Abstract
Planktonic copepods of the genus Calanus play a central role in North Atlantic/Arctic marine food webs. Here, using molecular markers, we redrew the distributional ranges of Calanus species inhabiting the North Atlantic and Arctic Oceans and revealed much wider and more broadly overlapping distributions than previously described. The Arctic shelf species, C. glacialis, dominated the zooplankton assemblage of many Norwegian fjords, where only C. finmarchicus has been reported previously. In these fjords, high occurrences of the Arctic species C. hyperboreus were also found. Molecular markers revealed that the most common method of species identification, prosome length, cannot reliably discriminate the species in Norwegian fjords. Differences in degree of genetic differentiation among fjord populations of the two species suggested that C. glacialis is a more permanent resident of the fjords than C. finmarchicus. We found no evidence of hybridization between the species. Our results indicate a critical need for the wider use of molecular markers to reliably identify and discriminate these morphologically similar copepod species, which serve as important indicators of climate responses.
Keywords: zooplankton, genetics, climate change, species identification, fjord, ecosystem shift
1. Introduction
Copepods of the genus Calanus are central in North Atlantic and Arctic pelagic food webs. Rich in lipids, they form a key link between primary producers and secondary consumers and predators. Four species of the genus Calanus occur throughout the North Atlantic and Arctic Oceans (figure 1): C. helgolandicus (Chel), C. hyperboreus (Chyp), C. finmarchicus (Cfin) and C. glacialis (Cgla); and there has been considerable effort to document and model their distributional changes [1,2]. Importantly, abundances and dynamics of fish stocks are strongly associated with Calanus species composition and abundances [3], and climate-driven changes in their biogeographical distributions (i.e. range shifts) can lead to ecosystem regime shifts and potential collapse of fish stocks such as cod [4]. However, distinguishing Calanus species is challenging due to their morphological similarity and lack of diagnostic characters. The usual method of species identification is body (prosome) length, although this approach has been questioned [5,6]. Misidentification may thus occur, impacting the reliability of our current knowledge of species distributions and preventing accurate assessment of species geographical range shifts in response to climate change.
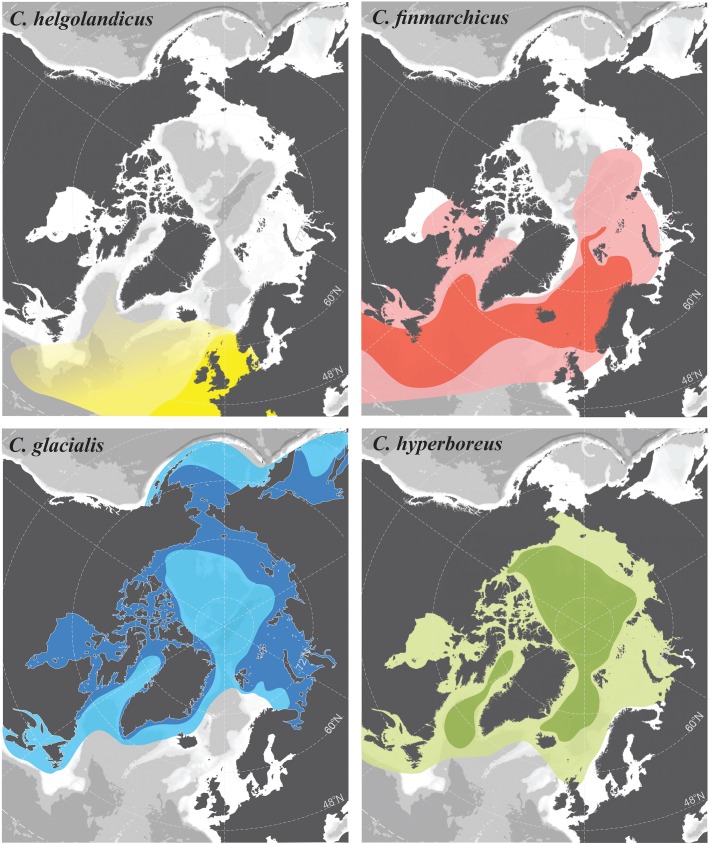
Figure 1.
Calanus species distributional ranges in the North Atlantic and Arctic Oceans based on morphological identification from previous studies (sources in electronic supplementary material, S8). For each panel, dark-shaded colour represents core area for each species, where reproduction is known to occur; light-shaded colour represents the total described distributional area.
Here we re-examine the distributional ranges of four co-occurring Calanus species in the North Atlantic and Arctic Oceans, using six molecular markers designed to ensure reliable species identification.
2. Material and methods
(a). Sample collection
Zooplankton samples were collected from 83 locations in the North Atlantic and Arctic Oceans (electronic supplementary material, S1) by vertical nets tows with 150–200 µm mesh sizes and preserved in 70–80% ethanol. A Folsom plankton splitter was used to make subsamples containing up to 150 Calanus individuals from developmental stage CIV–CVI (electronic supplementary material, S1). No species level morphological identification was performed for any individuals.
(b). Molecular species identification
DNA was extracted from the excised antennae of each specimen using the HotSHOT protocol [7], and molecular species identification of 4434 individuals was achieved using six nuclear markers type InDels (Insertion or Deletion motifs) [8] scored on a 3500xL genetic analyzer (Applied Biosystems). These bi-parentally inherited markers are easy to use and can potentially detect hybridization [9]. Their reliability was confirmed by the traditional, but more cost- and labour-intensive mitochondrial 16S rDNA sequencing (mtDNA) [10,11] of 159 individuals from 53 locations (electronic supplementary material, S2 and S3), following Smolina et al. [8]. In addition, 129 individuals from Saltfjord/Skjerstadfjord were measured (prosome length) and sequenced for the 16S (table 1; electronic supplementary material, S4 and S5). Identification of specimens from InDels and 16S rDNA sequences was congruent for all 677 individuals investigated (288 in present study (electronic supplementary material, S2–S4) and 389 in Nielsen et al. [9]). InDel markers were also used to test for the presence of hybrids between Cfin and Cgla [9] (electronic supplementary material, S6).
Table 1.
Comparison of Calanus finmarchicus (Cfin) and C. glacialis (Cgla) identification methods in Saltenfjord/Skjerstadfjord.
| prosome length range (μm) |
|||||||
|---|---|---|---|---|---|---|---|
| Saltenfjord/Skjerstadfjord | InDel species ID | 16S rDNA species ID | markers' congruence | N | stage CV | N | stage CVI female |
| Cfin | 89 | 89 | 100% | 26 | 1976.64–2717.76 | 14 | 2406.89–2747.02 |
| Cgla | 40 | 40 | 100% | 20 | 2119.40–2623.33 | 69 | 2150.68–3030.50 |
(c). Population differentiation
Population genetic analysis was carried out to distinguish between fjord resident and drifting (seasonally transient) species [12] (electronic supplementary material, S7). Focusing on Cfin and Cgla populations, genetic differentiation was measured using the global index of population differentiation, FST [13], based on 10 microsatellite DNA markers [14,15] assayed for 24 individuals per species from three locations: Isfjord, Saltfjord and Lurefjord.
3. Results and discussion
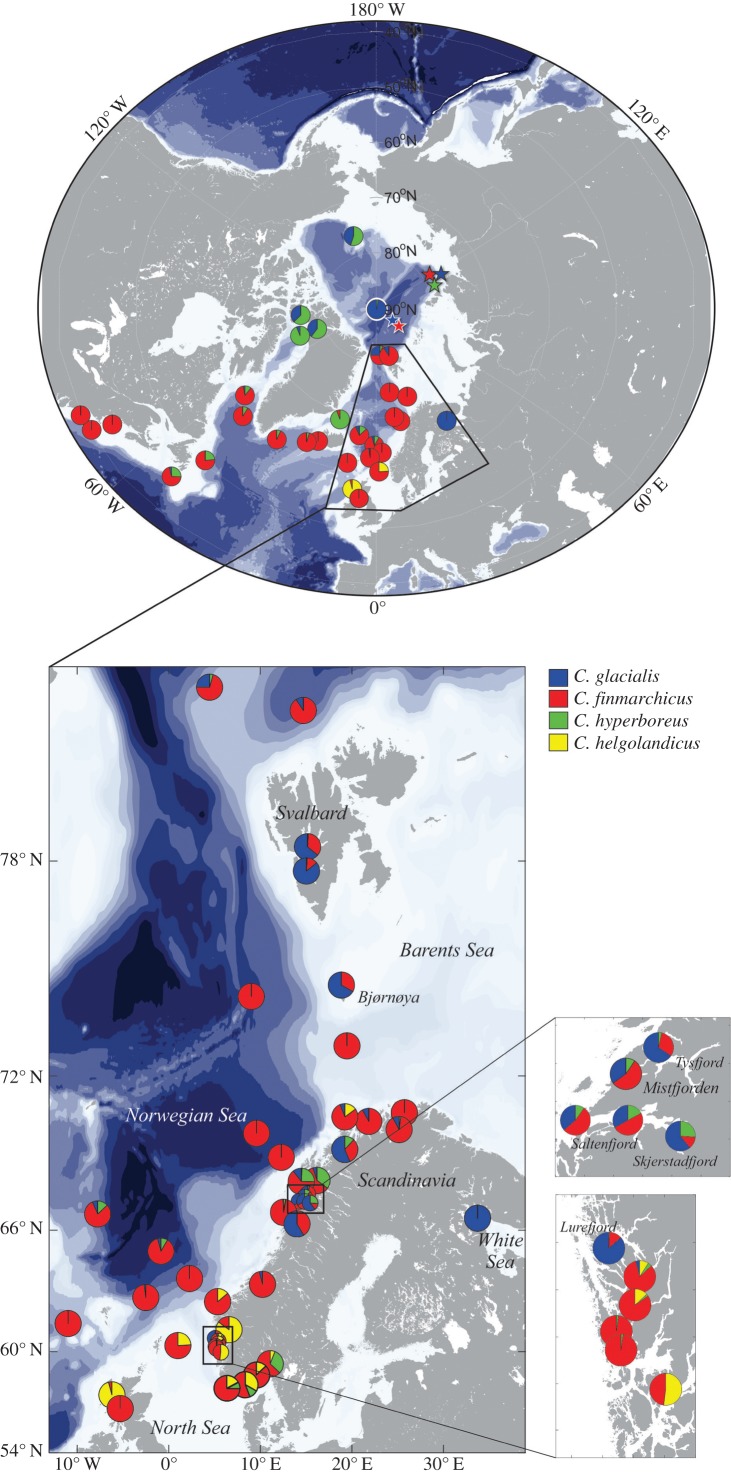
Identification of Calanus species using molecular markers revealed that all four species have much wider distributional ranges than previously reported (figures 1 and 2; electronic supplementary material, S1), as suggested by an earlier molecular study [6]. The distribution of Chel was known to extend from the Mediterranean Sea to the North Sea (58° N, figure 1) [16]. Here, we identified Chel in several Norwegian fjords and in the Norwegian Sea as far north as 70° N (figure 2). Specimens found in Myken stations (66° N) and near Tromsø (70° N) could result from transport in ocean frontal jet currents running from the North Sea along the Norwegian coast. However, the high prevalence (85%) of the species recorded in the relatively isolated Sognefjord (61° N) may represent a locally established population. It remains to be tested whether Chel has always been present in these fjords but misidentified, or whether our findings represent evidence of a recent biogeographical range shift.
Figure 2.
Calanus species distributional ranges in the North Atlantic and Arctic Oceans based on molecular species identifications. Pie charts represent relative frequencies of C. glacialis (blue), C. finmarchicus (red), C. hyperboreus (green) and C. helgolandicus (yellow) in each sample. Stars indicate non-quantitative species records.
Previous reports of the Arctic Chyp [17] occurring in the northern Norwegian Sea (figure 1) have been attributed to transport of individuals by Arctic intermediate waters [18]. Here, we detected the species in large proportions along the Norwegian coast, everywhere north of 58° N (figure 2; electronic supplementary material, S1). Whether the southern presence of Chyp results from advection from Arctic stocks or from self-reproducing populations remains to be investigated.
Calanus finmarchicus is currently considered to be an indicator species of North Atlantic water masses [17], and our results largely support this view (figure 2). The genetically confirmed species range extends as far north as 87° N and as far east in the Arctic as the eastern boarder of the Laptev Sea (78° N, 113° E, figure 2), regions of the Arctic Ocean affected by Atlantic inflow. It was proposed that Cfin may thrive in these Northern regions and replace Cgla in response to Arctic warming [19], however, at present the individuals recorded at these most northerly locations were likely transported from southern populations [19].
Calanus glacialis is regarded as a true Arctic shelf species, which serves as a circumpolar indicator of these waters [17] (figure 1). We rarely observed it offshore in Atlantic waters, but documented the species' occurrence in many Norwegian fjords, as far south as 60° N (figure 2), where it usually co-occurred with Cfin in fjords with deep basins separated from shelf waters by shallower sills (electronic supplementary material, S1). In several fjords, Cgla dominated over other Calanus species; we recorded a positive gradient of relative abundance of Cgla from the mouth to the innermost areas of some fjords (e.g. Ranfjord, electronic supplementary material, S1).
In the fjords, prosome length of Cgla and Cfin overlapped completely (table 1; electronic supplementary material, S5), which explains why Cgla's large occurrence has not been reported previously. Furthermore, recent work by our group shows that morphological characters cannot reliably distinguish between Cfin and Cgla throughout their range [20].
Some zooplankton species are long-term residents of Norwegian fjords, while others are replaced periodically with basin water exchanges [21]. Resident species are expected to show greater genetic differentiation among fjord populations than drifting species [12]. Our analysis found no significant genetic differentiation among fjord populations of Cfin (FST = 0.004n.s.), but Cgla populations did differ significantly (FST = 0.03*), suggesting lower rates of exchange (i.e. gene flow) for Cgla than for Cfin. These results support previous descriptions of Cfin as a drifting species [12] that is advected into and out of fjords seasonally [22]. Less gene flow—together with the absence of offshore populations—suggests that Cgla populations are resident [12]. In both the White Sea [23] and Lurefjord [24], Cgla is known to migrate in early summer from warm surface layers to colder deep water. This may explain the species' ability to maintain local populations and avoid transport out of fjords.
Hybridization between Cfin and Cgla has been suggested in the Northwest Atlantic [14] based on microsatellite markers developed for C. finmarchicus. Notably, no first-generation hybrids were found in our survey of 4434 individuals from samples collected throughout the Northeast Atlantic and Arctic Oceans (electronic supplementary material, S6). Based on the nature of the molecular characters (nuclear, co-dominant InDels) used for species identification and careful ground-truthing of our molecular results, we conclude that hybridization between the species, if it occurs at all, is rare or episodic.
4. Conclusion
Marine zooplankton have been regarded as sentinels of climate change [25] due to their short life histories and rapid responses to environmental variation. Development and use of molecular characters that can ensure accurate and reliable identification and discrimination of key indicator species, such as those within the Calanus genus, are critically needed. Only then can these species be used to document past, present and future patterns of biogeographical distributions, and detect and track responses of pelagic communities to climate change.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. Krogstad, E. Abramova, F. Norrbin, Ø. Leiknes, S. Basedow, T. Dale, T. Falkenhaug, A. Mailli, K. Last, S. Wells and the captains and crews of R/V Helmer Hanssen and G.O. Sars for their assistance with sampling. We are grateful to the ARCTOS network for support and useful discussions. We acknowledge two anonymous reviewers for constructive comments.
Data accessibility
Protocols are attached as the electronic supplementary material; genotypes and sequences have been deposited to public database, respectively Dryad (http://dx.doi.org/10.5061/dryad.tq71j) [26] and GenBank® (MF959702-MF959730 and MF972920-MF972922).
Authors' contributions
M.C. & G.H. designed the study, collected and analysed data, developed the method and wrote the first draft of the manuscript. M.H., W.M., S.S., K.E., A.B., K.K., J.E.S., A.K.S.D., S.K. and C.S. collected and analysed data. I.S. collected data and contributed to the development of the method. M.D. and V.T. analysed data and made the figures. All authors contributed significantly to the manuscript, approved the final version and agreed to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
M.C. was supported by the EU (FP7-EURO-BASIN-264933), Norwegian Research Council (216578; 227139; 246747) and Nord University. M.H. was supported by UNIS. K.K. was supported by the Russian Foundation for Basic Research (15-29-02447; 16-04-00375) and the Russian Scientific Foundation (14-50-00095). M.D. was supported by NRC-226417. S.K. was supported by the Polish–Norwegian Research Program (Pol-Nor/201992/93/2014).
References
- 1.Beaugrand G, Reid PC, Ibanez F, Lindley JA, Edwards M. 2002. Reorganization of North Atlantic marine copepod biodiversity and climate. Science 296, 1692–1694. ( 10.1126/science.1071329) [DOI] [PubMed] [Google Scholar]
- 2.Villarino E, Chust G, Licandro P, Butenschön M, Ibaibarriaga L, Larrañaga A, Irigoien X. 2015. Modelling the future biogeography of North Atlantic zooplankton communities in response to climate change. Mar. Ecol. Prog. Ser. 531, 121–142. ( 10.3354/meps11299) [DOI] [Google Scholar]
- 3.Beaugrand G, Kirby RR. 2010. Climate, plankton and cod. Glob. Change Biol. 16, 1268–1280. ( 10.1111/j.1365-2486.2009.02063.x) [DOI] [Google Scholar]
- 4.Beaugrand G, Brander KM, Lindley JA, Souissi S, Reid PC. 2003. Plankton effect on cod recruitment in the North Sea. Nature 426, 661–664. ( 10.1038/nature02164) [DOI] [PubMed] [Google Scholar]
- 5.Gabrielsen TM, Merkel B, Søreide JE, Johansson-Karlsson E, Bailey A, Vogedes D, Nygård H, Varpe Ø, Berge J. 2012. Potential misidentifications of two climate indicator species of the marine arctic ecosystem: Calanus glacialis and C. finmarchicus. Polar Biol. 35, 1621–1628. ( 10.1007/s00300-012-1202-7) [DOI] [Google Scholar]
- 6.Lindeque PK, Harris RP, Jones MB, Smerdon GR. 2004. Distribution of Calanus spp. as determined using a genetic identification system. Sci. Mar. 68, 121–128. ( 10.3989/scimar.2004.68s1121) [DOI] [Google Scholar]
- 7.Montero-Pau J, Gómez A, Muñoz J. 2008. Application of an inexpensive and high-throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnol. Oceanogr. Methods 6, 218–222. ( 10.4319/lom.2008.6.218) [DOI] [Google Scholar]
- 8.Smolina I, Kollias S, Poortvliet M, Nielsen TG, Lindeque P, Castellani C, Moller EF, Blanco-Bercial L, Hoarau G. 2014. Genome- and transcriptome-assisted development of nuclear insertion/deletion markers for Calanus species (Copepoda: Calanoida) identification. Mol. Ecol. Res 14, 1072–1079. ( 10.1111/1755-0998.12241) [DOI] [PubMed] [Google Scholar]
- 9.Nielsen TG, Kjellerup S, Smolina I, Hoarau G, Lindeque P. 2014. Live discrimination of Calanus glacialis and C. finmarchicus females: can we trust phenological differences? Mar. Biol. 161, 1299–1306. ( 10.1007/s00227-014-2419-5) [DOI] [Google Scholar]
- 10.Lindeque P, Harris R, Jones M, Smerdon G. 1999. Simple molecular method to distinguish the identity of Calanus species (Copepoda: Calanoida) at any developmental stage. Mar. Biol. 133, 91–96. ( 10.1007/s002270050446) [DOI] [Google Scholar]
- 11.Lindeque PK, Hay SJ, Heath MR, Ingvarsdottir A, Rasmussen J, Smerdon GR, Waniek JJ. 2006. Integrating conventional microscopy and molecular analysis to analyse the abundance and distribution of four Calanus congeners in the North Atlantic. J. Plankton Res. 28, 221–238. ( 10.1093/plankt/fbi115) [DOI] [Google Scholar]
- 12.Bucklin A, Kaartvedt S, Guarnieri M, Goswami U. 2000. Population genetics of drifting (Calanus spp.) and resident (Acartia clausi) plankton in Norwegian fjords. J. Plankton Res. 22, 1237–1251. ( 10.1093/plankt/22.7.1237) [DOI] [Google Scholar]
- 13.Weir BS, Cockerham CC. 1984. Estimating F-statistics for the analysis of population structure. Evolution 1984, 1358–1370. [DOI] [PubMed] [Google Scholar]
- 14.Parent GJ, Plourde S, Turgeon J. 2012. Natural hybridization between Calanus finmarchicus and C. glacialis (Copepoda) in the Arctic and Northwest Atlantic. Limnol. Oceanogr. 57, 1057–1066. ( 10.4319/lo.2012.57.4.1057) [DOI] [Google Scholar]
- 15.Provan J, Beatty GE, Keating SL, Maggs CA, Savidge G. 2009. High dispersal potential has maintained long-term population stability in the North Atlantic copepod Calanus finmarchicus. Proc. R. Soc. B 276, 301–307. ( 10.1098/rspb.2008.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnard R, et al. 2004. Continuous plankton records: plankton atlas of the North Atlantic Ocean (1958–1999). II. Biogeographical charts. Mar. Ecol. Prog. Ser. 2004, 11–75. [Google Scholar]
- 17.Conover R. 1988. Comparative life histories in the genera Calanus and Neocalanus in high latitudes of the northern hemisphere. In Biology of copepods (eds Boxshall GA, Schminke HK), pp. 127–142. Berlin, Germany: Springer. [Google Scholar]
- 18.Broms C, Melle W, Kaartvedt S. 2009. Oceanic distribution and life cycle of Calanus species in the Norwegian Sea and adjacent waters. Deep Sea Res. Part II 56, 1910–1921. ( 10.1016/j.dsr2.2008.11.005) [DOI] [Google Scholar]
- 19.Wassmann P, et al. 2015. The contiguous domains of Arctic Ocean advection: trails of life and death. Prog. Oceanogr. 139, 42–65. ( 10.1016/j.pocean.2015.06.011) [DOI] [Google Scholar]
- 20.Choquet M, Kosobokova K, Kwaśniewski S, Hatlebakk M, Dhanasiri AKS, Melle W, Daase M, Svensen C, Søreide JE, Hoarau G. Can morphology reliably distinguish between the copepods Calanus finmarchicus and C. glacialis, or is DNA the only way? In press. Limnology Oceanography: Methods. [Google Scholar]
- 21.Lindahl O, Hernroth L. 1988. Large-scale and long-term variations in the zooplankton community of the Gullmar fjord, Sweden, in relation to advective processes. Mar. Ecol. Prog. Ser. 43, 161–171. ( 10.3354/meps043161) [DOI] [Google Scholar]
- 22.Skreslet S, Rød NÅ. 1986. Advection of Calanus finmarchicus between habitats in Norwegian coastal waters. In The role of freshwater outflow in coastal marine ecosystems (ed. Skreslet S.), pp. 375–387. Berlin, Germany: Springer. [Google Scholar]
- 23.Pertsova N, Kosobokova K. 2010. Interannual and seasonal variation of the population structure, abundance, and biomass of the arctic copepod Calanus glacialis in the White Sea. Oceanology 50, 531–541. ( 10.1134/S0001437010040090) [DOI] [Google Scholar]
- 24.Niehoff B, Hirche H-J. 2005. Reproduction of Calanus glacialis in the Lurefjord (western Norway): indication for temperature-induced female dormancy. Mar. Ecol. Prog. Ser. 285, 107–115. ( 10.3354/meps285107) [DOI] [Google Scholar]
- 25.Hays GC, Richardson AJ, Robinson C. 2005. Climate change and marine plankton. Trends Ecol. Evol. 20, 337–344. ( 10.1016/j.tree.2005.03.004) [DOI] [PubMed] [Google Scholar]
- 26.Choquet M, et al. 2017. Data from: Genetics redraws pelagic biogeography of Calanus. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Choquet M, et al. 2017. Data from: Genetics redraws pelagic biogeography of Calanus. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Protocols are attached as the electronic supplementary material; genotypes and sequences have been deposited to public database, respectively Dryad (http://dx.doi.org/10.5061/dryad.tq71j) [26] and GenBank® (MF959702-MF959730 and MF972920-MF972922).