to anoxia to determine how long they were able to maintain activity and whether they adjusted Tb and metabolism in anoxia.
2. Abridged methodology
(a). Experimental design
NMRs were individually placed, unrestrained, into a custom-designed experimental chamber maintained at 30.3 ± 0.4°C (figure S1; for complete details regarding experimental methodology, see the electronic supplementary material). Behavioural parameters including movement speed (cm min−1), cumulative duration of activity (s min−1) and Tb were monitored throughout experiments. The experimental chamber was sealed and constantly ventilated with air (21% O2, balance N2) at a flow rate of 350–400 ml min−1. For control experiments (n = 9), NMRs were monitored for 6 h in normoxia. For hypoxic experiments (n = 8), NMRs were monitored for 1 h in normoxia followed by 1 h periods of progressive hypoxia (9, 7, 5 and 3% O2), followed by a 1 h recovery period in normoxia.
For anoxia experiments, NMRs were placed in a 200 ml chamber (figure S2), and gases were supplied at 500 ml min−1 to rapidly remove O2. Baseline activity, metabolic rate and Tb measurements were collected and then the chamber was rapidly switched to anoxia by supplying pure N2. Behaviour was monitored until the animals stopped moving and appeared to lose consciousness, at which point Tb was recorded and then the animal was removed from the chamber to recover.
3. Results and discussion
(a). Naked mole rats decrease physical activity and Tb in acute hypoxia but remain active
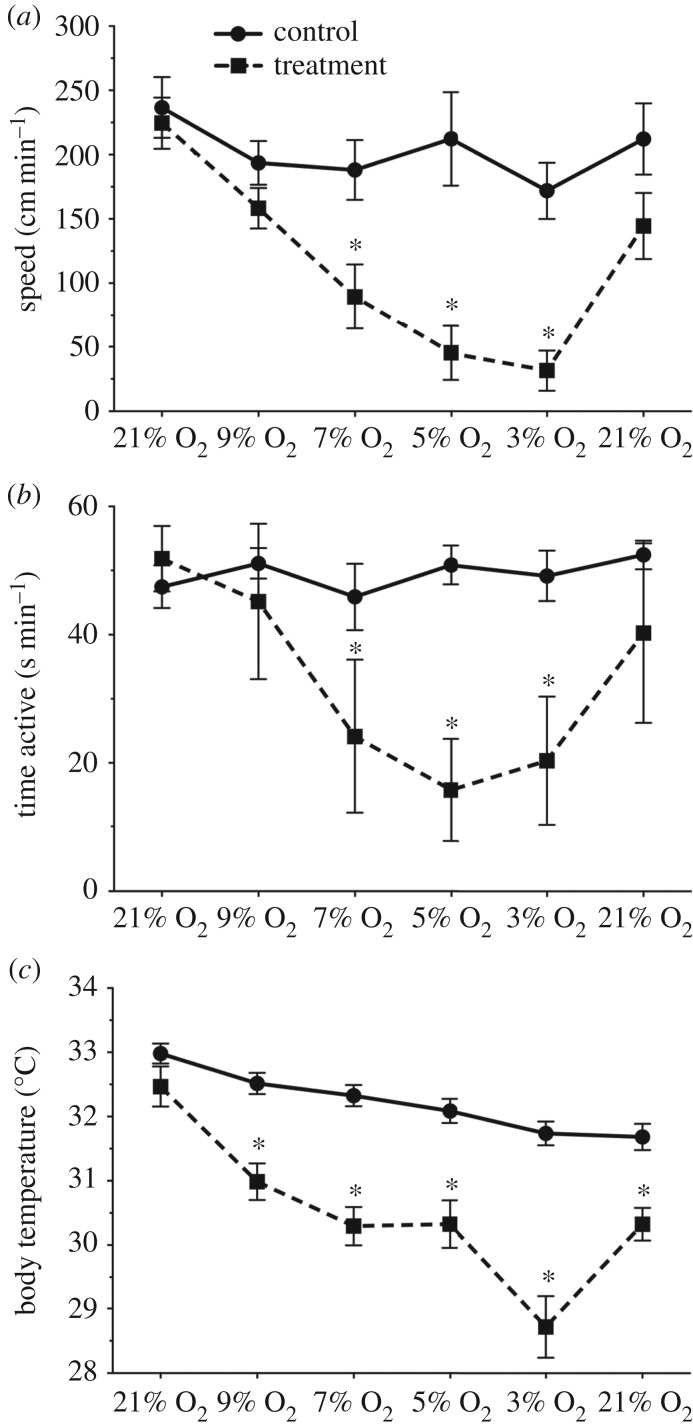
NMR movement speed and total time active were unchanged through 6 h of normoxia (figure 1a,b, circles) and were not significantly affected by hypoxia ≥9% O2 (figure 1a,b, squares). Conversely, both variables decreased in 7, 5 and 3% O2. The maximum suppression of speed occurred in 3% O2 (approx. 86% reduction), whereas time active was lowest in 5% O2 (approx. 70% reduction). Activity levels returned to baseline upon reoxygenation. Tb tended to decrease throughout control experiments but this trend did not reach significance (figure 1c). Conversely, relative to normoxia, Tb was significantly lowered by approximately 1.5–2°C within the range of 9–5% O2, and then dropped 1.5°C further in 3% O2. Tb recovered upon reoxygenation.
Figure 1.
NMRs decrease activity and Tb in hypoxia but remain awake and active. Summaries of NMR movement speed (a), time active (b) and Tb (c) during normoxia (n = 9) or progressive hypoxia (n = 8). Data are mean ± s.e.m. Asterisks (*) indicate significant differences between normoxia and hypoxia (p < 0.05).
This behavioural response of NMRs to acute hypoxia is similar to findings from other hypoxia- and anoxia-tolerant species. For example, the anoxia-tolerant crucian carp decreases locomotor activity but nonetheless remains active in nearly anoxic environments [7]. Most mammals use behavioural means to escape hypoxia, or if hypoxia cannot be avoided, to move to cooler regions to take advantage of anapyretic energy savings in hypoxia. Indeed, there is an inverse relationship between survival time in hypoxia and Tb in most small mammals [8]. Moving to colder environments facilitates a downward shift in the thermal set point, which contributes to this metabolic benefit. Conversely, hypoxia-tolerant mammals typically enter into a coma-like state when exposed to acute hypoxia, and remain inactive until normoxia is restored [8]. However, NMRs putatively live in chronic hypoxia in an environment in which Ta may fluctuate by as little as 1°C per year [5], and thus cannot readily escape hypoxia or move to cooler regions. Therefore, it is not surprising that NMRs exhibit a unique behavioural strategy in acute hypoxia, which is characterized by (i) a lower O2 threshold below which behaviour is effected by hypoxia, (ii) reduced activity in general, but (iii) maintenance of some activity and responsiveness to their environment.
Relative to their unique behavioural phenotype in hypoxia, the thermal response of NMRs to acute hypoxia is more consistent with that of other mammals, although the magnitude of this response is blunted by the limited thermal scope in their natural environment. For example, previous studies reported that mouse and hamster Tb decrease by 7°C and 3.5°C, respectively, when they are exposed to 5.5% O2 [9]. The thermoregulatory pattern we observe during hypoxia is notable within the context of the ecophysiology of this species. As NMRs likely live in chronic hypoxia in nature, 21% O2 in a laboratory setting presumably represents a hyperoxic environment, whereas 9% O2 may approach their natural burrow atmosphere. It is, therefore, notable that Tb plateaus at a reduced level in moderate hypoxia (9–5% O2) but then drops further in severe hypoxia (3% O2). An intriguing finding of our study is that NMR Tb dropped below Ta in 3% O2; however, the variability around the mean for this dataset overlaps with the measurement errors associated with the instruments used to measure Tb and Ta and this finding should be interpreted cautiously; it is likely that Tb drops to very near Ta, but not beyond. Nonetheless, the rapid drop in Tb in hypoxic NMRs is clear and remarkable.
Another important observation of our study is that NMR Tb remains elevated in normoxia at cold temperatures. There is disagreement in the literature as to whether or not NMRs are able to thermoregulate in normoxia at temperatures below their thermoneutral zone (TNZ: 31–34°C [6]). Specifically, one study found that NMR Tb tracks within less than 1–2°C of Ta [6], whereas others have reported that NMRs can maintain Tb at levels significantly higher (Tb – Ta ≤ 13.2°C) in Tas well below their TNZ [10,11]. Our results agree well with the earlier studies. However, differences in experimental approach between these studies shed light on the likely physiological mechanisms that facilitate rapid heat loss in NMRs in hypoxia. For example, in [11], the relative humidity was 100%, which would prevent evaporative water loss. Conversely, in [6], animals were exposed to a very high airflow rate, which would facilitate convective cooling, and 0% relative humidity, which would facilitate evaporative cooling (R. Buffenstein 2017, personal communication). In these experiments, evaporative water loss accounted for greater than 300% of metabolic heat production [6], which would explain the animals' inability to maintain Tb > Ta. Conversely, in our experiments, airflow through the chamber was slow but constant and the relative humidity level was approximately 50%. NMRs exposed to 3% O2 spent considerable periods of time (approx. 85%) lying on their backs, maximizing the exposure of abdominal skin surface to ambient air. When in this position, abdominal skin appeared very pink, indicating a high degree of blood flow, likely to facilitate heat transfer. Taken together, these observations from our laboratory and others suggest that NMRs may maximize peripheral circulation and use evaporative cooling, along with reduced behavioural activity, to rapidly reduce Tb in hypoxia.
(b). Naked mole rats maintain activity in acute anoxia and recover fully following reoxygenation
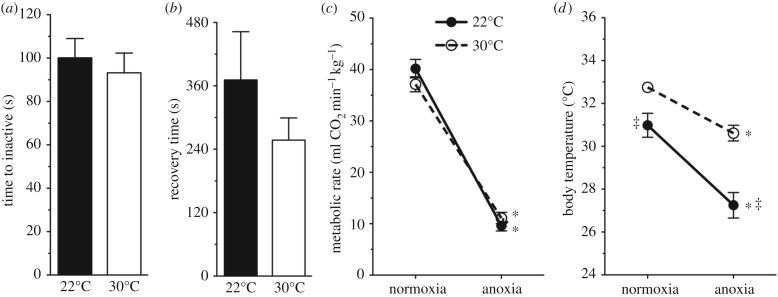
NMRs remained active in anoxia for approximately 100 s in both experimental temperatures before locomotor activity ceased (figure 2a). Animals recovered in normoxia and regained consciousness and mobility within 4–6 min of reoxygenation (figure 2b). Few animals can survive an anoxic challenge, and mammals, in particular, fare poorly under these conditions. For example, mice remain active for 26 s in anoxia at room temperature and do not recover [12]. In light of this, the ability of NMRs to maintain activity and consciousness in anoxia for up to 100 s is remarkable, as is the fact that animals recovered within a few minutes. Metabolic rate in anoxia decreased by approximately 75% in just 90 s (figure 2c). Tb also dropped rapidly in anoxia, decreasing by 2 and 4°C in Tas of 30°C and 22°C, respectively (figure 2d).
Figure 2.
NMRs tolerate short-term anoxia. (a,b) Summaries of time active in anoxia until cessation of movement (a), time elapsed in recovery until restoration of movement (b), metabolic rate (c) and Tb (d). Data are mean ± s.e.m., n = 7 per group. Asterisks (*) indicate significant differences between normoxia and anoxia, double daggers indicate significant differences between 22 and 30°C (p < 0.05).
A previous study did not report any change of NMR Tb in response to anoxia [3]. Although it is not specified in that paper, rectal Tb measurements were presumably taken after the animals had lost consciousness, as taking rectal temperature from active NMRs in a sealed jar is not a trivial task. In our experiments, NMR Tb dropped within 90 s of the onset of anoxia and had decreased by the time animals lost consciousness. Therefore, it is likely that these authors missed the anoxia-related decrease in Tb due to their experimental design. In support of this interpretation, our Tb measurements from NMRs that had lost consciousness in anoxia agree well with the Tb measurements in [3]. Interestingly, in our experiments, all animals were observed urinating on themselves with the onset of anoxia, which is a strategy that supports very rapid evaporative cooling in ectotherms [13], and likely contributed to the rapid heat loss in anoxic NMRs, along with higher rates of air flow in the chamber.
4. Conclusion
Taken together, our results indicate that NMRs use behavioural and thermoregulatory strategies that are consistent with reduced metabolic rate in acute hypoxia or anoxia. The degree to which these strategies are employed by NMRs is limited by their warm and constantly hypoxic burrow environment and appears to correlate with the severity of the hypoxic stress, such that at more extreme levels of hypoxia, NMRs enter into a coma-like state and cease all activity.
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
All procedures were conducted in accordance with the relative animal care and experimentation guidelines of the Canadian Council on Animal Care and were approved by the University of Ottawa Animal Care Committee (protocol no. 2535).
Data accessibility
Supporting data have been uploaded to Dryad and can be accessed at http://dx.doi.org/10.5061/dryad.11m62 [14].
Authors' contributions
M.E.P. conceived of and designed the study and wrote the manuscript. A.N.I. and A.M.K. performed the experiments, analysed all data and edited the manuscript. All authors gave final approval of the published version and agree to be accountable for all content therein.
Competing interests
We have no competing interests.
Funding
This work was supported by an NSERC Discovery grant, a Canada Research Chairship and a Parker B Francis PDF to M.E.P.
References
- 1.Pamenter ME, Dzal YA, Milsom WK. 2015. Adenosine receptors mediate the hypoxic ventilatory response but not the hypoxic metabolic response in the naked mole rat during acute hypoxia. Proc. R. Soc. B 282, 20141722 ( 10.1098/rspb.2014.1722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung D, Dzal YA, Seow A, Milsom WK, Pamenter ME. 2016. Naked mole rats exhibit metabolic but not ventilatory plasticity following chronic sustained hypoxia. Proc. R. Soc. B 283, 20160216 ( 10.1098/rspb.2016.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park TJ, et al. 2017. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356, 307–311. ( 10.1126/science.aab3896) [DOI] [PubMed] [Google Scholar]
- 4.Dzal YA, Jenkin SE, Lague SL, Reichert MN, York JM, Pamenter ME. 2015. Oxygen in demand: how oxygen has shaped vertebrate physiology. Comp. Biochem. Physiol. A 186, 4–26. ( 10.1016/j.cbpa.2014.10.029) [DOI] [PubMed] [Google Scholar]
- 5.Bennett NC, Jarvis JUM, Davies KC. 1988. Daily and seasonal temperatures in the burrows of African rodent moles. S. Afr. J. Zool. 23, 189–195. ( 10.1080/02541858.1988.11448101) [DOI] [Google Scholar]
- 6.Buffenstein R, Yahav S. 1991. Is the naked mole-rat Hererocephalus glaber an endothermic yet poikilothermic mammal. J. Therm. Biol. 16, 227–232. ( 10.1016/0306-4565(91)90030-6) [DOI] [Google Scholar]
- 7.Nilsson GE, Rosen P, Johansson D. 1993. Anoxic depression of spontaneous locomotor activity in crucian carp quantified by a computerized imaging technique. J. Exp. Biol. 180, 153–162. [Google Scholar]
- 8.Wood SC, Dupre RK, Hicks JW. 1985. Voluntary hypothermia in hypoxic animals. Acta Physiol. Scand. 124, 542. [Google Scholar]
- 9.Gordon CJ, Fogelson L. 1991. Comparative effects of hypoxia on behavioral thermoregulation in rats, hamsters, and mice. Am. J. Physiol. 260, R120–R125. [DOI] [PubMed] [Google Scholar]
- 10.Withers PC, Jarvis JUM. 1980. The effect of huddling on thermoregulation and oxygen consumption for the naked mole-rat. Comp. Biochem. Physiol. A 66, 215–219. ( 10.1016/0300-9629(80)90154-1) [DOI] [Google Scholar]
- 11.Mcnab BK. 1966. The metabolism of fossorial rodents: a study of convergence. Ecology 47, 712–733. ( 10.2307/1934259) [DOI] [Google Scholar]
- 12.Hartung J, Cottrell JE. 1987. Nitrous oxide reduces thiopental-induced prolongation of survival in hypoxic and anoxic mice. Anesth. Analg. 66, 47–52. ( 10.1213/00000539-198701000-00008) [DOI] [PubMed] [Google Scholar]
- 13.Tattersall GJ, Gerlach RM. 2005. Hypoxia progressively lowers thermal gaping thresholds in bearded dragons, Pogona vitticeps. J. Exp. Biol. 208, 3321–3330. ( 10.1242/jeb.01773) [DOI] [PubMed] [Google Scholar]
- 14.Ilacqua AN, Kirby AM, Pamenter ME.. 2017. Data from: Behavioural responses of naked mole rats to acute hypoxia and anoxia Dryad Digital Repository. ( 10.5061/dryad.11m62) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ilacqua AN, Kirby AM, Pamenter ME.. 2017. Data from: Behavioural responses of naked mole rats to acute hypoxia and anoxia Dryad Digital Repository. ( 10.5061/dryad.11m62) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Supporting data have been uploaded to Dryad and can be accessed at http://dx.doi.org/10.5061/dryad.11m62 [14].